FP tethering: a screening technique to rapidly identify compounds that disrupt protein–protein interactions†
Abstract
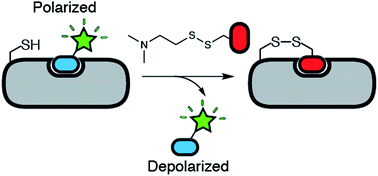
Tethering is a screening technique for discovering small-molecule fragments that bind to pre-determined sites via formation of a disulphide bond. Tethering screens traditionally rely upon mass spectrometry to detect disulphide bond formation, which requires a time-consuming liquid chromatography step. Here we show that tethering can be performed rapidly and inexpensively using a homogenous fluorescence polarization (FP) assay that detects displacement of a peptide ligand from the protein target as an indirect readout of disulphide formation. We apply this method, termed FP tethering, to identify fragments that disrupt the protein–protein interaction between the KIX domain of the transcriptional coactivator CBP and the transcriptional activator peptide pKID.

 Please wait while we load your content...
Please wait while we load your content...