Open Access Article
Open Access ArticleHeterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes†
Raisa
Haavikko
a,
Abedelmajeed
Nasereddin
b,
Nina
Sacerdoti-Sierra
b,
Dmitry
Kopelyanskiy
b,
Sami
Alakurtti
c,
Mari
Tikka
a,
Charles L.
Jaffe‡
b and
Jari
Yli-Kauhaluoma
*a
aFaculty of Pharmacy, Division of Pharmaceutical Chemistry, University of Helsinki, Viikinkaari 5 E, P. O. Box 56, FI-00014, Helsinki, Finland. E-mail: jari.yli-kauhaluoma@helsinki.fi; Tel: +358-9-191 59170
bDepartment of Microbiology and Molecular Genetics, IMRIC, Hebrew University-Hadassah Medical School, P. O. Box 12272, Jerusalem 91120, Israel. E-mail: cjaffe@cc.huji.ac.il; Tel: +972 26757435
cVTT Technical Research Centre of Finland, P. O. Box 1000 FI-02044 VTT, Espoo, Finland
First published on 8th January 2014
Abstract
The synthesis of heterocyclic betulin derivatives and their activity against Leishmania donovani is reported. Betulonic acid was used as a versatile intermediate. Several different fused heterocycles were introduced at the 2,3-position of the lupane skeleton including isoxazole, pyrazine, pyridine, indole and pyrazole rings. Also the 28-position was modified. Three compounds, 5, 8 and 25, showed low micromolar activity with IC50 values of 13.2, 4.3 and 7.2 μM, respectively. Compound 8 showed the best activity and selectivity, and its activity was tested on infected macrophages using a concentration, 5 μM, where no macrophage toxicity was exhibited. Interestingly, the activity of compound 8 on axenic amastigotes and Leishmania-infected macrophages was similar.
Introduction
Leishmaniasis is a spectrum of diseases caused by over 20 species of protozoan parasites belonging to the genus Leishmania. These diseases affect people in more than 88 countries. There are an estimated 1–2 million new cases every year, 12 million people currently infected, and 350 million people living in endemic areas at risk.1,2 During the past ten years leishmaniasis has spread considerably.2 It is transmitted by the bite of infected female Phlebotomine or Lutzomyia sandflies in the Old World and the New World, respectively.3There are three major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral disease.2,4 Cutaneous leishmaniasis is the mildest form of this disease and is characterized by skin ulcers on exposed areas at the site of the sand fly bite. The ulcers generally self-heal leaving scars after a few months to years. In the mucocutaneous form, which is difficult to treat, disfiguring lesions destroy the mucous membranes of the nose, mouth and throat cavity. Finally, visceral leishmaniasis (VL), the most severe form of the disease, is fatal if untreated. VL causes fever, weight loss, anaemia, and enlargement of the spleen and liver. Several treatments exist for leishmaniasis, but most of them have adverse effects. Pentavalent antimonials, the first-line treatment for leishmaniasis, have lost their efficacy in some regions endemic for VL,5 and liposomal amphotericin B is highly expensive. These treatments are administrated by injection and require clinical supervision or hospitalization. Miltefosine, the first effective orally administrated drug for leishmaniasis, is contraindicated in women of child-bearing age due to teratogenic effects.6 Hence, there is an urgent need to develop new, safe and effective treatments for these diseases.
Betulin is a plentiful naturally occurring lupane-type pentacyclic triterpene. Betulinic acid and other betulin derivatives show antiviral,7 anti-HIV,8 anti-inflammatory,9 anti-malarial,10 and anti-tumoral11 effects. Previously our group has shown that heterocyclic betulin derivatives have an effect against L. donovani amastigotes, which cause VL.12 In this study we describe a new set of heterocyclic betulin derivatives and their biological activity against Leishmania donovani amastigotes, as well as the structure–activity relationships of the compounds.
Results and discussion
Chemistry
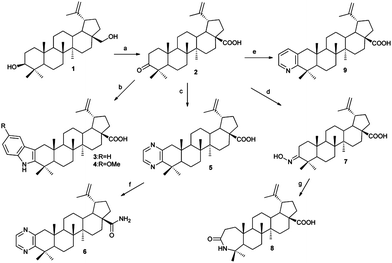
First, betulin 1 was subjected to Jones oxidation, and the resulting betulonic acid 2 was used as a key intermediate for the synthesis of several heterocyclic adducts (Scheme 1). The indole derivatives 3 and 4 were prepared by the Fischer indole synthesis in 21–42% yields. Letting betulonic acid react with ethylenediamine in the presence of sulfur and morpholine gave lupa-2,20(29)-dieno[2,3-b]pyrazin-28-oic acid 5 in 68% yield.13 This was treated with oxalyl chloride in dichloromethane (DCM) and the resulting acyl chloride was converted to the primary amide 6 quantitatively by aqueous ammonia in chloroform.14 3-Oximinolup-20(29)-en-28-oic acid 7 was obtained by refluxing betulonic acid in the presence of NH2OH·HCl and pyridine in methanol. The oxime 7 was further converted to 4-aza-3-oxohomolup-20(29)-en-28-oic acid 8 in 33% yield in the Beckmann rearrangement reaction by treating it with trifluoroacetic anhydride (TFAA) in DCM. Lupa-2,20(29)-dieno[2,3-b]pyridin-28-oic acid 9 was obtained from the reaction of betulonic acid and propargylamine in the presence of Cu(I)Cl in ethanol in 11% yield.15The corresponding isoxazole 10 and pyrazole 11 derivatives were synthesized via the 2-hydroxymethylene adduct 12 of betulonic acid 2 followed by the condensation/cyclization reaction with NH2OH·HCl16 or H2NNH2·H2O17 in 68% and 80% yields, respectively (Scheme 2). The carboxyl group of lupa-2,20(29)-dieno[2,3-d]isoxazol-28-oic acid 10 was converted to the primary amide functionality 13 as described above in the case of compound 6.14
20(29)-Dihydrolup-2-en[2,3-d]isoxazol-28-oic acid 14 was obtained from benzyl betulonate in three steps (Scheme 3). First, the carbon–carbon double bond of benzyl betulonate was reduced under a hydrogen atmosphere in the presence of palladium on carbon in ethyl acetate to give the corresponding dihydrobetulonic acid in 77% yield. The subsequent Claisen condensation with ethyl formate produced 2-(hydroxymethylene)-3-oxo-20(29)-dihydrolupen-28-oic acid in 56% yield. Finally, the treatment of the Claisen product with NH2OH·HCl in acetic acid gave the target 20(29)-dihydrolup-2-en[2,3-d]isoxazol-28-oic acid 14 in 90% yield.
28-Hydroxylupa-2,20(29)-dieno[2,3-d]isoxazole 15 was synthesized from betulin 1 in five steps (Scheme 4). First, the betulin C-28 hydroxy group was protected as a tetrahydropyranyl ether 16 in 80% yield, and the resulting THP ether was oxidized to the THP-protected betulonic alcohol with PCC in DCM18 in 46% yield. Subsequently, the same cascade of reactions as described above for lupa-2,20(29)-dieno[2,3-d]isoxazol-28-oic acid 10 was used to produce the isoxazole-fused 28-O-acetyl triterpene 17 in 26% yield over two steps (Scheme 4). In acidic conditions of the cyclization reaction the THP protecting group was cleaved and replaced with the acetoxy group. The acetoxy group was removed with p-TsOH in methanol in quantitative yield. Finally, 28-hydroxylupa-2,20(29)-dieno[2,3-d]isoxazole 15 was treated with 2-iodoxybenzoic acid in THF and DMSO to give 28-oxolupa-2,20(29)-dieno[2,3-d]isoxazole 18 in 51% yield.
Allobetulin 19 was obtained in 25% yield by refluxing betulin 1 in formic acid followed by refluxing the resulting intermediate formate ester in ethanolic solution of KOH in benzene (Scheme 5). The indole derivatives of allobetulin 20–21 were obtained with the same methodology as described above for betulonic acid (cf. synthesis of compounds 3 and 4) in 57–63% yields.
Betulonic aldehyde 22 was obtained from betulin 1 by PCC oxidation in DCM in 27% yield (Scheme 6). 28-Oxolupa-2,20(29)-dieno[2,3-b]pyrazine 23 was synthesized in 17% yield using the same methods as in the preparation of lupa-2,20(29)-dieno[2,3-b]pyrazin-28-oic acid 5. It was further reacted with NH2OH·HCl to give 28-oximinolupa-2,20(29)-dien[2,3-b]pyrazine 24 in 77% yield.
 | ||
Scheme 6
Reagents and conditions: (a) PCC, DCM, rt, 1 h, 27%; (b) ethylenediamine, sulfur, morpholine, reflux, 2.5 h, 17%; (c) NH2OH·HCl, pyridine–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), reflux, 16 h, 77%. 3), reflux, 16 h, 77%. | ||
3β-(3-Carboxy-3-methylbutanoyloxy)lup-20(29)-en-28-oic acid (bevirimat) 25 was synthesized from betulinic acid 26 by refluxing it in the presence of 2,2-dimethylsuccinic anhydride and DIPEA in DMF for 2 days in 5% yield (Scheme 7).
 | ||
| Scheme 7 Reagents and conditions: (a) NaBH4, 2-propanol, rt; (b) 2,2-dimethylsuccinic anhydride, DIPEA, DMF 170 °C, 2 days, 5%. DIPEA = N,N-diisopropylethylamine. | ||
Biology and structure–activity relationships
Previously we found a set of heterocyclic betulin derivatives to have promising activity against axenic amastigotes of L. donovani,12 and based on those results we synthesized a new set of fused heterocyclic adducts of betulin, betulinic acid and betulonic acid, and varied substituents at the position C-28 to explore effects of that position as well. Leishmanicidal activity of the modified compounds was assayed using the alamarBlue (AbD Serotec, Oxford, UK) viability assay on axenic amastigotes of L. donovani (Table 1).| Compound | % Inhibition ± s. e.b | IC50 μM ± s. e.c | ||
|---|---|---|---|---|
| 50 μM | 15 μM (μM ± s. e.) | 5 μM | ||
| a Precipitates at 50 μM, see crystals. b Average inhibition of triplicates. c Average of two experiments; amphotericin B is a positive control and was tested at 1 μM. | ||||
| 2 | 98.6 ± 0.1 | 45.9 ± 0.7 | — | — |
| 3 | 23.4 ± 2.2 | 27.4 ± 2.4 | 5.0 ± 4.4 | — |
| 4 | 29.3 ± 4.1 | 17.5 ± 2.7 | 14.0 ± 2.0 | — |
| 5 | 92.7 ± 0.1 | 79.7 ± 0.4 | 20.3 ± 0.4 | 13.2 ± 1.4 |
| 6 | 94.9 ± 0.2 | 35.2 ± 1.4 | — | — |
| 8 | 98.2 ± 0.1 | 75.2 ± 1.1 | 52.0 ± 1.2 | 4.3 ± 0.4 |
| 9 | 87.9 ± 0.5 | 25.6 ± 1.5 | — | — |
| 10 | 95.7 ± 0.3 | 15.6 ± 1.0 | — | — |
| 11 | 60.2 ± 0.7a | — | — | — |
| 13 | 84.3 ± 0.8 | 16.7 ± 2.0 | — | — |
| 14 | 2.4 ± 3.4 | — | — | — |
| 15 | 56.9 ± 0.4 | — | — | — |
| 17 | 6.1 ± 3.7 | — | — | — |
| 18 | 34.2 ± 2.0 | 21.1 ± 1.1 | — | — |
| 20 | 19.8 ± 2.4 | 18.5 ± 0.8 | — | — |
| 21 | 6.8 ± 0.7 | 10.2 ± 4.1 | 0.2 ± 1.3 | — |
| 24 | 2.8 ± 4.5 | — | — | — |
| 25 | 100.4 ± 0.1 | 69.1 ± 3.1 | 59.0 ± 0.4 | 7.2 ± 0.2 |
| 26 | 61.1 ± 0.6a | — | — | — |
| Amphotericin B | — | — | 99.9 ± 0.3 | — |
| Medium alone | 0.0 ± 4.6 | 1.5 ± 3.8 | 0.0 ± 0.9 | — |
Primary screening was performed at 50 μM concentration and compounds showing >70% inhibition were assayed at 15 μM concentration and finally most potent derivatives at 5 μM concentration (Table 1).
In the series of A-ring fused isoxazoles, the betulin-derived compound 15 had 57% inhibition of the growth at 50 μM concentration. The betulinic acid-derived isoxazole 10 inhibits 96% at 50 μM and 16% at 15 μM. Interestingly, the dihydrobetulinic acid-derived isoxazole 14 had only 3% inhibition at 50 μM. In our earlier studies we found a similar effect, but not this strong, between betulonic acid and dihydrobetulonic acid.19 The primary amide derivative of the betulinic acid-derived isoxazole 13 inhibits 84% of the growth at 50 μM concentration, but only 17% at 15 μM concentration. On the other hand, the betulinic aldehyde-derived isoxazole 18 has lower activity (34%) at 50 μM but slightly better activity (21%) at 15 μM concentration compared to 13. This may be due to solubility, as the aldehyde might not be completely soluble at high concentration. The least active isoxazole derivative, 28-O-acetylbetulin-derived isoxazole 17 inhibited only 6% of the growth at 50 μM concentration. It has been suggested that the carboxyl group in the triterpenoid skeleton enhances the observed antiprotozoal effects.20 However, among these A-ring fused isoxazole derivatives of betulin, the compounds 18 and 13 were more active than the betulinic acid-derived isoxazole 10. All isoxazole derivatives were less active than betulonic acid 2 (99% at 50 μM, 46% at 15 μM).
The A-ring fused pyrazine derivative of betulinic acid 5 showed 93% at 50 μM, 80% at 15 μM, and 20% inhibition at 5 μM concentration, whereas for the corresponding primary amide 6, we observed inhibition of 95% at 50 μM, and 35% at 15 μM concentration, and for its 28-oximino derivative 24 only 3% at 50 μM. Interestingly, the A-ring fused pyridine derivative of betulinic acid 9 inhibited 88% at 50 μM, but only 26% at 15 μM concentration. Here, with pyrazine derivatives, we could see the importance of the carboxyl group for antileishmanial activity.
The A-ring fused 5′-methoxyindole derivative of betulinic acid 4 was the most active indole derivative. At 5 μM, the lowest concentration tested, inhibition was 14%, whereas the corresponding unsubstituted indole derivative 3 inhibited only 5%. Also with the related indole derivatives the importance of the carboxyl group can be seen as the A-ring fused indole derivative of allobetulin 20 and the corresponding 5′-fluoroindole derivative 21 did not have activity at all. One factor affecting this might be the reduced solubility; allobetulin derivatives are not that soluble under the assay conditions. In addition, the A-ring fused pyrazole derivative of betulinic acid 11 and betulinic acid 26 precipitated at 50 μM in these assays.
4-Aza-3-oxohomobetulinic acid 8 displayed very good activity (98%) at 50 μM concentration and even at 5 μM concentration (inhibition 52%), whereas the A-ring fused pyrazole derivative of betulinic acid 11 displayed moderate 60.2% inhibition at 50 μM concentration. In addition, potent anti-HIV betulinic acid derived compound 25, bevirimat, displayed very good inhibition: 100% inhibition at 50 μM concentration and 59% at 5 μM concentration. The best compounds after primary screening were compounds 5, 8 and 25 that significantly inhibited parasite growth when tested at lower concentrations. The IC50 values for 5, 8 and 25 were 13.2, 4.3 and 7.2 μM, respectively, with compound 8 showing the best activity. Cytotoxicity IC50 values of 8 and 25 against THP-1 cell line were 55.5 and 54.0 μM, respectively. Compound 25 (bevirimat) showed the highest activity among the compounds tested in this study. It showed 100% inhibition at 50 μM, 69% at 15 μM, and 59% at 5 μM. Interestingly, bevirimat 25 also showed good activity against HIV-infected patients in a recent phase II study.21 Only 4-aza-3-oxohomobetulinic acid 8 showed a similar level of activity with 98% inhibition at 50 μM, 75% at 15 μM, and 52% at 5 μM. The third most active compound was the A-ring fused pyrazine derivative of betulinic acid 5. Cytotoxicity (Table 2) using the human macrophage cell line THP-1 was determined for 8 and 25, and found to be similar (IC50ca. 50 μM) for both compounds. Compound 8 had the best selectivity index (IC50 THP-1/IC50 axenic amastigotes; SI = 12.9), and its activity was tested on L. donovani-infected macrophages at low 5 μM concentration, where no macrophage toxicity was observed. Interestingly, activity of the compound 8 against Leishmania infected macrophages (54.0 ± 4.8% inhibition) was similar to that seen for axenic amastigotes (52.0 ± 1.2% inhibition).
| Compound | Toxicity for THP-1 cellsa IC50 (μM ± s. e.) | SIb | % inhibition ± s. e. of parasites in iMΦc 5 μM |
|---|---|---|---|
| a Average of two experiments. b Selectivity index = IC50 THP-1/IC50 axenic amastigotes. c Average of three experiments; nd – not done; amphotericin B is a positive control and was tested on infected macrophages at 1 μM. | |||
| 8 | 55.5 ± 1.8 | 12.9 | 54.0 ± 4.8 |
| 25 | 54.0 ± 1.7 | 7.5 | nd |
| Amphotericin B | nd | nd | 96.7 ± 0.7 |
Experimental section
Chemistry
Biology
L. donovani (MHOM/SD/1962/1S-Cl2d) was used in all bioassays. Axenic amastigotes were grown at 37 °C in a 5% CO2 incubator as described in complete RPMI 1640 containing 20% fetal calf serum, pH 5.5. Screening of the compounds for leishmanicidal activity was carried out using the alamarBlue (AbD Serotec, Oxford, UK) viability assay similar to that reported for leishmanial promastigotes. Standardization and optimization of the assay for axenic amastigotes has been described elsewhere.25 Compounds to be assayed were diluted to twice the final concentration used in the assays in the complete amastigote medium, containing 1% DMSO, and were aliquoted in triplicate (125 μL per well) into 96-well flat-bottom plates (Nunc, Roskilde, Denmark). IC50 was determined using serial two-fold dilutions of the test compounds from 50 to 0.4 μM. Amastigotes (5.0 × 105 cells mL−1; 125 μL per well) were added to each well and incubated for 24 h at 37 °C in a 5% CO2 incubator. The alamarBlue viability indicator was added (25 μL per well) and the plates incubated for an additional 24 h at which time the fluorescence (λex = 544 nm; λem = 590 nm) was measured in a microplate reader (Fluoroskan Ascent FL, Finland). Complete medium both with and without DMSO was used as negative controls (0% inhibition of amastigote growth). Amphotericin B (Sigma-Aldrich, St Louis MO), a drug used to treat VL, was included as a positive control on each plate and gave >90% inhibition of parasite growth at 1 μM. Toxicity was measured on the human leukaemia monocyte cell line (THP-1 6.4 × 104 cells per well) using the alamarBlue viability indicator as previously described.26 IC50 was determined using serial two-fold dilutions of the test compounds in triplicate from 500 to 0.25 μM. Inhibition of intracellular amastigote growth in infected THP-1 cells (1.0 × 105 cells per well) was carried out using transgenic Ld:pSSU-int/LUC promastigotes that express luciferase essentially as previously described.26 Amphotericin B (1 μM) was included as a positive control on each plate. Complete medium both with and without DMSO was used as negative controls. Calculation of the IC50's and statistical analysis were carried out using GraphPad Prism Version 6.0b (GraphPad Software, Inc. San Diego, CA).Conclusions
A set of betulin, betulinic acid and dihydrobetulinic acid derivatives, including eight new A-ring fused heterocycles, was synthesized and tested against L. donovani. Two heterocyclic compounds 5, 8, and potent anti-HIV drug candidate 25 had significant inhibition on parasite growth even at 5 μM concentration. Compound 8 had the best selectivity index, and showed similar good activity on Leishmania-infected macrophages and axenic amastigotes. Further improvement and optimization are needed to get more potent betulin derivatives against L. donovani.Acknowledgements
This study was supported by the European Commission (Contract no. EU-KBBE-227239-ForestSpeCs), the Academy of Finland (projects 252308, 264020, 265481) and The Finnish Cultural Foundation. DK was supported in part by the Michael and Penny Feiwel Foundation. We also thank Minni Pirttimaa, Dr Vânia Moreira and Dr Päivi Uutela for valuable discussions.Notes and references
- J. Alvar, I. D. Velez, C. Bern, M. Herrero, P. Desjeux, J. Cano, J. Jannin and M. den Boer, PLoS One, 2012, 7, e35671 CAS.
- WHO, Leishmaniasis, World Health Organization, http://www.who.int/leishmaniasis/en/index.html Search PubMed.
- World Health Organization, Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26, March 2010, 2010, No. 949 Search PubMed.
- P. Desjeux, Comp. Immunol. Microbiol. Infect. Dis., 2004, 27, 305–318 CrossRef CAS PubMed.
- S. L. Croft, S. Sundar and A. H. Fairlamb, Clin. Microbiol. Rev., 2006, 19, 111–126 CrossRef CAS PubMed.
- T. P. C. Dorlo, M. Balasegaram, J. H. Beijnen and P. J. de Vries, J. Antimicrob. Chemother., 2012, 67, 2576–2597 CrossRef CAS PubMed.
- T. Kanamoto, Y. Kashiwada, K. Kanbara, K. Gotoh, M. Yoshimori, T. Goto, K. Sano and H. Nakashima, Antimicrob. Agents Chemother., 2001, 45, 1225–1230 CrossRef CAS PubMed.
- J. F. Mayaux, A. Bousseau, R. Pauwels, T. Huet, Y. Henin, N. Dereu, M. Evers, F. Soler, C. Poujade, E. De Clercq and J. B. Le Pecq, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 3564–3568 CrossRef CAS.
- P. K. Mukherjee, K. Saha, J. Das, M. Pal and B. P. Saha, Planta Med., 1997, 63, 367–369 CrossRef CAS PubMed.
- J. C. P. Steele, D. C. Warhurst, G. C. Kirby and M. S. J. Simmonds, Phytother. Res., 1999, 13, 115–119 CrossRef CAS.
- E. Pisha, H. Chai, I. S. Lee, T. E. Chagwedera, N. R. Farnsworth, G. A. Cordell, C. W. W. Beecher, H. H. S. Fong, A. D. Kinghorn, D. M. Brown, M. C. Wani, M. E. Wall, T. J. Hieken, T. K. Das Gupta and J. M. Pezzuto, Nat. Med., 1995, 1, 1046–1051 CrossRef CAS.
- S. Alakurtti, T. Heiska, A. Kiriazis, N. Sacerdoti-Sierra, C. L. Jaffe and J. Yli-Kauhaluoma, Bioorg. Med. Chem., 2010, 18, 1573–1582 CrossRef CAS PubMed.
- J.-F. Li, Y. Zhao, M.-M. Cai, X.-F. Li and J.-X. Li, Eur. J. Med. Chem., 2009, 44, 2796–2806 CrossRef CAS PubMed.
- O. B. Flekhter, E. I. Boreko, L. R. Nigmatullina, E. V. Tret'yakova, N. I. Pavlova, L. A. Baltina, S. N. Nikolaeva, O. V. Savinova, V. F. Eremin, F. Z. Galin and G. A. Tolstikov, Russ. J. Bioorg. Chem., 2004, 30, 80–88 CrossRef CAS.
- G. Abbiati, A. Arcadi, G. Bianchi, S. Di Giuseppe, F. Marinelli and E. Rossi, J. Org. Chem., 2003, 68, 6959–6966 CrossRef CAS PubMed.
- H. Parra-Delgado, C. M. Compadre, T. Ramírez-Apan, M. J. Muñoz-Fambuena, R. L. Compadre, P. Ostrosky-Wegman and M. Martínez-Vázquez, Bioorg. Med. Chem., 2006, 14, 1889–1901 CrossRef CAS PubMed.
- F. Fernández, O. Caamaño, M. D. García, I. Alkorta and J. Elguero, Tetrahedron, 2006, 62, 3362–3369 CrossRef PubMed.
- Y. Wei, C.-M. Ma and M. Hattori, Eur. J. Med. Chem., 2009, 44, 4112–4120 CrossRef CAS PubMed.
- S. Alakurtti, P. Bergström, N. Sacerdoti-Sierra, C. L. Jaffe and J. Yli-Kauhaluoma, J. Antibiot., 2010, 63, 123–126 CrossRef CAS PubMed.
- M. del Rayo Camacho, R. Mata, P. Castaneda, G. C. Kirby, D. C. Warhurst, S. L. Croft and J. D. Phillipson, Planta Med., 2000, 66, 463–468 CrossRef CAS PubMed.
- P. F. Smith, A. Ogundele, A. Forrest, J. Wilton, K. Salzwedel, J. Doto, G. P. Allaway and D. E. Martin, Antimicrob. Agents Chemother., 2007, 51, 3574–3581 CrossRef CAS PubMed.
- V. Kumar, N. Rani, P. Aggarwal, V. K. Sanna, A. T. Singh, M. Jaggi, N. Joshi, P. K. Sharma, R. Irchhaiya and A. C. Burman, Bioorg. Med. Chem. Lett., 2008, 18, 5058–5062 CrossRef CAS PubMed.
- M. Urban, J. Sarek, M. Kvasnica, I. Tislerova and M. Hajduch, J. Nat. Prod., 1997, 70, 526–532 CrossRef PubMed.
- J. Xu, Z. Li, J. Luo, F. Yang, T. Liu, M. Liu, W.-W. Qiu and J. Tang, J. Med. Chem., 2012, 55, 3122–3134 CrossRef CAS PubMed.
- O. Shimony and C. L. Jaffe, J. Microbiol. Methods, 2008, 75, 196–200 CrossRef CAS PubMed.
- C. Reichwald, O. Shimony, U. Dunkel, N. Sacerdoti-Sierra, C. L. Jaffe and C. Kunick, J. Med. Chem., 2008, 51, 659–665 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, 1H and 13C NMR spectra. See DOI: 10.1039/c3md00282a |
| ‡ CLJ holds the Michael and Penny Feiwel Chair of Dermatology. |
| This journal is © The Royal Society of Chemistry 2014 |