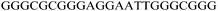Open Access Article
Open Access ArticleDiminazene or berenil, a classic duplex minor groove binder, binds to G-quadruplexes with low nanomolar dissociation constants and the amidine groups are also critical for G-quadruplex binding†
Jie
Zhou
a,
Vu
Le
b,
Dimpy
Kalia
a,
Shizuka
Nakayama
a,
Clinton
Mikek
b,
Edwin A.
Lewis
*b and
Herman O.
Sintim
*ac
aDepartment of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742, USA. E-mail: hsintim@umd.edu
bDepartment of Chemistry, Mississippi State University, Mississippi State, MS 39762, USA. E-mail: elewis@chemistry.msstate.edu
cProgram in Oncology, University of Maryland Marlene and Stewart Greenebaum Cancer Center, 22 S. Greene Street, Baltimore, Maryland 21201, USA
First published on 23rd July 2014
Abstract
G-quadruplexes have shown great promise as chemotherapeutic targets, probably by inhibiting telomere elongation or downregulating oncogene expression. There have been many G-quadruplex ligands developed over the years but only a few have drug-like properties. Consequently only a few G-quadruplex ligands have entered clinical trials as cancer chemotherapeutic agents. The DNA minor groove ligand, berenil (diminazene aceturate or DMZ), is used to treat animal trypanosomiasis and hence its toxicological profile is already known, making it an ideal platform to engineer into new therapeutics. Herein, using a plethora of biophysical methods including UV, NMR, MS and ITC, we show that DMZ binds to several G-quadruplexes with a Kd of ∼1 nM. This is one of the strongest G-quadruplex binding affinities reported to date and is 103 tighter than the berenil affinity for an AT-rich duplex DNA. Structure–activity-relationship studies demonstrate that the two amidine groups on DMZ are important for binding to both G-quadruplex and duplex DNA. This work reveals that DMZ or berenil is not as selective for AT-rich duplexes as originally thought and that some of its biological effects could be manifested through G-quadruplex binding. The DMZ scaffold represents a good starting point to develop new G-quadruplex ligands for cancer cell targeting.
Introduction
Both DNA and RNA can form many secondary structures, such as G-quadruplex,1–3 triplex,4,5 i-motif,6,7 and a biological role for these polymorphs, especially G-quadruplexes,8–10 has been suggested. There are ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760
760![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 guanine-rich regions in the human genome, which have the potential to form G-quadruplexes,11,12 including those at the telomere end13 and promoter regions of some cancer-related genes.10 Guanine tracts in RNA are known to form G-quadruplexes in vivo3,14–16 but the formation of G-quadruplexes in chromosomal DNA has been a matter of debate due to the fact that the guanine tracts in chromosomal DNA can also form duplexes with complementary tracts of cytosines. After many years of fierce debate regarding a biological role for DNA G-quadruplexes in vivo, acceptance is now growing that DNA G-quadruplexes might indeed form in vivo and that there could be biological consequences of G-quadruplex formation in chromosomal DNA. Firstly, it has been demonstrated that fluorogenic G-quadruplex-specific ligands could become fluorescent inside cells, especially during cell division, when single stranded regions of chromosomal DNA are created during DNA replication.17,18 Secondly, seminal works by the laboratories of Pluckthun19 and Balasubramanian20 resulted in the engineering of G-quadruplex-specific antibodies, which have been used to provide compelling evidence that G-quadruplexes form in vivo. Additionally, biophysical approaches (mainly NMR21,22 and DEER/ENDOR23,24) have demonstrated that synthetic G-rich oligonucleotides could form G-quadruplex structures in vivo.
000 guanine-rich regions in the human genome, which have the potential to form G-quadruplexes,11,12 including those at the telomere end13 and promoter regions of some cancer-related genes.10 Guanine tracts in RNA are known to form G-quadruplexes in vivo3,14–16 but the formation of G-quadruplexes in chromosomal DNA has been a matter of debate due to the fact that the guanine tracts in chromosomal DNA can also form duplexes with complementary tracts of cytosines. After many years of fierce debate regarding a biological role for DNA G-quadruplexes in vivo, acceptance is now growing that DNA G-quadruplexes might indeed form in vivo and that there could be biological consequences of G-quadruplex formation in chromosomal DNA. Firstly, it has been demonstrated that fluorogenic G-quadruplex-specific ligands could become fluorescent inside cells, especially during cell division, when single stranded regions of chromosomal DNA are created during DNA replication.17,18 Secondly, seminal works by the laboratories of Pluckthun19 and Balasubramanian20 resulted in the engineering of G-quadruplex-specific antibodies, which have been used to provide compelling evidence that G-quadruplexes form in vivo. Additionally, biophysical approaches (mainly NMR21,22 and DEER/ENDOR23,24) have demonstrated that synthetic G-rich oligonucleotides could form G-quadruplex structures in vivo.
If G-quadruplex formation in vivo has a biological consequence, then small molecules that target and stabilize these structures could have therapeutic value. In animal chromosomes, the telomerase enzyme (which is up-regulated in certain cancers) is responsible for maintaining the telomere length thereby rendering cancer cells immortal.25,26 The telomere is G-rich and has been shown via many biophysical experiments to be capable of forming G-quadruplexes.27–30 Many compounds that bind to G-quadruplexes have been shown to inhibit the extension of the DNA substrate by telomerase and some have even shown interesting anti-proliferative properties when added to cancer cells.31–35 In addition to telomeres, G-quadruplexes are present in the promoter regions of a number of cancer-related genes such as c-myc,36BCL-2,37KRAS,38 c-kit39 and VEGF,40 where they are involved in the regulation of transcription of these genes by disrupting binding of transcription factors.41,42
In light of these potential important biological roles of G-quadruplexes, there is high interest in discovering G-quadruplex-selective ligands for both fundamental studies (for example, fluorescent ligands that will allow for studying G-quadruplexes in vivo) and also drug-like molecules that will allow for selective targeting of G-quadruplexes related to cancer17,32,43,44 and other diseases.45 In this manuscript, we reveal that DMZ, which has been shown to bind to the minor groove of AT-rich DNA with a micromolar dissociation constant, binds to G-quadruplexes with a nanomolar dissociation constant, i.e. 3 orders of magnitude stronger affinity for G-quadruplexes than AT-rich duplexes. The DMZ scaffold is therefore a good starting point to develop potent G-quadruplex ligands.
Material and methods
General
4-Aminobenzamidine dihydrochloride, aniline and diminazene aceturate (DMZ or Berenil) were purchased from Aldrich. All triazenes were stored at 4 °C and in dark since significant decomposition of triazenes was observed if stored at room temperature and in presence of light. Sequences of DNA used in this study are shown in Table 1. Purified oligonucleotides 22-mer c-kit1, 18-mer VEGF, 23-mer bcl-2 2345, 15-mer TBA and 20-mer 8bp AT were purchased from IDT and analyzed for purity before use. Purified WT 27-mer bcl-2, WT 24-mer c-myc, WT 22-mer human telomere hTel were purchased from Midland Oligos (Midland, TX) and used without further purification.DNA stock solutions were prepared by reconstituting the lyophilized oligonucleotide into 20 mM Tris buffer with a salt concentration of 100 mM KCl and a pH of 7.2. Approximately 2 mL of the oligonucleotide was dialyzed (1000 Mw cutoff membrane) against three changes of buffer solution (1 L, 24 h each) at 4 °C. The concentrations of stock DNA solutions were verified using UV-Vis. G-Quadruplex DNAs were annealed by quickly heating the sample to 100 °C, holding at 100 °C for ten minutes then slowly cooling to 5 °C, over a three hour period. Molar extinction coefficients of the DNAs were determined using a nearest-neighbor method for single stranded DNA.48 The extinction coefficients at 260 nm for the WT 27-mer bcl-2, WT 24-mer c-myc, WT 22-mer human telomere hTel sequences are 267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1, 248
200 M−1 cm−1, 248![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1 cm−1, and 228
100 M−1 cm−1, and 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1 respectively.49–51 The extinction coefficient at 260 nm for the 7 base pair hairpin with A2T2 (7bp HP·AT) and the 7 base pair hairpin without A2T2 (7bp HP) are 165
500 M−1 cm−1 respectively.49–51 The extinction coefficient at 260 nm for the 7 base pair hairpin with A2T2 (7bp HP·AT) and the 7 base pair hairpin without A2T2 (7bp HP) are 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1 and 159
200 M−1 cm−1 and 159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1 respectively. Cell culture grade water purchased from Corning was used for all experiments.
200 M−1 cm−1 respectively. Cell culture grade water purchased from Corning was used for all experiments.
Synthesis of Triazene-1 and Triazene-2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 20 mL) to afford analytically pure product (1.2 g, 84%) as an acetate salt. 1H NMR (DMSO-d6, 400 MHz) δ 7.87–7.83 (m, 2H), 7.57–7.47 (m, 4H), 7.47–7.42 (m, 2H), 7.31–7.26 (m, 1H), 1.72 (s, 3H). 13C NMR (DMSO-d6, 100 MHz,) δ 177.1, 165.8, 148.9, 148.4, 130.2, 130.1, 127.4, 123.4, 120.5, 116.1, 25.8. HRMS (ESI+) m/z calcd. for C13H14N5 [M + H]+ 240.1249, found 240.1248.
2, 20 mL) to afford analytically pure product (1.2 g, 84%) as an acetate salt. 1H NMR (DMSO-d6, 400 MHz) δ 7.87–7.83 (m, 2H), 7.57–7.47 (m, 4H), 7.47–7.42 (m, 2H), 7.31–7.26 (m, 1H), 1.72 (s, 3H). 13C NMR (DMSO-d6, 100 MHz,) δ 177.1, 165.8, 148.9, 148.4, 130.2, 130.1, 127.4, 123.4, 120.5, 116.1, 25.8. HRMS (ESI+) m/z calcd. for C13H14N5 [M + H]+ 240.1249, found 240.1248.
NMR spectra for synthesized compounds were recorded with a Bruker AV-400 or Bruker DRX-400. 1H-NMR chemical shifts are reported as (δ) in ppm and are calibrated according to residual solvent peaks. 1H NMR coupling constants (J values) are reported in Hertz (Hz). 13C-NMR chemical shifts are reported as ppm relative to residual solvent peak. High-resolution mass spectra (HRMS) for synthesized compounds were recorded with JEOL AccuTOF-CS (ESI positive, needle voltage 1800–2400 eV).
UV titration studies of DNA with the triazene ligands, DMZ and Triazene-1
UV measurements for titration studies were done using a JASCO V-630 spectrophotometer. Fluorescence measurements for the displacement assay were done using a Varian Cary Eclipse fluorimeter. The molar extinction coefficients at 260 nm for 22-mer c-kit1, 18-mer VEGF, 23-mer bcl-2 2345, 15-mer TBA and 20-mer 8bp AT are as follows 226![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1, 169
700 M−1 cm−1, 169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1, 227
800 M−1 cm−1, 227![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1, 143
300 M−1 cm−1, 143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1 and 205
300 M−1 cm−1 and 205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1, respectively. The concentration of ligands used in this study was 10 μM and the DNA concentrations were 0, 0.25, 1.5, 3, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 100, and 150 μM. The buffer contained 250 mM KCl, 50 mM Tris-HCl (at pH 7.5). The following quadruplex DNA were tested: c-kit1, VEGF, bcl-2 2345, and TBA. Duplex DNA (8bp AT) was also tested. The sample was first heated up to 95 °C for 5 min without the ligand and then cooled down to room temperature in 15 min. The ligand was then added and the mixture was incubated at 4 °C for ∼12 h before recording the data. In case of Triazene-1 and Triazene-2, the sample mixture contained 1% DMSO.
500 M−1 cm−1, respectively. The concentration of ligands used in this study was 10 μM and the DNA concentrations were 0, 0.25, 1.5, 3, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 100, and 150 μM. The buffer contained 250 mM KCl, 50 mM Tris-HCl (at pH 7.5). The following quadruplex DNA were tested: c-kit1, VEGF, bcl-2 2345, and TBA. Duplex DNA (8bp AT) was also tested. The sample was first heated up to 95 °C for 5 min without the ligand and then cooled down to room temperature in 15 min. The ligand was then added and the mixture was incubated at 4 °C for ∼12 h before recording the data. In case of Triazene-1 and Triazene-2, the sample mixture contained 1% DMSO.
NMR measurements for DNA binding of triazene ligands
NMR measurements for the binding studies were done using Bruker Avance III HD 800 spectrometer equipped with Cryo-Probe. The procedures used in the binding studies were described previously.46 The ligand concentration was 150 μM, and that of the DNA (c-kit1 or 8bp AT) was 300 μM. The buffer used was 10 mM potassium phosphate (pH 7.5) containing 137 mM NaCl, 1 mM EDTA, and also contained 10% D2O. The sample was initially heated up to 95 °C for 5 min without the ligand and then cooled down to room temperature in 15 min. Subsequently, the sample was incubated at 4 °C for ∼12 h without the ligand (for G-quadruplex formation). Then, the ligand was added and incubated for 2 h before the NMR measurement (25 °C). (Note: because of the high concentrations being used for the NMR, incubating the DNA/ligand for longer periods caused precipitation, probably due to G-quadruplex polymer formation, which is catalyzed by the ligand).Isothermal titration calorimetry
ITC experiments were performed using a VP-ITC calorimeter (GE-Heathcare). A typical ITC experiment involved the addition of 28 (10 μL) injections of a nominal 1 mM ligand solution into ∼1.5 mL of a dilute DNA solution (10 μM). All ITC titration experiments were done at 25 °C. Corrected titration curves were obtained by subtracting the blank titration data from the ITC-data for the ligand–DNA titrations. The corrected ITC titrations were fit to a multiple independent sites binding model using a nonlinear regression algorithm, CHASM, developed in our laboratory.47Circular dichroism (CD)
CD titration experiments were performed with an Olis DSM-20 spectropolarimeter (Bogart, GA). All measurements were done at 25 °C using a 1 cm quartz cuvette and covering a spectral range of 220–420 nm. All DNA samples were prepared such that they had a nominal absorbance of less than 1.0 at 260 nm. Stock solutions of the ligand were added in small amount to reach a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 10
1, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of ligand per equivalence of DNA.
1 of ligand per equivalence of DNA.
Electrospray ionization mass spectrometry
ESI-MS experiments on the triazene–DNA complexes were carried out on a Bruker MicrOTOFQ mass spectrometer. Data acquisition was set to operate in negative ion mode. All experiments were performed in 50 mM ammonium acetate buffer containing 20% HPLC grade methanol and adjusted with 1 N KOH solution to reach pH of 7.0. The WT hTel 22-mer G-quadruplex sample was prepared at a concentration of approximately 10 μM in the ammonium acetate buffer and was exhaustedly dialyzed. Stock solutions of DMZ and Triazene-1 were prepared in the final dialysate buffer at concentration as high as 300 μM. The ESI-MS samples were prepared by mixing the DNA and ligand stock solutions to yield a mixture containing excess of each ligand per equivalence of DNA. The MS capillary voltage was set to +3500 V, dry N2 gas flow was adjusted to 0.5 L min−1 at 110 °C, and the G-quadruplex/ligand samples were directly infused into the MS by using a kD Scientific syringe pump set to a flow rate of 200 μL per hour. Data processing was performed by using the Bruker Daltonics Data Analysis program.Results and discussion
Recently, there has been considerable interest in repurposing drugs for new therapeutic indications.52,53 Along this line, others have been interested in repurposing DNA duplex minor groove binders for targeting G-quadruplexes.54–57 Compounds that bind minor grooves of DNA duplexes have a track record of clinical efficacy for many indications including animal trypanosomiasis and babesiosis.58 Because there are numerous toxicological data for these drugs, using them as starting points for the development of G-quadruplex-selective ligands appears to be a reasonable proposition. Curiously, although several duplex minor groove binders have been tested for G-quadruplex binding, DMZ (Fig. 1) a prototypical AT-rich minor groove binder, which is used clinically to treat animal trypanosomiasis has not been thoroughly investigated for G-quadruplex binding. To date there is only a single report, from our laboratory, that demonstrated that DMZ aggregates the dinucleotide, c-di-GMP, into an ill-defined supramolecular aggregate (probably containing G-quadruplexes) via an uncharacterized mechanism.59DMZ as a G quadruplex ligand
 | ||
| Fig. 3 DMZ interactions with duplex DNA (AT-rich). The amidine groups of DMZ make important hydrogen-bonding interactions with both DNA residues and water molecules in the minor groove of DNA (structure is from PDB#2DBE). | ||
We synthesized two DMZ analogs (Triazene-1, which has one of the amidine groups in DMZ deleted and Triazene-2,61 which does not contain any amidine group, see Fig. 3). These DMZ analogs were then evaluated on the basis of their affinity for binding to both duplex and G-quadruplex DNA. The synthesis of Triazene-1 and Triazene-2, following precedent,61 is outlined in Scheme 1. First, the diazonium salts of p-aminobenzamidine and aniline were generated by treating with sodium nitrite followed by their coupling with aniline to give Triazene-1 and Triazene-2 in good yields. Both of the synthesized triazenes were characterized by mass and NMR spectroscopy (see ESI† for spectral data).
UV spectroscopy studies of Triazene-1 binding to G quadruplexes and duplex DNA
In qualitative UV-visible titration experiments, the absorbance maxima of Triazene-1 (10 μM) displayed a red shift with increasing concentrations of various G-quadruplexes (Fig. 4 and Fig. S3, ESI†), implying binding while Triazene-2, exhibited self-aggregation (data not shown here) and was not considered further. In contrast, when Triazene-1 was incubated with duplex DNA (Fig. S3E, ESI†), there was no evidence for a robust interactions between the Triazene-1 and an AT rich duplex DNA. Visual inspection of the UV titration experiments (compare Fig. 2, Fig. S2 (ESI†) and Fig. 4, Fig. S3, ESI†) revealed that DMZ had a higher affinity to the G-quadruplexes than Triazene-1, the DMZ analog having only one amidine group. The differences in affinity (selectivity) between DMZ and Triazene-1 for binding to duplex AT minor groove sites vs. G-quadruplex stacking or intercalation interactions were quantified using ITC experiments, vide infra.NMR analysis of binding of DMZ and Triazene-1 to DNA
To provide direct evidence for the binding of DMZ and Triazene-1 to c-kit1 (G-quadruplex) and duplex DNA, we performed 1H-NMR titration experiments. Xu and co-workers used NMR to investigate the binding of a cyanine dye to c-kit1 so we decided to start with c-kit1, based on this precedent.18,46 In general, the guanine imino protons resonate between 10.0 to 12.5 ppm in a quadruplex matrix and this represents guanine NH⋯O hydrogen bonds in the Hoogsteen alignments of G-quartets. Upon binding of small molecules, the chemical environment around these imino protons changes, resulting in changes in their NMR chemical shifts. Fig. 5A shows the NMR spectra of the guanine imino protons of c-kit1 in presence of the ligands, DMZ and Triazene-1 in 90% H2O/D2O. The chemical shifts for the guanine imino protons in the spectrum of c-kit1 alone (Fig. 5A), ([ligand]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [c-kit1] = 0
[c-kit1] = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was assigned based on the earlier work.46 The NMR titration spectra of DMZ and Triazene-1 with c-kit1 show significant decreases in the peak intensities and line-broadening in the imino proton spectra (Fig. 5A). These results demonstrate direct binding of DMZ and Triazene-1 to c-kit1 (NMR resonances between 11.1 and 11.4 ppm are different between the case with no ligand added and when DMZ or triazene are added), consistent with our UV-visible titration experiments (Fig. 2, Fig. S2 (ESI†) and Fig. 4, Fig. S3, ESI†). When DMZ or Triazene-1 was added to c-kit1, the chemical shifts associated with the imino protons of G6, G10 and G2 did not change, probably excluding end-stacking with the 5′-terminal tetrad of the quadruplex. Other NMR titration experiments with other G-quadruplexes, such as VEGF and bcl-2 2345 did not give stable baselines (data not shown), probably due to aggregation of the ligand–DNA complex. Despite this technical difficulty, the c-kit1 NMR titration data provide good evidence that DMZ does indeed bind to G-quadruplex DNA.
1) was assigned based on the earlier work.46 The NMR titration spectra of DMZ and Triazene-1 with c-kit1 show significant decreases in the peak intensities and line-broadening in the imino proton spectra (Fig. 5A). These results demonstrate direct binding of DMZ and Triazene-1 to c-kit1 (NMR resonances between 11.1 and 11.4 ppm are different between the case with no ligand added and when DMZ or triazene are added), consistent with our UV-visible titration experiments (Fig. 2, Fig. S2 (ESI†) and Fig. 4, Fig. S3, ESI†). When DMZ or Triazene-1 was added to c-kit1, the chemical shifts associated with the imino protons of G6, G10 and G2 did not change, probably excluding end-stacking with the 5′-terminal tetrad of the quadruplex. Other NMR titration experiments with other G-quadruplexes, such as VEGF and bcl-2 2345 did not give stable baselines (data not shown), probably due to aggregation of the ligand–DNA complex. Despite this technical difficulty, the c-kit1 NMR titration data provide good evidence that DMZ does indeed bind to G-quadruplex DNA.
To further explore the interaction of the DMZ and Triazene-1 ligands with duplex DNA, NMR experiments in which 8bp-AT was incubated with the triazene ligands were performed as described for c-kit1 above. In the case of duplex DNA (8bp AT), only DMZ (but not Triazene-1) showed significant changes in the imino region of spectrum of the duplex DNA (see Fig. 5B). This is consistent with the UV titration data, which showed that Triazene-1 did not bind to AT-rich duplex DNA (see Fig. 4 and Fig. S3, ESI†).
NMR titration experiments have demonstrated that both DMZ and Triazene-1 bind to the c-kit1 G-quadruplex. However, a definitive identification of the DMZ and Triazene-1 binding sites was not possible with the current NMR data (Fig. 5A). Future work will be focused on obtaining structural information for G-quadruplex DNA/DMZ (and/or DMZ analog) complexes via X-ray crystallography and/or NMR. Such studies would provide important information that will be used to design next generation triazene analogs that could have higher affinity and selectivity for G-quadruplex DNA.
Initial screening experiments employing UV and NMR titration methods, provided qualitative evidence that DMZ binds to both duplex and G-quadruplex DNA whereas Triazene-1 binds only to G-quadruplex DNA (and not AT rich duplex DNA) albeit with lower affinity than DMZ. However, neither the UV nor NMR methods are sensitive enough for the accurate determination of sub-micromolar binding constants. By employing ITC, we were able to determine the energetics for the interactions of DMZ and Triazene-1 with several commonly studied G-quadruplex constructs hTel, c-myc, bcl-2. We also used ITC to study the interactions between DMZ and Triazene-1 and two hairpin duplex DNA constructs, one having an A2T2 binding site, 7bp HP-AT, and one without the A2T2 site, 7bp HP. The sequences for these DNA targets were shown in Table 1.
DMZ–Triazene-1–hTel22 complexes identified via mass spectroscopy
We have previously reported on the thermodynamic properties for the binding of several porphyrins and porphyrin derivatives to a variety of oncogene promoter sequence G-quadruplexes.62,63 We have repeatedly shown that the cationic porphyrin TMPyP4 binds to a number of G-quadruplexes and forms complexes having a saturation stoichiometry of (n + 1) where n is the number of G-tetrads in the G-quadruplex motif. In the case of the c-myc, bcl-2, and kras promoter sequence G-quadruplexes the saturation stoichiometry was determined to be 4 moles of TMPyP4 per mole of G4-DNA.62,63 The DMZ (or Triazene-1) ligands are approximately half the size of TMPyP4 and we would speculate that two molecules of DMZ (or Triazene-1) could pi-stack with one G-tetrad (see Fig. 6). The ESI mass spectra for a solution containing hTel22 DNA in an excess amount of DMZ (panel A) and/or an excess amount of Triazene-1 (panel B) indicate that up to 8 molecules of these ligands can bind to one G-quadruplex. Three important features can be observed from panel A: first, there are no observable m/z peaks for free DNA; second, there are multiple m/z peaks indicating stoichiometry values larger than 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; and third, DMZ·hTel22 complexes are observed with stoichiometries up to 8
1; and third, DMZ·hTel22 complexes are observed with stoichiometries up to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as evidenced from the m/z peaks at 1843.3 and 2314.0. Similarly, the ESI mass spectrum shown in Fig. 7 panel B for a solution containing hTel22 in an excess amount of Triazene-1 also suggests a maximum stoichiometry of 8
1 as evidenced from the m/z peaks at 1843.3 and 2314.0. Similarly, the ESI mass spectrum shown in Fig. 7 panel B for a solution containing hTel22 in an excess amount of Triazene-1 also suggests a maximum stoichiometry of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, there are notable differences between the data shown in panels A and B. The mass spectrum shown in panel B clearly exhibits the presence of some free or uncomplexed DNA as evidenced by m/z peaks at 1751.5 and 2328. The presence of free DNA even in excess amounts of Triazene-1 ligand suggests a low binding affinity for the interaction of Triazene-1 with the hTel22 G-quadruplex DNA.
1. However, there are notable differences between the data shown in panels A and B. The mass spectrum shown in panel B clearly exhibits the presence of some free or uncomplexed DNA as evidenced by m/z peaks at 1751.5 and 2328. The presence of free DNA even in excess amounts of Triazene-1 ligand suggests a low binding affinity for the interaction of Triazene-1 with the hTel22 G-quadruplex DNA.
 | ||
| Fig. 6 Chemical structures of Diminazene aceturate (DMZ) and Triazene-1 in comparison to a chemical representation of a G-quartet. | ||
Determination of DMZ and Triazene-1 binding constants to DNA using ITC
Typical raw ITC data for the titration of DMZ into five different DNA samples, 7bp HP·AT, 7 bp HP, 27mer bcl-2, 24mer c-myc, and 22mer hTel, are shown in Fig. S4 (ESI†). The thermodynamic parameters for the interaction of DMZ with these DNAs were obtained directly from the nonlinear regression fits of the raw ITC data to a one site, fractional one site, or three competitive sites thermodynamic binding model, depending on the number of species indicated in the thermograms. As shown in Fig. S4 (ESI†), the binding of DMZ to 7bp HP·AT exhibits two different but independent overlapping binding modes and is reminiscent of the binding of netropsin (another AT minor groove binder).64–67 The interaction of DMZ with the 7bp HP sequence, lacking the A2T2 site, exhibits weaker complex formation (and only one binding mode). The interactions between DMZ and 27mer bcl-2 or 24mer c-myc are best described by at least three different binding processes. Typical nonlinear regression fits for the analysis of the binding of DMZ to 7bp HP, 7bp HP-AT and bcl-2, and c-myc are shown in Fig. 8. The solid lines in Fig. 8 represent the model lines using the best fit thermodynamic parameters for these ligand DNA interactions. | ||
| Fig. 8 Typical nonlinear regression fits for the DMZ titrations of 7bp HP, and 7bp HP·AT are shown in panel A. The 7bp HP titration data are fit to a one site model and the 7bp HP·AT data are fit to a fractional two site model. Typical nonlinear regression fits for the DMZ titrations of 27mer bcl-2 and 24mer c-myc are shown in panel B. Both the 27mer bcl-2 and the 24mer c-myc DMZ titration data are fit to a three site model. The best fit parameters for the data shown in panels A and B are listed in Table 2. | ||
The thermodynamic parameters obtained from the ITC experiments (Fig. S4, ESI† and fits Fig. 8) reveal that DMZ binds to some G-quadruplex DNAs (for example bcl-2 and c-myc) with dissociation constants as low as ∼1 nM. This is of a similar affinity to that exhibited by guanidine phthalocyanines, considered to be one of the tightest binders of G-quadruplexes with Kd of ∼2 nM.17 Also the ligand TMPyP4, a classic G-tetrad binding ligand, binds to G-quadruplexes with Kd ≈ 2 × 10−7 M,62,68 so the fact that DMZ binds to G-quadruplexes with 1 nM dissociation constant is interesting. Of note is the observation that even the second dissociation constant, Kd2 ≈ 5 × 10−8 M, for the DMZ·c-myc complex is an order of magnitude smaller than the dissociation constant (Kd ≈ 1 × 10−6 M) obtained for the DMZ·7bp HP-AT complex. Our Kd value for the DMZ·7bp HP-AT complex is very similar to the dissociation constant (Kd ≈ 1 × 10−6 M) determined in NMR experiments previously reported by Jenkins et al. for DMZ interactions with several types of B-DNAs containing the AT rich sequences.69 Interestingly, Jenkins et al. also mentioned the two overlapping events exhibited by the interactions of DMZ with AT rich sequences.69 The phenomenon of two overlapping sites observed for the AT rich sequence has been well documented and several possible mechanisms have been suggested, including: (1) two binding orientations of the minor groove binder in the DNA minor groove or (2) the formation of a complex in which one or more water molecules are trapped between the ligand binding in the minor groove and the floor of the DNA groove.64–66
The ITC heat data for the titrations of Triazene-1 to four different DNA sequences (a DNA hairpin and three G-quadruplex DNAs) are shown in Fig. S5 (ESI†). Again, from visual inspection, it seems that the titration of Triazene-1 into Na+hTel22 is calorimetrically silent while the addition of Triazene-1 to K+hTel22 results in a significant exothermic signal. Notable differences between the DMZ and the Triazene-1 DNA titration experiments are that DMZ exhibits a much tighter binding affinity than Triazene-1, at least for the preferred G-quadruplexes, and Triazene-1 exhibits only a single binding event for its interactions with the bcl-2, and c-myc G-quadruplexes. In addition, the binding affinity for Triazene-1 interacting with K+hTel22 is similar to the binding affinities for Triazene-1 interacting with either the bcl-2 or c-myc G-quadruplexes. This result is in sharp contrast to DMZ which exhibits selectivity for the bcl-2 or c-myc G-quadruplexes over K+hTel22. Triazene-1 binds to all of the tested G-quadruplexes with significantly lower affinity (4–5 orders of magnitude lower, compare Tables 2 and 3) than does DMZ. The binding affinity for of Triazene-1 with 7bp HP-AT is also reduced by a factor of approximately 30. Furthermore, the second binding event observed in the DMZ binding to 7bp HP-AT is now eliminated in of the case of Triazene-1 interacting with the duplex DNA, 7bp HP-AT. Clearly, the removal of one amidine group from the parent DMZ compound to create the Triazene-1 ligand results in the loss of A2T2 recognition in the minor groove of the DNA. This was expected as the presence of two amidine groups is a common feature of several minor groove binders such as distamycin,70 netropsin,64,66,70 DAPI71 and pentamidine.72 However, it remains to be answered why the loss of A2T2 recognition in the minor groove as exhibited by Triazene-1 also results in much lower binding affinities for the interactions of Triazene-1 with different G-quadruplexes.
| Sequences | K 1 (M−1) | ΔH1 (kcal mol−1) | K 2 (M−1) | ΔH2 (kcal mol−1) | K 3 (M−1) | ΔH3 (kcal mol−1) |
|---|---|---|---|---|---|---|
| Nd = not determined. Note: K1, K2 and K3 are association constants. Dissociation constants, Kd = 1/(K1 or K2 or K3). | ||||||
| 7bp HP·AT | 9.8 × 105 | −2.2 | 4.5 × 104 | −8.7 | — | — |
| 7bp HP | — | — | 1.8 × 104 | −5.3 | — | — |
| 27mer bcl-2 | 8.8 × 108 | −4.5 | 1.9 × 106 | −2.2 | 6.4 × 104 | −7.3 |
| 24mer c-myc | 7.6 × 108 | −5.5 | 1.9 × 107 | −2.5 | 2.8 × 105 | −4.7 |
| 22mer hTel Na+ | Nd | Nd | Nd | Nd | Nd | Nd |
| 22mer hTel K+ | — | — | — | — | 5.5 × 104 | −3.8 |
| Sequences | K 1 (M−1) | ΔH1 (kcal mol−1) |
|---|---|---|
| 7bp HP-AT | 2.9 × 104 | −1.9 |
| 7bp HP | Nd | Nd |
| 27mer bcl-2 | 3.5 × 103 | −1.8 |
| 24mer c-myc | 3.6 × 104 | −1.1 |
| 22mer hTel Na+ | Nd | Nd |
| 22mer hTel K+ | 2.1 × 103 | −2.7 |
DMZ binding to G quadruplexes studied by circular dichroism (CD)
To determine the structural effects of the interactions between the DMZ and G-quadruplex DNAs, we performed a series of CD titration experiments in which DMZ was added to different G-quadruplexes. Fig. 9 and 10 show the CD spectra for the titration of DMZ into three different G-quadruplexes, bcl-2, c-myc, and hTel22. These CD titrations were performed in K+ Tris buffer. Under these experimental buffer conditions (K+ salt), the CD spectra for the free WT bcl-2, WT c-myc, and hTel22 G-quadruplexes exhibited characteristic CD spectral features that are similar to those previously reported in the literature.49–51,62 However, upon complexation with DMZ, the CD signals for both 27mer WT bcl-2 and 24mer WT c-myc dramatically attenuated as DMZ is added. A plausible explanation for this is that the inherent G-tetrad π–π stacking interactions in both the 27mer WT bcl-2 and 24mer WT c-myc G-quadruplexes is weakened upon DMZ binding. In comparison to TMyP4, DMZ does not have the extended π-surfaces, which can interact extensively with the π system of the G-tetrad in either an end-stacking or intercalation mode. Perhaps the explanation for the surprisingly tight binding to specific G-quadruplexes is that DMZ is binding in the G-quadruplex groove or interacting in some way with loop bases. In the case of DMZ binding to K+hTel22, the addition of the DMZ appears to have very little effect on the parallel structure of hTel22 G-quadruplex, see Fig. 10. In the case of binding DMZ to duplex DNA, an induced DMZ CD signal is observed (see Fig. S6, ESI†). However, there was no evidence for an induced ligand CD signal when DMZ was bound to the different G-quadruplexes. This lack of an induced CD is suggestive of end stacking or intercalative binding but not groove binding.73 A more detailed structural study of the DMZ G-quadruplex complexes could clarify these observations and help in the development of even more potent DMZ-based G-quadruplex binders. | ||
| Fig. 9 CD spectra obtained from titration experiments for the additions of DMZ into a 27mer WT bcl-2 (4.5 μM, panel A) and into 24mer WT c-myc (3.9 μM, panel B). | ||
 | ||
| Fig. 10 CD spectra obtained from titration experiment for the additions of DMZ into a hTel22 (5.1 μM). | ||
Conclusion
The finding that DMZ binds tightly to G-quadruplexes DNA is exciting for the following reasons: (a) DMZ does not readily form π-aggregates74 and hence it is not as prone to non-specific binding to other biomolecules as other aromatic-containing G-quadruplex ligands; (b) the amidine groups on DMZ, improve aqueous solubility (an important factor for drugs); (c) unlike other structurally complex minor groove binders such as netropsin, DMZ has a simple structure and could be readily diversified and synthesized cheaply on a large scale and (d) the amidine group, which is protonated at physiological pH, would facilitate drug permeation across lipid membranes that are externally decorated with anionic phosphates.This work suggests that some of the toxicity or even the clinical benefits of DMZ may be due to its binding to G-quadruplexes, which has so far been overlooked. It is ironic that a ligand such as DMZ, which has long been considered as AT-rich specific minor groove binder does in fact strongly bind to G-quadruplexes. Future work will concentrate on making structural variants of DMZ that will include expanded aromatic systems and increased amidine groups for specific and potent G-quadruplex targeting. New classes of G-quadruplex ligands, which have low affinity for duplex DNA, are predicted to have potential applications in cancer therapy.75–77
Acknowledgements
Camille Dreyfus foundation and University of Maryland Graduate Dean’s Dissertation Fellowship (JZ) supported this work.References
- M. Gellert, M. N. Lipsett and D. R. Davies, Proc. Natl. Acad. Sci. U. S. A., 1962, 48, 2013 CrossRef CAS.
- M. L. Bochman, K. Paeschke and V. A. Zakian, Nat. Rev. Genet., 2012, 13, 770 CrossRef CAS PubMed.
- S. Kumari, A. Bugaut, J. L. Huppert and S. Balasubramanian, Nat. Chem. Biol., 2007, 3, 218 CrossRef CAS PubMed.
- M. D. Frank-Kamenetskii and S. M. Mirkin, Annu. Rev. Biochem., 1995, 64, 65 CrossRef CAS.
- V. Sklenar and J. Feigon, Nature, 1990, 345, 836 CrossRef CAS PubMed.
- K. Gehring, J. L. Leroy and M. Gueron, Nature, 1993, 363, 561 CrossRef CAS PubMed.
- T. A. Brooks, S. Kendrick and L. Hurley, FEBS J., 2010, 277, 3459 CrossRef CAS PubMed.
- G. W. Collie and G. N. Parkinson, Chem. Soc. Rev., 2011, 40, 5867 RSC.
- M. Duchler, J. Drug Targeting, 2012, 20, 389 CrossRef CAS PubMed.
- S. Balasubramanian, L. H. Hurley and S. Neidle, Nat. Rev. Drug Discovery, 2011, 10, 261 CrossRef CAS PubMed.
- A. K. Todd, M. Johnston and S. Neidle, Nucleic Acids Res., 2005, 33, 2901 CrossRef CAS PubMed.
- J. L. Huppert and S. Balasubramanian, Nucleic Acids Res., 2005, 33, 2908 CrossRef CAS PubMed.
- Y. Xu, Chem. Soc. Rev., 2011, 40, 2719 RSC.
- M. Wieland and J. S. Hartig, Chem. Biol., 2007, 14, 757 CrossRef CAS PubMed.
- M. Wieland and J. S. Hartig, Nat. Protoc., 2009, 4, 1632 CrossRef CAS PubMed.
- J. D. Beaudoin and J. P. Perreault, Nucleic Acids Res., 2010, 38, 7022 CrossRef CAS PubMed.
- J. Alzeer, B. R. Vummidi, P. J. Roth and N. W. Luedtke, Angew. Chem., Int. Ed., 2009, 48, 9362 CrossRef CAS PubMed.
- Q. Yang, J. Xiang, S. Yang, Q. Zhou, Q. Li, Y. Tang and G. Xu, Chem. Commun., 2009, 1103 RSC.
- C. Schaffitzel, I. Berger, J. Postberg, J. Hanes, H. J. Lipps and A. Pluckthun, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8572 CrossRef CAS PubMed.
- G. Biffi, D. Tannahill, J. McCafferty and S. Balasubramanian, Nat. Chem., 2013, 5, 182 CrossRef CAS PubMed.
- P. Agrawal, E. Hatzakis, K. Guo, M. Carver and D. Yang, Nucleic Acids Res., 2013, 41, 10584 CrossRef CAS PubMed.
- W. J. Chung, B. Heddi, M. Tera, K. Iida, K. Nagasawa and A. T. Phan, J. Am. Chem. Soc., 2013, 135, 13495 CrossRef CAS PubMed.
- I. T. Holder, M. Drescher and J. S. Hartig, Bioorg. Med. Chem., 2013, 21, 6156 CrossRef CAS PubMed.
- M. Azarkh, V. Singh, O. Okle, D. R. Dietrich, J. S. Hartig and M. Drescher, ChemPhysChem, 2012, 13, 1444 CrossRef CAS PubMed.
- N. W. Kim, M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich and J. W. Shay, Science, 1994, 266, 2011 CAS.
- A. M. Zahler, J. R. Williamson, T. R. Cech and D. M. Prescott, Nature, 1991, 350, 718 CrossRef CAS PubMed.
- Y. Wang and D. J. Patel, Structure, 1993, 1, 263 CrossRef CAS.
- G. N. Parkinson, M. P. Lee and S. Neidle, Nature, 2002, 417, 876 CrossRef CAS PubMed.
- G. W. Collie, S. M. Haider, S. Neidle and G. N. Parkinson, Nucleic Acids Res., 2010, 38, 5569 CrossRef CAS PubMed.
- C. Bazzicalupi, M. Ferraroni, A. R. Bilia, F. Scheggi and P. Gratteri, Nucleic Acids Res., 2013, 41, 632 CrossRef CAS PubMed.
- Q. Li, J. F. Xiang, H. Zhang and Y. L. Tang, Curr. Pharm. Des., 2012, 18, 1973 CrossRef CAS.
- T. Vy Thi Le, S. Han, J. Chae and H. J. Park, Curr. Pharm. Des., 2012, 18, 1948 CrossRef.
- I. Mender, S. Senturk, N. Ozgunes, K. C. Akcali, D. Kletsas, S. Gryaznov, A. Can, J. W. Shay and Z. G. Dikmen, Int. J. Oncol., 2013, 42, 1709 CAS.
- S. M. Gowan, J. R. Harrison, L. Patterson, M. Valenti, M. A. Read, S. Neidle and L. R. Kelland, Mol. Pharmacol., 2002, 61, 1154 CrossRef CAS.
- J. I. Roh, Y. H. Sung and H. W. Lee, OncoTargets Ther., 2013, 6, 1161 Search PubMed.
- A. Siddiqui-Jain, C. L. Grand, D. J. Bearss and L. H. Hurley, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11593 CrossRef CAS PubMed.
- J. Dai, T. S. Dexheimer, D. Chen, M. Carver, A. Ambrus, R. A. Jones and D. Yang, J. Am. Chem. Soc., 2006, 128, 1096 CrossRef CAS PubMed.
- S. Cogoi and L. E. Xodo, Nucleic Acids Res., 2006, 34, 2536 CrossRef CAS PubMed.
- S. Rankin, A. P. Reszka, J. Huppert, M. Zloh, G. N. Parkinson, A. K. Todd, S. Ladame, S. Balasubramanian and S. Neidle, J. Am. Chem. Soc., 2005, 127, 10584 CrossRef CAS PubMed.
- D. Sun, W. J. Liu, K. Guo, J. J. Rusche, S. Ebbinghaus, V. Gokhale and L. H. Hurley, Mol. Cancer Ther., 2008, 7, 880 CrossRef CAS PubMed.
- M. Gunaratnam, S. Swank, S. M. Haider, K. Galesa, A. P. Reszka, M. Beltran, F. Cuenca, J. A. Fletcher and S. Neidle, J. Med. Chem., 2009, 52, 3774 CrossRef CAS PubMed.
- K. I. McLuckie, Z. A. Waller, D. A. Sanders, D. Alves, R. Rodriguez, J. Dash, G. J. McKenzie, A. R. Venkitaraman and S. Balasubramanian, J. Am. Chem. Soc., 2011, 133, 2658 CrossRef CAS PubMed.
- R. Martinez and L. Chacon-Garcia, Curr. Med. Chem., 2005, 12, 127 CrossRef CAS.
- T. M. Ou, Y. J. Lu, J. H. Tan, Z. S. Huang, K. Y. Wong and L. Q. Gu, ChemMedChem, 2008, 3, 690 CrossRef CAS PubMed.
- W. I. Sundquist and S. Heaphy, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 3393 CrossRef CAS.
- Q. Yang, J. Xiang, S. Yang, Q. Li, Q. Zhou, A. Guan, X. Zhang, H. Zhang, Y. Tang and G. Xu, Nucleic Acids Res., 2010, 38, 1022 CrossRef CAS PubMed.
- V. H. Le, R. Buscaglia, J. B. Chaires and E. A. Lewis, Anal. Biochem., 2013, 434, 233 CrossRef CAS PubMed.
- G. E. Plum, Current Protocols in Nucleic Acid Chemistry, 2001, ch. 7, unit 7.3 DOI:10.1002/0471142700.nc0703s00.
- N. Nagesh, R. Buscaglia, J. M. Dettler and E. A. Lewis, Biophys. J., 2010, 98, 2628 CrossRef CAS PubMed.
- J. M. Dettler, R. Buscaglia, V. H. Le and E. A. Lewis, Biophys. J., 2011, 100, 1517 CrossRef CAS PubMed.
- J. S. Hudson, L. Ding, V. Le, E. Lewis and D. Graves, Biochemistry, 2014, 53, 3347 CrossRef CAS PubMed.
- Z. Dong, S. Ekins and J. E. Polli, Mol. Pharmaceutics, 2013, 10, 1008 CrossRef CAS PubMed.
- P. B. Madrid, S. Chopra, I. D. Manger, L. Gilfillan, T. R. Keepers, A. C. Shurtleff, C. E. Green, L. V. Iyer, H. H. Dilks, R. A. Davey, A. A. Kolokoltsov, R. Carrion, Jr., J. L. Patterson, S. Bavari, R. G. Panchal, T. K. Warren, J. B. Wells, W. H. Moos, R. L. Burke and M. J. Tanga, PLoS One, 2013, 8, e60579 CAS.
- S. Cosconati, L. Marinelli, R. Trotta, A. Virno, S. De Tito, R. Romagnoli, B. Pagano, V. Limongelli, C. Giancola, P. G. Baraldi, L. Mayol, E. Novellino and A. Randazzo, J. Am. Chem. Soc., 2010, 132, 6425 CrossRef CAS PubMed.
- S. Cosconati, A. Rizzo, R. Trotta, B. Pagano, S. Iachettini, S. De Tito, I. Lauri, I. Fotticchia, M. Giustiniano, L. Marinelli, C. Giancola, E. Novellino, A. Biroccio and A. Randazzo, J. Med. Chem., 2012, 55, 9785 CrossRef CAS PubMed.
- L. Martino, A. Virno, B. Pagano, A. Virgilio, S. Di Micco, A. Galeone, C. Giancola, G. Bifulco, L. Mayol and A. Randazzo, J. Am. Chem. Soc., 2007, 129, 16048 CrossRef CAS PubMed.
- A. K. Jain and S. Bhattacharya, Bioconjugate Chem., 2011, 22, 2355 CrossRef CAS PubMed.
- M. P. Barrett, C. G. Gemmell and C. J. Suckling, Pharmacol. Ther., 2013, 139, 12 CrossRef CAS PubMed.
- I. Kelsey, S. Nakayama and H. O. Sintim, Bioorg. Med. Chem. Lett., 2012, 22, 881 CrossRef CAS PubMed.
- D. G. Brown, M. R. Sanderson, J. V. Skelly, T. C. Jenkins, T. Brown, E. Garman, D. I. Stuart and S. Neidle, EMBO J., 1990, 9, 1329 CAS.
- W. W. Hartman and T. B. Dickey, Org. Synth., 1934, 24 CAS.
- V. H. Le, N. Nagesh and E. A. Lewis, PLoS One, 2013, 8, e72462 CAS.
- G. B. Rowland, K. Barnett, J. I. Dupont, G. Akurathi, V. H. Le and E. A. Lewis, Bioorg. Med. Chem., 2013, 21, 7515 CrossRef CAS PubMed.
- M. W. Freyer, R. Buscaglia, D. Cashman, S. Hyslop, W. D. Wilson, J. B. Chaires and E. A. Lewis, Biophys. Chem., 2007, 126, 186 CrossRef CAS PubMed.
- M. W. Freyer, R. Buscaglia, B. Nguyen, W. D. Wilson and E. A. Lewis, Anal. Biochem., 2006, 355, 259 CrossRef CAS.
- M. W. Freyer, R. Buscaglia, A. Hollingsworth, J. Ramos, M. Blynn, R. Pratt, W. D. Wilson and E. A. Lewis, Biophys. J., 2007, 92, 2516 CrossRef CAS PubMed.
- E. A. Lewis, M. Munde, S. Wang, M. Rettig, V. Le, V. Machha and W. D. Wilson, Nucleic Acids Res., 2011, 39, 9649 CrossRef CAS PubMed.
- M. W. Freyer, R. Buscaglia, K. Kaplan, D. Cashman, L. H. Hurley and E. A. Lewis, Biophys. J., 2007, 92, 2007 CrossRef CAS PubMed.
- T. Jenkins, A. Lane, S. Neidle and D. Brown, Eur. J. Biochem., 1993, 213, 1175 CrossRef CAS PubMed.
- M. L. Kopka, C. Yoon, D. Goodsell, P. Pjura and R. E. Dickerson, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 1376 CrossRef CAS.
- W. Wilson, F. Tanious, H. Barton, L. Strekowski, D. Boykin and R. Jones, J. Am. Chem. Soc., 1989, 111, 5008 CrossRef CAS.
- I. Donkor, R. Tidwell and S. Jones, J. Med. Chem., 1994, 37, 4554 CrossRef CAS.
- M. Carvlin, N. Dattagupta and R. Fiel, Biochem. Biophys. Res. Commun., 1982, 108, 66 CrossRef CAS.
- D. S. Pilch, M. A. Kirolos, X. Liu, G. E. Plum and K. J. Breslauer, Biochemistry, 1995, 34, 9962 CrossRef CAS.
- R. Haudecoeur, L. Stefan, F. Denat and D. Monchaud, J. Am. Chem. Soc., 2013, 135, 550 CrossRef CAS PubMed.
- O. P. Cetinkol, A. E. Engelhart, R. K. Nanjunda, W. D. Wilson and N. V. Hud, ChemBioChem, 2008, 9, 1889 CAS.
- T. A. Brooks and L. H. Hurley, Genes Cancer, 2010, 1, 641 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Competition assay between DMZ and NMM for G-quadruplex binding, UV titration studies of DNA with DMZ and Triazene-1, CD spectra of DMZ and Triazene-1 binding to duplex DNA and NMR spectra of Triazene-1 and Triazene-2 are available. ITC raw data for the DMZ and Triazene-1 titrations of the HP and G-quadruplex DNAs. See DOI: 10.1039/c4mb00359d |
| This journal is © The Royal Society of Chemistry 2014 |