Research highlights: measuring and manipulating cell migration
Anja
Kunze
,
Ivan
Pushkarsky
,
Harsha
Kittur
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@ucla.edu
First published on 24th September 2014
Abstract
Microfabricated systems and microfluidic tools are well-suited to interface with cells because of the matching length scales. In this issue, we highlight three recent papers in which unique tools were used to control or measure cell migration. Cell migration is a key biological process involved in normal physiology (e.g. in embryonic development, immune response, and wound healing) which can go awry in diseases such as cancer. We highlight work applying electric fields and surface patterning to direct the collective migration of epithelial cells using galvanotaxis, in which surprisingly larger patches of cells respond more uniformly to electric fields. Such a platform may yield insights into or be co-opted to control tissue formation. We also describe recent results on developing a simple system to measure the migration of neutrophils in response to chemoattractants and on using this system to discriminate between neutrophils from asthmatic and non-asthmatic patients. Finally, a micropillar system is highlighted in which the epithelial-to-mesenchymal transition is quantified at the single-cell level, and may aid in our understanding of the plastic process of progression to a malignant phenotype. Cell migration is a wonderfully complex process in which microscale systems can not only contribute to a better understanding, but might also improve its recording and manipulation for practical applications.
A galvanotactic platform to direct collective cell migration
Cell migration is often stimulated by a gradient resulting in a chemical cue. Besides chemical cues, cells also respond to mechanical stimuli, e.g. when a cell physically links to a neighboring cell that is migrating. In this case, the migration direction and speed depend on neighboring cell movements in competition with gradients in the environment. The process of migration in contact with cell neighbors is called collective cell migration and plays a role in wound healing, embryonic development and metastatic cancer migration.1To better understand the mechanism underlying the collective cell migration in vitro, technologies have been developed to track cells; including monolayer stress microscopy2 and cell imaging velocimetry,3,4 however, techniques to control and guide collective migrations have not been previously demonstrated. Cohen et al. developed a microsystem to study collective cell migrations under the influence of a direct current (DC) electric field, a process called galvanotaxis.5 The galvanotactic behavior had been discovered a century ago, however, this effect was only studied with respect to individual cells, not whole cell populations. To examine the ability to control the orientation and direction of cell migration in a physically connected cell population, they used MDCK-II cells, which have physical cell–cell junctions. Fig. 1A shows the galvanotactic cell migration setup. Using a cell-seeding microstencil, Cohen and co-workers locally controlled (i) the shape and size of the epithelial cell population and (ii) the position of the cell population with respect to the applied DC field (Fig. 1B1). MDCK-II cells were seeded into the openings of the polydimethylsiloxane (PDMS) stencil and cultured for up to 12 h until the cells in the monolayer had formed physical cell junctions.5 During control experiments, the cell population displayed a random pattern of cell migration, which Cohen et al. visualized using time-lapse imaging and quantified using particle image velocimetry (PIV) and line integral convolution (LIC), shown in Fig. 1B2. To induce a galvanotactic guidance cue, the cells were exposed for 1.5 h to a DC current density of 0.3 mA mm−1, which corresponds to ~5 V cm−1. The galvanotactic stimuli induced a distinct shift in the LIC pattern, shown in Fig. 1B3. To test the impact of the stimuli on the directionality of border versus bulk migration, two pattern geometries were selected. The larger geometry with an area of 25 mm2 had a lower perimeter to surface area ratio as compared to the smaller area of 5 mm2. Thus, Cohen et al. assumed that cells within the smaller cell population would be more resistant to external guidance stimuli. Indeed, they observed that the larger cell population more easily changed the direction of migration in response to the electric field.
 | ||
| Fig. 1 Galvanotaxis-controlled collective cell migration. (A) Schematic description of the assembly of the galvanotactic cell culture setup, which is placed in a standard culture Petri dish. (B1) Phase contrast image shows a square-sized (5 mm2) patterned MDCK-II cell patch, which performs random cell migration. (B2 and B3) Line integral convolution (LIC) post-processed vector fields (derived from a particle image velocimetry analysis: PIV) demonstrate random (B2) and cathodically guided (B3) cell migration patterns. (C1 and C2) Cell layer population size seems to impact the galvanotaxis-controlled cell migration effect. (C1) The LIC processed vector field indicates more galvanotactic-guided cell migration in the larger cell patch (right: 25 mm2) compared to the smaller cell patch (left: 5 mm2). (C2) The Φ diagram shows the degree of ordered cell migration parallel to the applied DC field. After the DC field is switched on, the larger cell patch shows highly ordered cell migration parallel to the DC field. Reproduced from ref. 5 with permission from the Nature Publishing Group®. | ||
In summary, Cohen et al. demonstrated that the migration patterns of cells in epithelial monolayers seem to be highly plastic and controllable via galvanotactic cues. Electric fields are naturally present in wounds and these results could shed light on future mechanisms to improve the migration of connected cell sheets for wound healing, or further in the future for guiding tissue morphogenesis.
Measuring altered neutrophil chemotaxis in asthmatics
Impairments in neutrophil migration could be used as a biomarker to help in diagnosing asthma, however, previously demonstrated experiments that have found correlations between neutrophil migration and asthma were not conducted in a time period suitable for diagnostic use. Using a simple microfluidic implementation, Sackmann et al. found that neutrophils, taken from the blood of asthmatic patients, exhibited slower chemotactic migration velocities than those taken from healthy patients.6 In the study, the authors describe an all-in-one handheld microfluidic platform that isolates neutrophils from a droplet of blood, measures their chemotactic properties automatically with custom software and uses the results to discriminate between asthmatic and non-asthmatic patients with a sensitivity and specificity of 96% and 73%, respectively.This chemotaxis assay device, first presented in a previous work,7 is made up of two parts which are used to isolate the neutrophils from whole blood and to form the chemoattractant gradient, separately. The first part is the base of the device that contains micro-channels, where the neutrophils are isolated, and the second is a multifunctional lid which holds the chemoattractant. In the first step of the experiment, a droplet of whole blood is added to the micro-channels in the base of the device. The polystyrene surface of these channels is coated with P-selectin allowing for the neutrophils present in the blood to adhere and the remainder of the blood’s contents to be cleared with subsequent washes. A micropipette is used for loading all solutions. Once the purification step is complete, the lid containing a hydrogel–chemoattractant (H–CA) mixture is placed onto the base, forming a contact between the H–CA droplet and the microchannels, thus allowing the chemoattractant to diffuse from the hydrogel and create a concentration gradient in the channel (Fig. 2A).
 | ||
| Fig. 2 The asthma characterization device. (A) Comparison of the purification and chemotaxis protocols for the asthma characterization device (bottom) and the transwell assay (top). (B) COMSOL modeling of the chemical gradient formation. (C) Experimental results showing the gradient formation of the Alexa Fluor 488 dye. (D) Experiment and modeling, found in close agreement, for the formation of the chemical gradient. (E) Experiment and modeling showing that the purification of neutrophils from erythrocytes is heavily dependent on the aspect ratio of the microfluidic device. Reproduced from ref. 6 with permission (copyright © the National Academy of Sciences). | ||
Neutrophil chemotaxis in response to the concentration gradient was recorded using time-lapsed imaging and processed using a custom written software, which tracked individual taxing cells and output several metrics. The authors tested the blood of 23 asthmatic and 11 healthy patients and found that the chemotactic velocity, defined as the taxing speed in the direction of increasing chemoattractant concentration, was significantly reduced for asthmatic patients (P < 0.002). To check for possible effects of corticosteroids on chemotaxis, the authors performed the assay on asthmatic individuals not taking these medications and found a similar reduction in the chemotactic velocity. As further validation, the authors took other commonly used clinical measurements of the patients, finding that low fractions of exhaled nitric oxide (FeNo) measurement values (which correlate positively with the numbers of inflammatory cells present in the lungs) coincided with higher chemotactic velocities. Lastly, the authors determined a chemotactic velocity measurement threshold that could be used to correctly classify 22 out of their 23 tested asthmatic patients and 8 out of the 11 non-asthmatic patients.
In general, the device is simple and could be an attractive diagnostic tool due to its ease-of-use and because it does not need a fluid-driving system. The cell phenotyping nature of this diagnostic approach could perhaps be an improvement over traditional asthma diagnostic methods which rely on clinical observations and commonly over-diagnose patients with confounding variables and under-diagnose the elderly.
Automated analysis of EMT-induced cells in micropillar arrays
The dispersal of individual invasive cells from a tumor is generally indicative of malignancy. The origin of these invasive cells is strongly linked to the epithelial–mesenchymal transition (EMT), a transformation by which cells lose collective migration/invasion characteristics and gain individual invasion characteristics such as decreased cell–cell adhesion and increased motility. Additionally, cells that have undergone EMT tend to have a distinct fitness advantage due to an increased drug resistance and the activation of anti-apoptotic pathways. In studying the complex phenomena associated with malignant tumor cell invasion, in vitro platforms such as transwell migration assays have provided experimental control and high throughput for understanding the migration of various bulk populations upon the activation of EMT-related pathways such as Snail,8 but so far they have not been able to resolve the heterogeneity within a population at a single-cell level.Wong, et al. have developed a simple microfluidic motility assay that uses rows of pillars (Fig. 3A) to enhance cell–surface interactions as means of competing for and disrupting cell–cell contacts, and thereby delineating individually scattered highly motile cells from a unidirectional, collectively advancing front.9 They tracked the cell migration using automated time-lapse microscopy (Fig. 3B), and sought to establish a distinction between the epithelial and the mesenchymal cells by determining the average lifetime number of the nearest neighbors for each cell (Fig. 3C). First, they used a breast cancer model in which the behavior of the MCF-10A cells describes a homogeneous epithelial population (N ~ 4.5 ± 1.3 nearest neighbors), while that of the MDA-MB-231 cells describes a homogeneous mesenchymal population (N ~ 1.1 ± 0.8 nearest neighbors). Next, they transfected MCF-10A cells with a stable Snail construct, representing heterogeneous, EMT-induced cells. This was confirmed by fluorescence immunostaining of the epithelial (E-cadherin) and mesenchymal (vimentin) characteristics, as shown in Fig. 3D–F. Taken together, a cutoff was set by which cells with N > 2.5 neighbors are marked as epithelial, and cells with N < 2.5 neighbors are regarded as mensenchymal. From here, three other parameters (final Y position, velocity, and straightness) were plotted for each cell as a function of number of cell neighbors, as seen in the Gaussian mixture models in Fig. 3G. These characteristics were shown to further delineate the two subpopulations, as the vimentin-expressing MCF-10A Snail cells showed increased velocities with straighter trajectories that better matched the MDA-MB-231 cells, while the E-cadherin-expressing cells show similarities to the basic MCF-10A cells in that they exhibit slower, more tortuous paths.
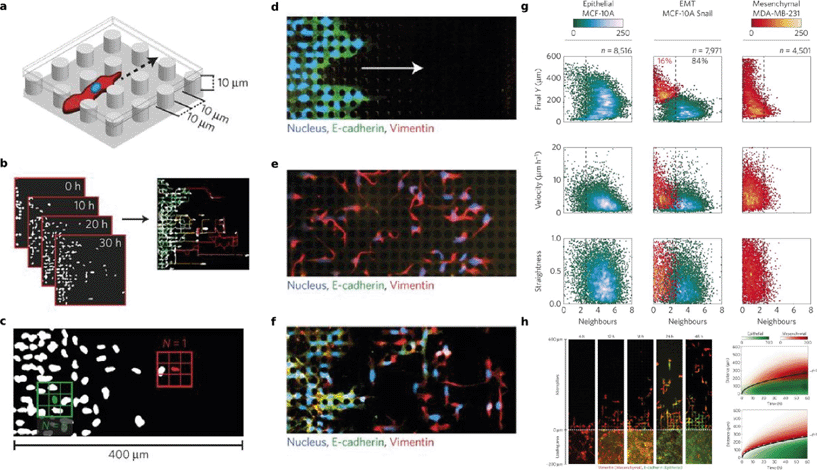 | ||
| Fig. 3 EMT and migration through pillar arrays. (A) Depiction of a simple pillar array disrupting the cell–cell contacts for the separation of the mesenchymal cells from an epithelial front. (B) Time-lapse images and subsequent automated analysis are used to quantify the key parameters of the migration that distinguish subpopulations, such as average lifetime of nearest neighbors (C) as well as final Y position, velocity, and straightness (G). (D–F) indicate that MCF-10A cells provide a suitable epithelial baseline (D), MDA-MB-231 cells describe a mesenchymal baseline (E), and MCF-10A cells induced with Snail represent EMT-induced cells that display both phenotypes. (G) Mesenchymal cells move in quick and straight paths as opposed to the slow and tortuous moving epithelial cells. The MCF-10A Snail cells were classified using Gaussian mixture models to have both sub-populations. (H) The MCF-10A Snail cells started with the mesenchymal phenotype (>95%), but over the course of two days, most cells reverted back to the epithelial phenotype. These observations were modeled (bottom right) and appeared to be in agreement with the experimental data (top right). Reproduced from ref. 9 with permission from the Nature Publishing Group®. | ||
Interestingly, the MCF-10A Snail cells initially showed >95% mesenchymal biomarker expression, but by the end of the experiment, only 16% retained those qualities while 84% had reverted back to the epithelial phenotype, as qualitatively seen in Fig. 3H. This phenotypic plasticity during migration may be the result of high cell–cell contacts that induce the reverse process, mesenchymal–epithelial transition (MET). The authors relate this scenario to the unidirectional solidification of a binary mixture, whereby in a mixture of solid and liquid phase, the solid front rejects solutes at the interface and causes them to diffuse away into the more soluble liquid phase, resulting in further cooling and solidification at the interface. Similarly, the slow-moving epithelial front becomes saturated, and rejects interactions with the individual cells that retain their mesenchymal phenotype and continue to migrate faster downstream. From such an analogy,10 they developed a model to describe their observations. Fig. 3H shows that the experimental results (top right) agree with their solidification model (bottom right) and that the epithelial front collectively migrates at a rate proportional to the square root of the time.
Finally, Wong et al. applied a small panel of kinase-inhibiting drugs to knock down migration and proliferation. They found that the drug FMK-MEA successfully inhibited the invasion and proliferation in both epithelial and mesenchymal subpopulations as opposed to other drugs like BID-1870 and U0126, which only significantly affected the epithelial front. Taken together, these data demonstrate the power of this microfluidic approach to image and analyze the behavior of thousands of cells at single-cell resolution, which can be used as a drug screening tool to provide insight into their effects on subpopulations, which is coupled with the solidification model for understanding the complex interactions between epithelial and mesenchymal cells that may occur at the tumor front.
References
- P. Friedl and D. Gilmour, Collective cell migration in morphogenesis, regeneration and cancer, Nat. Rev. Mol. Cell Biol., 2009, 10(7), 445–457 CrossRef CAS PubMed.
- D. T. Tambe, et al., Monolayer Stress Microscopy: Limitations, Artifacts, and Accuracy of Recovered Intercellular Stresses, PLoS One, 2013, 8(2), e55172 CAS.
- F. Milde, et al., Cell Image Velocimetry (CIV): boosting the automated quantification of cell migration in wound healing assays, Integr. Biol., 2012, 4(11), 1437–1447 RSC.
- S. R. Vedula, et al., Epithelial bridges maintain tissue integrity during collective cell migration, Nat. Mater., 2014, 4(1), 87–96 Search PubMed.
- D. J. Cohen, W. J. Nelson and M. M. Maharbiz, Galvanotactic control of collective cell migration in epithelial monolayers, Nat. Mater., 2014, 13(4), 409–417 CrossRef CAS PubMed.
- E. K.-H. Sackmann, et al., Characterizing asthma from a drop of blood using neutrophil chemotaxis, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5813–5818 CrossRef PubMed.
- E. K. Sackmann, et al., Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays, Blood, 2012, 120, e45–e53 CrossRef CAS PubMed.
- S. Javaid, J. Zhang, E. Anderssen, J. C. Black, B. S. Wittner, K. Tajima, D. T. Ting, G. A. Smolen, M. Zubrowski, R. Desai, S. Maheswaran, S. Ramaswamy, J. R. Whetstine and D. A. Haber, Cell Rep., 2013, 5, 1679–1689 CrossRef CAS PubMed.
- I. Y. Wong, S. Javaid, E. A. Wong, S. Perk, D. A. Haber, M. Toner and D. Irimia, Nat. Mater., 2014 DOI:10.1038/nmat4062.
- M. G. Worster, J. Fluid Mech., 1986, 167, 481–501 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |
