Research highlights: increasing paper possibilities
Chueh-Yu
Wu
,
Oladunni
Adeyiga
,
Jonathan
Lin
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@ucla.edu
First published on 21st July 2014
Abstract
In this issue we highlight three recent papers that demonstrate new strategies to extend the capabilities of paper microfluidics. Paper (a mesh of porous fibers) has a long history as a substrate to perform biomolecular assays. Traditional lateral flow immunoassays (LFAs) are widely used for rapid diagnostic tests, and perform well when a yes or no answer is required and the analyte of interest is at relatively high concentrations. High concentrations are required because usually only a small volume of analyte-containing fluid flows past the detection region, leading to a limited signal. Further, the small pores within paper matrices prevent the use of paper to control the flow of larger particles and cells, limiting the use of paper microfluidics for cell-based diagnostics. The work we highlight addresses these important unmet challenges in paper microfluidics: enriching low concentration analytes to a higher concentration in a smaller volume that can be processed effectively, and using paper to pump flows in larger channels amenable to cells. Applying these new approaches may allow diagnosis of disease states currently technically unachievable using current LFA systems, while maintaining many of the “un-instrumented” advantages of an assay on self-wicking paper.
Electrokinetic concentration on paper
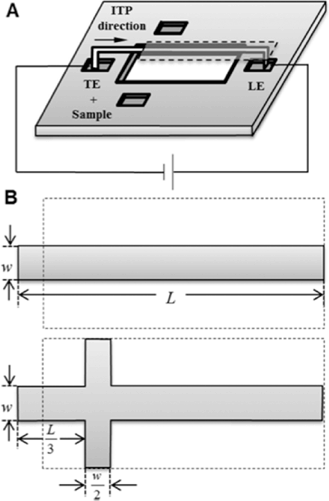
Although electrokinetic techniques have been used to concentrate sample in microfluidic systems for decades, recent work has largely focused on glass chips and powerful techniques such as isotachophoresis (ITP), which takes advantage of differential electrophoretic mobility to concentrate analyte ions into a moving ITP plug, have not yet been adapted for paper microfluidics.1,2Moghadam et al. demonstrate an electrokinetic, ITP-based technique for preconcentrating analyte on nitrocellulose for use in lateral flow assays.3 In this study, the authors used current-controlled peak-mode ITP to achieve up to 900-fold concentration of Alexa Fluor 488 with HCl as the leading electrolyte (LE) and HEPES as the trailing electrolyte (TE) (Fig. 1A, B).
By examining the paper ITP system parametrically, the authors provide useful insights into the design of paper ITP systems. They demonstrate that the stacking ratio, the ratio of analyte concentration in the ITP plug to analyte concentration in the sample reservoir, is improved by decreasing TE concentration, increasing electrical current and increasing the length of the membrane. Changing these parameters, however, results in increases in electro-osmotic flow and evaporation, which act to reduce the stacking ratio. Thus, optimization is required for these ITP systems and the authors have provided useful intuition for approaching this process. In characterizing the system, the authors achieved a maximum stacking ratio of 760 with 50% extraction using a 500 μA current on a 40 mm membrane.
The authors also characterize a cross-shaped membrane design in order to combat evaporation, which is significant at high currents (Fig. 1B). In this design, water is introduced into the arms of the cross to improve membrane wetting. With this device, the authors achieved a current of 1 mA resulting in a stacking ratio of 900 with 60% sample extraction at runtimes of less than 90 seconds.
Additionally, the authors demonstrate the ability to perform paper ITP with consumer AA and button batteries, achieving stacking ratios of 564 and 541 respectively. The ability to use batteries instead of conventional power supplies represents a dramatic improvement in cost effectiveness and portability of the technology, making the technology more suitable for point-of-care, resource-poor setting applications.
Moghadam et al. have demonstrated a powerful, rapid, on-paper sample concentration technique, addressing one of the core constraints on lateral flow assays. This technique provides not only up to 900-fold concentration, but also compatibility with large sample volumes (~100 μl). The authors provide valuable insights into optimizing these systems as well as propose means by which the capabilities of the technique can be improved and cost decreased. Next steps will require applying these approaches to increase the volume interrogated (and therefore decrease the detection limit) by lateral flow immunoassays starting from complex bio-samples.
Two-phase in flow concentration on paper
Another group has tackled the problem of low sensitivity inherent to lateral flow immunoassays (LFA) by concentrating targets from a larger volume by speeding up intrinsic thermodynamic partitioning in aqueous two phase systems.4 Previously, this group has reported using a micellar aqueous two phase separation (ATPS) system to separate and concentrate a specific biomarker into a smaller volume. This concentrated volume may then be added to a lateral flow immunoassay (LFA) for further downstream analysis. In this work, the authors construct a polymer–salt aqueous two phase separation system that is combined with a 3D fiberglass paper based LFA to both concentrate and detect biomarkers of interest as a complete system – with separation occurring during flow through the paper (Fig. 2). The ATPS used here consists of polyethylene glycol (PEG) with potassium phosphate salt and functionalized gold nanoparticles mixed into a background solution of Dulbecco's phosphate-buffered saline.The degree of separation and concentration of a particle (e.g. biomarker) in this system is based on size and hydrophilicity of the particle. For example, small, hydrophobic particles are more likely to be retained in the PEG rich region, given the large size and hydrophobic nature of PEG particles. On the contrary, larger, hydrophilic (e.g. functionalized gold nanoparticles) are more likely to be retained in the salt (or PEG poor) region. In this work, gold nanoparticles functionalized to bind the protein transferrin were used as a proof of concept.
Interestingly, using this ATPS system in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PEG
1 (PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt) volume ratio within a 2D paper based device reduces the separation time in comparison with the macroscopic separation that occurs within a vial. While the phenomenon diminished as the PEG
salt) volume ratio within a 2D paper based device reduces the separation time in comparison with the macroscopic separation that occurs within a vial. While the phenomenon diminished as the PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt ratio increased to 9
salt ratio increased to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by stacking fiberglass paper, the authors note an ability to preserve this enhanced separation at the 9
1, by stacking fiberglass paper, the authors note an ability to preserve this enhanced separation at the 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEG
1 PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) salt ratio, concentrate samples by 10-fold, and additionally achieve a 10-fold increase in detection limit. This 3D design also allows for more volume to be processed, and therefore measurement of a larger total number of target analyte molecules for a given concentration, decreasing the lower concentration limit of detection. In a 1
salt ratio, concentrate samples by 10-fold, and additionally achieve a 10-fold increase in detection limit. This 3D design also allows for more volume to be processed, and therefore measurement of a larger total number of target analyte molecules for a given concentration, decreasing the lower concentration limit of detection. In a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system, volume processed can increase from 20 μl to 40 μl with a 2-fold increase in detection limit. In a 9
1 system, volume processed can increase from 20 μl to 40 μl with a 2-fold increase in detection limit. In a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system volumes processed can increase from 20 μl to 200 μl with a 10-fold increase in detection limit.
1 system volumes processed can increase from 20 μl to 200 μl with a 10-fold increase in detection limit.
Moving forward it will be important to characterize how the combined ATPS and LFA system handles more complex sample matrices, such as plasma, sputum, or food products. It will also be interesting to understand how the paper matrix enhances the ATPS separation so this can be further engineered.
Laser micromachined hybrid open/paper microfluidic chips
Paper-based microfluidic systems have garnered interest due to certain advantages including ease-of-use in operating a self-contained platform that often requires no power or external instrumentation. However, it remains challenging to transport suspensions of discrete objects, such as cells or microparticles that are conventionally used in many microfluidic applications. Chumo et al. recently developed a hybrid open/paper microfluidic chip, where the liquid carrier was transported in Mylar microchannels but driven by a paper channel, with quantitative flow control.5 The hybrid chips were fabricated using a stack of polymer and paper sheets, shown in Fig. 3(a), designed using SolidWorks and micromachined using a CO2 laser. On the hybrid chip an acrylic reservoir, conventional open channel, paper channel, and wick of Kimwipe were connected in series.The geometry of the paper channel was modified to control the flow rate due to the large flow resistance of the paper. The flow can be modeled using Darcy's law and Kirchoff's current law. The experimental results showed that in a simple straight channel the flow rate was reversely proportional to the length of the paper channel. Also, a hybrid chip with a Y junction was shown to generate a laminar co-flow pattern with stream width controlled by the length ratio of the paper channels at the outlet. Moreover, core and sheath lamina were combined to achieve a stable flow-focusing geometry. It is important to note that flow focusing was stably performed with beads and cells for >10 min, demonstrating the utility for applications in microfluidic cell- or particle-based assays.
References
- P. Smejkal, et al., Microfluidic isotachophoresis: a review, Electrophoresis, 2013, 34, 1493 CrossRef CAS PubMed.
- Y. Oyama, et al., A glass fiber sheet-based electroosmotic lateral flow immunoassay for point-of-care testing, Lab Chip, 2012, 12, 5155 RSC.
- B. Y. Moghadam, K. T. Connelly and J. D. Posner, Isotachophoretic preconcentration on paper-based microfluidic devices, Anal. Chem., 2014, 86, 5829 CrossRef CAS PubMed.
- R. Y. Chiu, E. Jue, A. T. Yip, A. R. Berg, S. J. Wang, A. R. Kivnick, P. T. Nguyen and D. T. Kamei, Lab Chip, 2014, 14, 3021–3028 RSC.
- B. Chumo, M. Muluneh and D. Issadore, Biomicrofluidics, 2013, 7, 064109 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |