Research highlights: microfluidics meets big data
Peter
Tseng
,
Westbrook M.
Weaver
,
Mahdokht
Masaeli
,
Keegan
Owsley
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@seas.ucla.edu
First published on 28th January 2014
Abstract
In this issue we highlight a collection of recent work in which microfluidic parallelization and automation have been employed to address the increasing need for large amounts of quantitative data concerning cellular function – from correlating microRNA levels to protein expression, increasing the throughput and reducing the noise when studying protein dynamics in single-cells, and understanding how signal dynamics encodes information. The painstaking dissection of cellular pathways one protein at a time appears to be coming to an end, leading to more rapid discoveries which will inevitably translate to better cellular control – in producing useful gene products and treating disease at the individual cell level. From these studies it is also clear that development of large scale mutant or fusion libraries, automation of microscopy, image analysis, and data extraction will be key components as microfluidics contributes its strengths to aid systems biology moving forward.
Microfluidic flow cytometry of microRNA
MicroRNAs (or miRNA) are small, non-coding molecules that regulate genetic expression. These RNAs play a critical role in single cell development, and manifest in larger scale biological processes including immune response, inflammation, and cancer. Proper quantification of miRNA levels in cells can potentially serve multiple purposes, including in disease diagnosis, and could elucidate links between genes and cellular development. This detection is accomplished with a number of methods, including most commonly northern blotting, and more recently by microarray technologies, quantitative PCR, and in situ hybridization (quantification by fluorescent tags or gel electrophoresis).1 Both northern blotting and microarray approaches lack single cell resolution due to cell lysing and homogenization steps, thus obscuring the response of individual cells. Single-cell qPCR and in situ hybridization, although capable of single-cell resolution, can be difficult to execute and has low throughput, or suffers from low reproducibility. Key needs of miRNA detection technologies are repeatable, sensitive, non-destructive and multiplexed methods of analysis given small numbers of molecules to read out.1To resolve the above challenges, Singh and colleagues2 utilized an integrated, microfluidic platform to trap Jurkat cells, and subsequently perform two tasks: (1) in situ hybridization, and (2) protein immunostaining on chip. Upon completion, cell attachment was cleaved, and single cells were analyzed by microfluidic cytometry directly on chip.
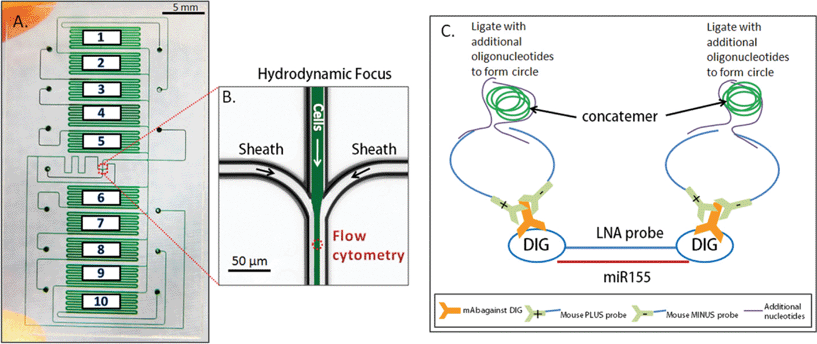
The chip was composed of 10 fluidically-isolatable chambers in order to test 10 different experimental conditions (Fig. 1). Within these chambers, fixed Jurkat cells were initially captured using Cell Tak™ solution, and in situ hybridization was assayed using a unique combination of locked nucleic acid probes (LNA, commonly used to improve hybridization sensitivity), coupled with rolling circle amplification to amplify signal to be visible in single cells. In this study, intracellular miR155 (a miRNA that plays roles in various pathological processes) was hybridized with their tagged probe, and ligated with oligos for signal amplification and fluorescent detection. The authors additionally modified the cell fixation and staining process to allow for fluorescent visualization of protein, in addition to the above miRNA visualization.
Cell characterization was performed directly on chip with microfluidic flow cytometry, as cells (~200) from individual chambers were cleaved using elastase, focused to centerlines using sheath flow, and assayed by fluorescence excitation, and scatter detection. The authors demonstrated the potential of this approach by quantifying temporal dynamics between the protein CD69 and miR155 expression during cell stimulation by lonomycin. The protein CD69 had a detectable increase in expression within 8 hours, while upregulation of miR155 lagged behind these events, becoming noticeable at 16 hours.
This microfluidic platform yielded numerous benefits over traditional in situ hybridization. This approach can reduce sample variability by giving users direct control over input analyte into the system, and additionally consumes low quantities of reagent. The authors estimated that the approach yields a ~100 fold reduction in cost, to below $1.50 per sample, significantly improving the accessibility of in situ hybridization assays.
In summary, an integrated, multiplexed, microfluidic approach to miRNA detection can serve to reduce variability and cost, while allowing for simultaneous quantification of both protein expression and miRNA regulation in single cells. This platform could potentially be used in systems biology as a device to uncover temporal dynamics, and interplay between sets of miRNA and proteins.
Printing cell arrays for high-content microfluidic screening
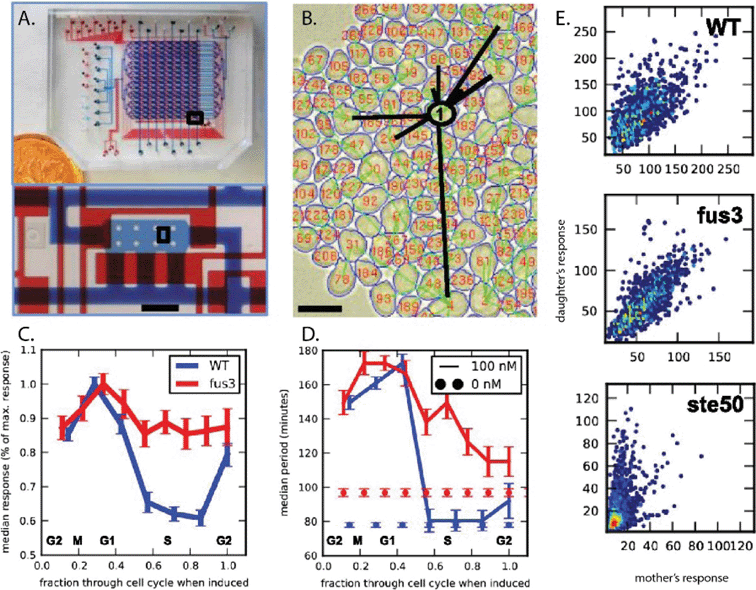
Transcriptional analysis and proteomics have become powerful tools in mapping cellular pathways, often employing high content screening (HCS) as a powerful image analysis and post processing technique in microtiter plate format for cell phenotype readouts including cell shape, size, motility, protein expression, and growth rate, often coupled with fluorescence readout. Microtiter formats, however, suffer from reduced control of the dynamic cellular environment, and difficulty in imaging of cell multilayers that can form for organisms like yeast.Dénervaud et al.3 have approached this challenge using an integrated microfluidic chemostat, effectively converting a microtiter plate platform to a microfluidic format with tunable microenvironments and single-plane imaging. Each device contains 1152 micro-chemostats, subdivided into three separately addressable 384 chemostat arrays, allowing for three separate experiments on chip. Each single chemostat is inoculated with a clonal population of Saccharomyces cerevisiae cultures by (i) printing of cellular arrays on epoxy-silane glass slides using a commercial DNA micro-arrayer (Qarray2, Genetix), (ii) alignment of PDMS channels and bonding, and (iii) rehydration of cultures by injecting media into the chemostat array, where culture spots remained dry for 20–30 minutes. Subsequently, the spotted cultures are grown to a confluent population in the chemostat, while a button valve is actuated above the culture chambers, resulting in a 5 μm chamber height to ensure a monolayer of cells for automation of image analysis.4 Sieve channels in the bottom of each chemostat (1.5 μm tall) ensure that no cells escape from the bottom culture layer, and cells overgrowing the monolayer exit the chamber through the top under an actuated valve. This geometry leads to steady state growth and removal of cells, as well as replenishment of fresh media – key for uniform analysis conditions and reduction of extrinsic biological noise.
The integration of large-scale fluidic networks with printed cell arrays makes this platform a very powerful tool for systems biology approaches to proteomics. Here, Dénervaud et al. utilize a library of 4085 S. cerevisiae GFP fusion strains to quantitatively characterize their spatiotemporal stress response to various chemical stresses and UV irradiation. In particular, this technique is strengthened by the quantitative nature of analysis, enabled by high level, custom automated image acquisition and analysis of cell size, shape, growth rates and GFP localization. In a single experiment, spatiotemporal information regarding GFP localization can be extracted from ~108 cells, with 20 min time resolution and ~1 μm spatial resolution (using a 90× objective) over a 12 hour period.
This particular study identified 118 proteins that change either abundance or subcellular localization in response to UV irradiation or exposure to methyl methanesulfonate (MMS), involved in direct DNA damage of DNA replication stress, respectively. Within that class, 33 proteins (representing the largest subclass) were involved in the formation of cytoplasmic foci and were identified as proteins involved in mRNA processing body (P-body) formation, which is a suspected effector in the DNA damage response. Further, a deletion library consisting of 560 strains was generated to investigate regulators of this response, in which new regulators of P-body response were identified. Further, they show for the first time that response regulators can act by either affecting the abundance of Rnr4 (a critical P-body subunit), or simply by inhibiting its cellular localization.
This novel and complete platform (from device construction through custom, well-built image analysis software) has the potential to become the gold standard for high throughput HCS by incorporating the multiplexing of microarraying platforms or microtiter plates with the precision control of the growth environment and visualization enabled by large-scale microfluidics. From this data it is clear that both cellular localization of proteins as well as total abundance play a critical role in cellular response and phenotype. Combining this approach with deletion libraries, Dénervaud et al. have taken a more quantitative proteomic approach to systems biology.
Tracking whether daughters take after their mother
Microfluidic platforms offer relevant throughputs required for obtaining statistically meaningful information on the response of heterogeneous cell populations to external stimuli. Using a similar integrated microfluidic platform described above to control the growth environment of yeast cells and maintain imaging in a single plane, Ricicova et al.5 performed studies to understand one aspect of biological noise – i.e. how cell lineage played a role in cell signaling response.To understand how cells detect external cues and the contribution of non-genetic heritability, the authors track and reconstruct full lineage trees of multiple generations of cells using automated software. Previous microfluidic platforms have used confinement to develop lineage relationships6 but relied on manual or quasi-automated lineage tree reconstruction which limits the number of cells that can be practically analyzed. To address this issue, Ricicova et al. have implemented a high-throughput fully automated live-cell imaging microfluidic platform that enables simultaneous measurement of gene expression, cell-cycle periods and lineage information under a variety of time-dependent medium conditions.
The microfluidic chip is designed to be loaded through 8 columns, each of which may be loaded with a different yeast strain and programmable chemical sequences are delivered through 16 rows for a total of 128 simultaneous live cell imaging experiments. The response of single cells and cell-lineage relationships of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells are tracked for up to eight generations. By selecting a subarray of chambers to be imaged and defining a time-dependent chemical condition, the user initiates automated perfusing and imaging of the array of chambers. Using MATLAB, the lineages are reconstructed by extracting images from thousands of cells. Following a complete segmentation of all the cells from the captured images for consequent time points, cells and lineages are then tracked and the mother–daughter pairs are identified.
000 cells are tracked for up to eight generations. By selecting a subarray of chambers to be imaged and defining a time-dependent chemical condition, the user initiates automated perfusing and imaging of the array of chambers. Using MATLAB, the lineages are reconstructed by extracting images from thousands of cells. Following a complete segmentation of all the cells from the captured images for consequent time points, cells and lineages are then tracked and the mother–daughter pairs are identified.
The transcriptional response of the yeast Saccharomyces cerevisiae to pheromone was analyzed using this system and heterogeneity in cell response as well as the role of seven different genes was investigated. The study specifically reveals that fus3Δ cells exhibit the highest heritability in their capacity to respond to pheromone (Fig. 2). Using cell tracking, the authors observed differences in the distribution of cell-cycle periods in wildtype cell populations compared to fus3Δ cells in the presence of pheromone. Interestingly, in the absence of pheromone significant differences between cell-cycle kinetics of wildtype and fus3Δ cells were also observed. Using these observations, the authors concluded that the enhanced heritability of fus3Δ cells is a result of attenuating two key sources of noise that is usually introduced during the process of cellular division: (1) cell-cycle inhibition of MAPK signaling and (2) asymmetry in the cell-cycle kinetics of mother–daughter pairs. Additionally, the high heterogeneity observed in the response of a ste50Δ mutant strain to pheromone was shown to originate from a unique asymmetry between mother and daughter response.
Using the yeast pheromone response as a model, the authors show the capability of this platform in studying how signaling networks are primed by non-genetic heritability and highlight the importance of quantitative cell-cycle and lineage tracking in understanding the nonrandom sources of heterogeneity in cellular response – identifying alterations of cell cycle and age symmetry as two key factors. Simultaneous collection of gene expression, age, cell-cycle and cell-lineage relationships from thousands of single cells under controlled conditions is suggested to improve our ability to answer major biological questions regarding cell population responses to external cues.
Single-cell response to dynamic signals
Typically, cellular responses are thought to be encoded in the amplitude or location of an intracellular signal, but recent evidence has shown that additional information can be encoded in the dynamics of these signals. Msn2, a zinc finger transcription factor in the yeast stress response, encodes the type of response in its translocation dynamics; in this way, a single transcription factor can activate different response pathways appropriate to the stress.Hansen et al.7 make use of a microfluidic platform for probing signal transduction pathways that respond to the dynamics of a signal. A yeast strain is modified with an analog-sensitive protein kinase A (PKA), an Msn2 phosphorylator, to enable selective inhibition of PKA activity with 1-NM-PP1. The ORFs of seven Msn2-activated promoters are replaced with CFP and YFP, enabling measurement of gene activation using quantitative microscopy. Msn2 is fused with mCherry to visualize its localization. These custom strains of yeast are then seeded onto a microfluidic device with straight channels and computer-controlled three-way valves connected to the inlets, allowing the researchers to deliver controlled pulses of media with and without 1-NM-PP1. The device is placed onto a microscope, allowing direct visualization of the response of individual cells to different temporal signals. Similar microfluidic devices have been previously developed for temporal control of soluble input signals applied to cells during culture.8
The combination of microbiology techniques, microfluidic networks, and quantitative microscopy allows a high-throughput study of single-cell response to signal dynamics. Here, Hansen et al. measure the response of seven different promoters to the translocation dynamics of a single transcription factor. They find that, while all of the promoters are activated by sustained, high-amplitude nuclear localization of Msn2, some promoters are also activated by oscillatory localization. Different promoters may be preferentially activated by controlling the oscillatory behavior of the Msn2 signal.
Because the technique allows imaging of individual cells, the authors are able to identify a mechanism for promoters to decode the signal dynamics: those promoters that respond to sustained signals had significantly more expression noise than those that also responded to oscillatory signals. In general, an oscillatory signal results in a noisier response, which is filtered by the downstream promoter based on its activation time. Slower promoters filter out the noisy signal, while fast promoters respond. This comes with a trade-off; slower promoters also tend to have a higher intrinsic noise. This hypothesis is validated by a mathematical model, which shows that promoters can generally fall into four classes for differentiating between transcription factor dynamics, depending on the promoter's activation threshold (high or low) and response time (slow or fast). Of those tested, three were low-threshold, fast-responding promoters, with three high-threshold, slow-responding promoters opposite; one promoter was fast-responding, high-threshold.
Using this platform, researchers can more extensively characterize signal transduction behavior, including the study of individual promoters as signal-processing units. An integrated microfluidic system with quantitative microscopy enables Hansen et al. to characterize the behavior of several promoters at the single-cell level, extracting information about how noise characteristics affects response to a dynamic input.
References
- K. A. Cissell and S. K. Deo, Trends in microRNA detection, Anal. Bioanal. Chem., 2009, 394, 1109–1116 CrossRef CAS PubMed.
- M. Wu, M. Piccini, C.-Y. Koh, K. S. Lam and A. K. Singh, Single Cell MicroRNA Analysis Using Microfluidic Flow Cytometry, PLoS One, 2013, 8, e55044 CAS.
- N. Dénervaud, et al., A chemostat array enables the spatio-temporal analysis of the yeast proteome, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15842–15847 CrossRef PubMed.
- P. J. Lee, N. C. Helman, W. A. Lim and P. J. Hung, A microfluidic system for dynamic yeast cell imaging, BioTechniques, 2008, 44, 91–95 CrossRef CAS PubMed.
- M. Ricicova, et al., Dissecting genealogy and cell cycle as sources of cell-to-cell variability in MAPK signaling using high-throughput lineage tracking, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11403–11408, DOI:10.1073/pnas.1215850110.
- A. C. Rowat, J. C. Bird, J. J. Agresti, O. J. Rando and D. A. Weitz, Tracking lineages of single cells in lines using a microfluidic device, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18149–18154 CrossRef CAS PubMed.
- A. S. Hansen and E. K. O'Shea, Promoter decoding of transcription factor dynamics involves a trade-off between noise and control of gene expression, Mol. Syst. Biol., 2013, 9, 704 CrossRef CAS PubMed.
- K. R. King, S. Wang, A. Jayaraman, M. L. Yarmush and M. Toner, Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments, Lab Chip, 2008, 8, 107–116 RSC.
| This journal is © The Royal Society of Chemistry 2014 |