Effects of methane addition to nebulizer gas on polyatomic interferents and ion sensitivity in inductively coupled plasma mass spectrometry
Rui
Santos
UCTM-Lab LNEG, Rua da Amieira – Apartado 1089, S. Mamede de Infesta, Portugal. E-mail: rui.santos@lneg.pt
First published on 9th October 2013
Abstract
The addition of methane to nebulizer gas was assessed as a method for the reduction of polyatomic interferents in inductively coupled plasma mass spectrometry (ICP-MS). The effects of nebulizer gas flow rate, RF power and methane flow were studied for a range of analytes and polyatomic ions. The analyte sensitivity, especially for Be, Br and I, was enhanced by a factor of 7–12 with methane addition. Polyatomic ions, such as ArCl+, ArO+, ClO+ and ArArH+, were reduced between 61 and 92% when compared to those of an unmodified plasma. Such reduction allowed better quantification limits for V, As, Se, Br and I, as well as 90% BaO+ polyatomic reduction. Finally, the optimized conditions were evaluated in successful recovery tests for As, Se and V in different matrices with high chloride content. The Fe accuracy was evaluated in several reference materials.
Introduction
Analytical quantification of some elements by ICP-MS may be limited by the formation of polyatomic interferents, especially for those below m/z 80.1–4 Assigned as polyatomic, the ions typically come from Ar support gas, atmospheric gases (nitrogen and oxygen) or from the sample matrix (O, OH, Cl, S and P). Since the development of this analytical technique, different methods have been developed to resolve, attenuate or even eliminate this type of interferents, some of them successfully. These approaches range from the most inexpensive and simple ones to attenuate some of the interferents, like careful optimization of the instrument parameters such as nebulizer gas flow rate and RF power (cited as power throughout the text),4–6 and solvent removal by the Peltier effect in spray chambers, to more expensive and time consuming methods to eliminate some of the interferents, such as hydride generation,7 the use of collision cells8−10 or, finally, the use of a high resolution ICP-MS11,12 to resolve the analyte signal from the interferents. The aims of all these strategies are the reduction of polyatomic interferents. Another expedient method for reduction/elimination of this set of interferents is to use another inert gas, like nitrogen, and mix it with one of the three flows of argon.Some researchers use different gases and types of combinations, since the major advantage of mixed-gas plasmas for liquid sample introduction is the reduction of certain acid-related polyatomic interferents, such as ArCl+ and ClO+.12,13 A wide variety of additional gases for the Ar plasma gas supply, such as nitrogen,14–17 oxygen,18 hydrogen,17,19 helium20–22 and carbon-containing solvents, have been studied using solution nebulization ICP-MS. For example, Sheppard et al.22 found that addition of He produces a plasma capable of ionizing elements with high ionization potential more efficiently than pure Ar plasma. Evans and Ebdon23,24 reported the addition of nitrogen and oxygen to the nebulizer gas with a significant reduction of ArCl+. Lam and Horlick25 reported that polyatomic interferents can also be reduced and the sensitivity of analytes can be improved by adding nitrogen to the auxiliary gas flow. Lam and McLaren26 found that the UO+/U+ ratio and ArO+ intensities were reduced by adding 8% nitrogen to the auxiliary gas, and they also reported interferents reduction on Fe and Se. The optimization of nitrogen addition to the nebulizer and auxiliary gas flows, reported by Hill et al.,27 revealed a dramatic reduction of ArCl+ and ClO+ interferents on As and V, respectively.
More recently, most studies have focused on the addition of other gases. Smith et al.28 investigated the addition of Xe to the nebulizer gas and reported a reduction in polyatomic interferents. Allain et al.29 added methane to the nebulizer gas and verified improvement in the sensitivity of most analytes. However, addition of organic compounds to the ICP, either as admixtures to the gas phase or C-based additives to the sample solution, has a disadvantage: they will produce other kinds of polyatomic interferents, such as CN+, CO+, ArC+, etc., especially below m/z 53.
Since the first use of mixed gases, it has been observed that even small amounts of additives change significantly the Ar plasma properties.30 Among other effects, the shape or geometry of the plasma is affected in these mixed gas plasmas,13,25 which subsequently affects the ion extraction or sampling efficiency of ions through the sampler cone. It has also been reported that an increased amount of hydrogen in carbon containing plasmas, and its related plasma chemistry,19,27,31–34 might be responsible for the reduction of these interferents.19,32,34,35
This paper presents experiments for a range of polyatomic interferents and elements with different masses and ionization potentials with methane addition. Our results show that signal enhancement of analytes and polyatomic reduction, such as ArCl+, ClO+, ArO+, ArArH+ and BaO+, could be achieved through instrument parameter optimization, such as nebulizer gas flow and power, with different methane flows. After optimization of these parameters, the accuracy achieved in the determination of V, As, Se and Fe, through successful recovery tests, Reference Materials (RMs) and Standard Reference Materials (SRMs), and the quantification limits (LOQ) determination achieved, for all the elements tested, including the elements with high ionization potential, such as Br and I, were evaluated.
Experimental
Instrumentation
All data in this study were obtained with an ICP-QMS instrument PQExCell (VG Elemental) from LNEG – Geosciences Laboratory, Oporto, Portugal. The ICP-QMS was installed in a clean (class 100–1000) and thermostatically maintained room (±2 °C) and the sample solutions were introduced via a peristaltic pump (Spetec Perimax 12) and an auto-sampler (50/60 Cetac ASX500). A standard sample introduction system, consisting of a cross-flow nebulizer, a Scott-type double-path spray chamber Peltier cooled at 4 °C and a Fassel quartz torch were used. The instrument warm-up was 2–3 hours before measurements to ensure system stability according to factory specifications. Methane (N55, 99.9995%, Air Liquide, Portugal) was added to the nebulizer gas flow through a T-connection via a mass flow controller (F200DV-AGD-33V, Bronkhorst High-Tech, Netherlands) calibrated and able to increase by 0.1 mL min−1 and regulate flows of methane up to 10 mL min−1.Reagents and standards
For the experimental testing of the formation of polyatomic interferents, the samples were prepared as two different blank solutions (2% HCl and 2% HNO3) using ultra pure acids obtained by double sub-boiling distillation in a Duopor system (Milestone) and deionised water produced with a Milli-Q Elemental system (Millipore), with resistivity better than 18 MΩ cm. The different standards used were prepared by successive dilutions from mono-element standards in 1000 mg L−1 stocks (AlfaAesar). These solutions were volumetrically prepared using Eppendorf fixed volume micropipettes that were externally calibrated every 6 months and internally verified between calibrations. The five reference materials of the proficiency testing scheme (LGC-Aquacheck), distributions AQ380, 388, 392, 396, 400 and 404 from the years 2010 and 2011, and the SRM NIST 1643d, were used to assess the accuracy of the elements.Analytical procedure
Each working day began with optimization of instrumental conditions in order to obtain the best sensitivity for 115In in the commercial solution MS-2 at 2% HNO3 without methane addition. After instrument stabilization (≈2 hours) the criteria of RSD <1% for the masses 9Be, 59Co, 115In, 138Ba, 208Pb, 209Bi and 238U and for oxide formation BaO+/Ba2+ <0.2% were achieved using the instrument parameters noted in Table 1. The analytical determinations carried out in order to establish the influence of instrument parameters (nebulizer gas flow and power) and the amount of methane added on the different species (analytes and polyatomic interferents) were performed in triplicate on standard solutions containing various species (Be, Co, Br, In, I, Ba, Pb, Bi and U) with a concentration of 1 μg L−1 and in chloride standard of 1000 mg L−1. These experiments were performed with and without methane gas addition to the nebulizer gas in accordance with the following instrumental conditions: gas coolant at 13 L min−1; auxiliary gas at 0.8 L min−1; the nebulizer gas from 0.6 to 1.2 L min −1; power: from 1200 to 1700 W; and CH4 addition: from 0 to 3 mL min−1.| ICP-MS normal mode | |
|---|---|
| R. F. Power | 1350 W |
| Coolant gas | 13 L min−1 |
| Auxiliary gas | 0.8 L min−1 |
| Nebulizer gas | 0.8 L min−1 |
| Sampling depth | 400 mm |
| Sampling cone | 1.0 mm |
| Skimmer cone | 0.7 mm |
| Sample uptake time | 120 s |
| Washout time | 180 s |
| Extractor | −658 V |
| Lens 1 | −5.5 V |
| Lens 2 | −74.4 V |
| Lens 3 | −57.7 V |
| Focus | 18.0 V |
| Deflector | −50.5 V |
| Pole bias | 3.0 V |
| Acquisition parameters | |
|---|---|
| Settling time | 100 μs |
| Channels per mass | 3 |
| Sweeps number | 250 |
| Total acquisition time | 60 s |
| Dead time | 35 ns |
| Detector | Electron multiplier |
| Mass acquisition | Pulse/analog modes |
| Methane addition | |
|---|---|
| A | 1 mL min−1 |
| B | 2 mL min−1 |
| C | 3 mL min−1 |
| Instrumental conditions | I | II | III |
|---|---|---|---|
| Power | 1350 W | 1700 W | 1300 W |
| Methane addition | 0 mL min−1 | 1 mL min−1 | 3 mL min−1 |
| Nebulizer gas | 0.8 L min−1 | 0.8 L min−1 | 0.8 L min−1 |
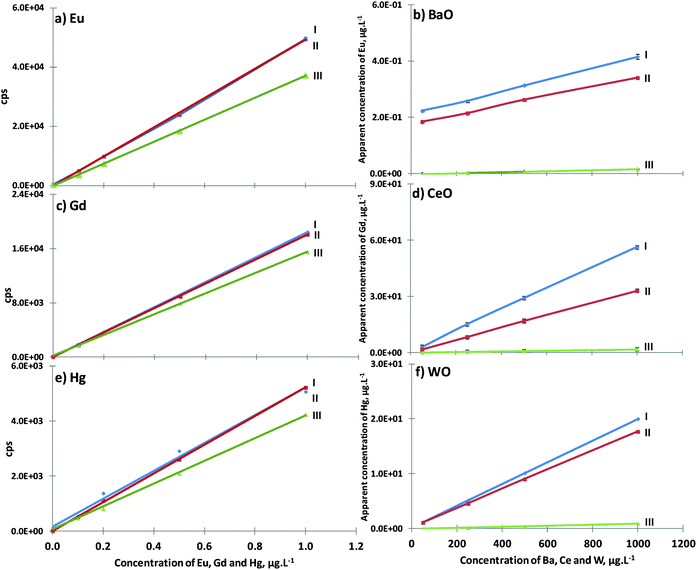
The instrumental conditions defined for reducing ArCl+ and ClO+ interferents were tested in two standards, one with 2 μg L−1 of V and As and the other with 10 μg L−1 of Se, both in the presence of increasing chloride concentrations (0, 50, 250, 500 and 1000 mg L−1). Oxide species minimization, such as BaO+vs. Eu, CeO+vs. Gd and WO+vs. Hg, was tested in the presence of increasing concentrations of Ba, Ce and W (0, 50, 250, 500 and 1000 mg L−1) on 0.2 μg L−1 Eu, Gd and Hg standards. The evaluation of ArO+ reduction, after adjusting instrumental conditions, was tested using the accuracy achieved in the measurement of 54Fe and 56Fe isotopes on the five RMs and SRM NIST 1643d. Finally, the optimization of the ionization of elements with high ionization potential, like Br and I, was tested against ArArH+ interferent reduction but also with its ionization maximization. In fact, polyatomic reduction and ionization improvement led to a better LOQ of such elements.
Unlike Park et al.,36 who chose to add methane to the coolant or auxiliary gas, it was demonstrated that much higher flows are needed to produce similar effects to those observed with the addition of methane to the nebulizer gas. During this optimization, no carbon deposits were found on the surfaces of cones that were able to clog them.
Results and discussion
Polyatomic ion identification
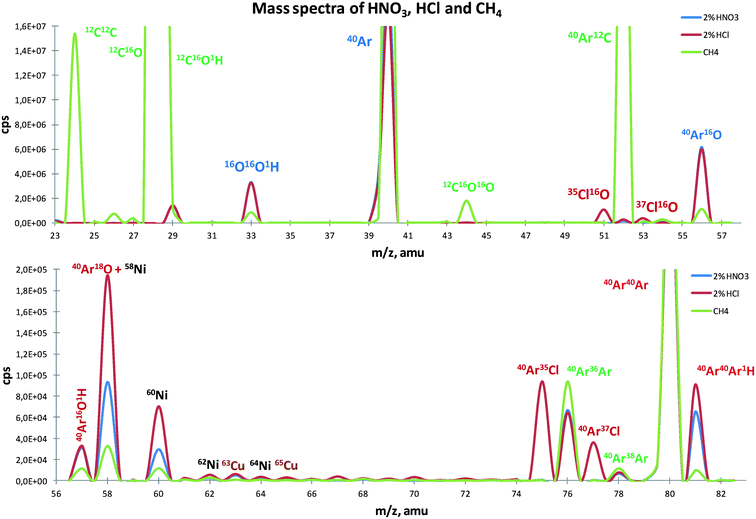
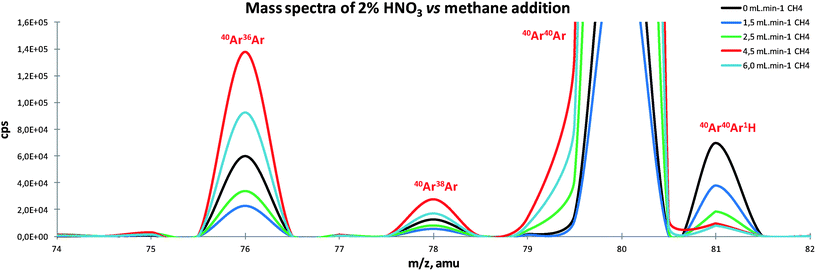
When mixed plasma was used, new interferents were formed and needed a thorough investigation, especially when carbon compounds were introduced into the plasma. Therefore, three mass spectra under three different conditions (2% HNO3, 2% HCl without methane addition, and with 1.5 mL min−1 CH4) were acquired. It is clear that very different polyatomic species are present under each condition (Fig. 1). Usually, at 2% HNO3, polyatomic ions like OOH+, ArO+, ArOH+, ArAr+ and ArArH+ are the main interferents. However, at 2% HCl new combinations occur, giving rise to new polyatomic ions, such as ClO+ and ArCl+, and those that already occur at 2% HNO3 also increase (ArO+ and ArArH+). The introduction of small amounts of CH4 leads to profound changes in the mass spectrum and polyatomic species present. Since carbon becomes one of the major ions in the plasma, carbon combinations like CC+, CO+, COH+ and ArC+ become dominant in the spectrum, as expected. In addition, the introduction of methane not only favors ionic reactions with carbon but also the reaction between Ar+ ions (ArAr+).30 On the other hand, the formation of the corresponding hydrides ArArH+ and, most significantly, the presence of species such as ArOH+ and ArO+ are reduced.Effect of nebulizer gas flow
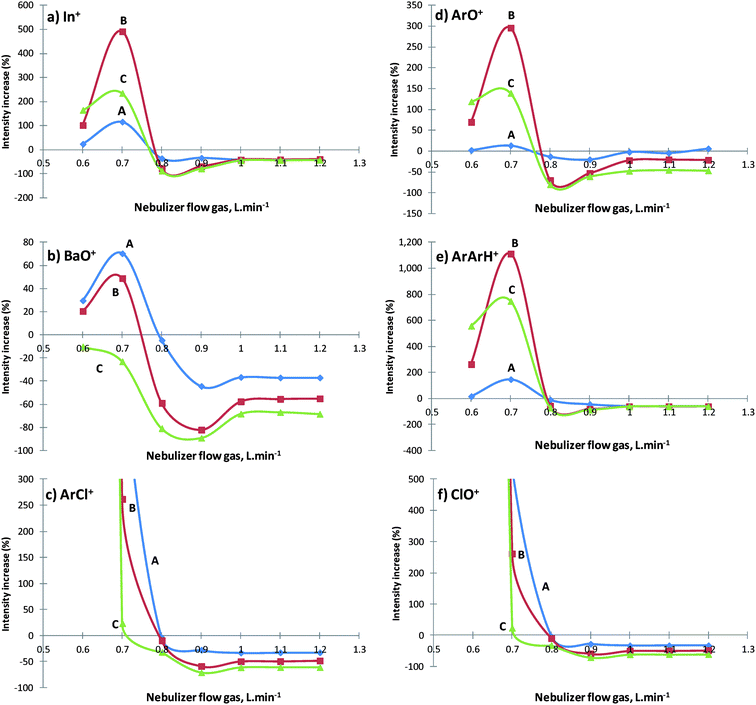
Before any consideration about the results obtained, it has been established that the position of the maximum ion intensity in the central channel of the ICP is strongly dependent on the nebulizer gas flow37 and ion transmission. However, it was possible to evaluate whether the maximum ion transmission was affected by methane addition by changing the nebulizer gas flow.The data illustrated in Fig. 2 show the nebulizer gas flow influence on (a) In+; (b) BaO+; (c) ArCl+; (d) ArO+; (e) ArArH+ and (f) ClO+ intensities (cps) with three CH4 additions: 1, 2 and 3 mL min−1. The influence of methane addition in all the scenarios tested was similar, although the intensity increase (%) was strongly dependent on the methane amount introduced.
All the analytes tested increased their ionization yield from 4 to 12 fold with the addition of 2 mL min−1 CH4 but only to a maximum nebulizer gas flow of 0.7 L min−1. After 0.8 L min−1 nebulizer gas flow all the analytes lost their intensity. In fact, the addition of 1 mL min−1 CH4 caused more than 25% loss of intensity for most elements if the nebulizer gas flow was higher than 0.8 L min−1.
The distribution of atomic and polyatomic species in plasma is not uniform due to different mobilities between ions and electrons and space charge effects. Another aspect to consider is a possible modification of the geometry of the plasma with methane addition, which could have significant influence on the sampling efficiency. When different methane amounts were added, more hydrogen atoms and electrons were delivered to the plasma, narrowing the central channel due to the electron density and the presence of hydrogen atoms around it, which could be one of the probable and reasonable explanations for this phenomenon leading to a better sampling efficiency. The equilibrium between methane addition and plasma stability reached below 0.8 L min−1 of nebulizer gas flow. In fact, some authors38,39 have suggested that a charge transfer reaction occurs between positively charged carbon species and elements in the central channel of the plasma, which could be another reason for ionization enhancement. This transfer mechanism, but not this mechanism alone, may also explain the tendency of a greater signal enhancement for elements with high ionization potential in the presence of carbon. Of course, elements with different masses and different ionization potentials felt these changes in different ways, especially those with higher ionization potential, like Be (9.32 eV), Br (11.81 eV) and I (10.45 eV), or with lower masses, like Be, which reached 12 fold in ionization yield. Another possible reason, linked to the ionization degree, could be the rise of peripheral plasma temperatures due to the change in the thermal conductivity of the plasma or the electron temperature19 as a consequence of the increased amount of hydrogen. In fact, the ionization yield still increased for higher power (Fig. 4a) as a consequence of the ICP higher temperature available.
There is no doubt that for a nebulizer gas flow higher than 0.7 L min−1 all the polyatomic interferent intensities dropped after methane addition (Fig. 2b–f). Some aspects mentioned in the literature, like ICP temperature, may also influence the vaporization efficiency; asymmetric charge transfer reactions between carbon and certain analyte atoms,38,39 dissociation of polyatomic species caused by concurrent reactions in the presence of methane12,40 and, finally, modification of the central channel plasma geometry tend to relate the influence of methane to polyatomic reduction and better ionization yields. However, in our opinion, none of the items listed above, individually, may be the only reasons for these effects, but a mix of them could be considered a reasonable possibility.
Fig. 2 shows clearly that the introduction of methane leads to a very significant oxide polyatomic reduction, such as BaO+, ArO+ and ClO+ (Fig. 2b, d and f), which had reductions of 90, 80 and 71%, respectively, for 3 mL min−1 CH4 at 0.8–0.9 L min−1 nebulizer gas flow. The reduction is proportional to the amount of methane added, and one of the possible reasons for this could be the polyatomic species dissociation, due to a likely concurrent formation of CO+ and a charge transfer reaction with carbon species, as has been shown in the literature.27,40
Regarding the effect of ArArH+ (Fig. 2e) on 81Br, a significant 87% reduction was noted, especially for higher nebulizer gas (0.9 L min−1) and with 3 mL min−1 of CH4 added, which would be expected to increase under these conditions. Methane additions in ICP mixed plasmas had direct influence on plasma chemical reactions. In fact, it was expected that polyatomic hydride formation could be favored, like ArArH+, because of the increased amount of H atoms due to methane (Fig. 3). However, the alteration of plasma chemistry and/or other interaction phenomena led to different behavior according to the methane amount introduced. Fig. 3 shows that ArAr+ decreased until a certain amount of methane was added (2.5 mL min−1), but with higher amounts of methane, its intensities became more pronounced when compared to the absence of methane. This behavior can be explained by the temperature change that occurs in the central channel of the plasma, since Ar ionization is very sensitive to temperature changes,39 and since the enhancement is not proportional to the amount of methane added. The carbon charge transfer reaction is also not applicable, because Ar and carbon have a considerable energy deviation (4.5 eV) and for that reason, this process is less favored to occur.41 The ArArH+ signal decreases faster than ArAr+ because a combination of three atoms is involved, instead of two. A possible explanation for this could be the higher electron temperature in hot plasma, which disfavors atom recombination. Another cause could be the influence of the sampling position as a consequence of the plasma geometry change.
 | ||
| Fig. 3 Polyatomic behavior of ArAr+ and ArArH+ in 2% HNO3 for different methane additions: 0, 1.5, 2.5, 4.5 and 6 mL min−1. | ||
By contrast, and as predicted, the addition of methane ultimately caused a dramatic increase in ArC+, which became one of the most abundant polyatomic interferents in the mass spectrum at m/z 52, causing a partial overlap (tailing effect) in the mass 51V. The largest difference occurred at a gas flow rate of 0.7 L min−1, where the addition of 3 mL min−1 CH4 caused a response of 20 Mcps compared to just 6500 cps in the absence of methane.
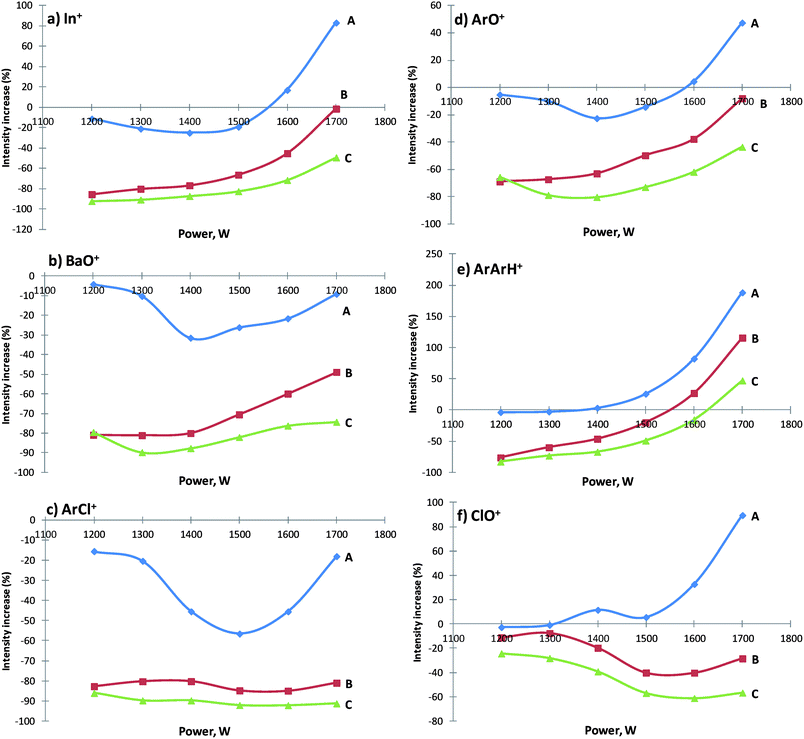
Effect of power
Once again, the ionization efficiency of In (Fig. 4a) was affected by methane addition, like the other elements tested. The elements with high ionization potential (Be and I) showed a 125 and 156% ionization increase, respectively, for 1700 W with 1 mL min−1 CH4 addition. The results show that signal enhancement of hardly ionizable analytes is not due to improvement in the nebulization transport of the sample but more related to the carbon amount added to the plasma, as observed by Hu et al.39 In fact, this is in accordance with Hill et al.,35 who reported ionization improvements only for applied power greater than 1500 W with methane addition of 1 mL min−1. On the opposite side, less available energy, at lower power, and the cooling effect on the central channel by methane addition are the main reasons for the ionization decrease of the elements. A lower ionization yield continued to occur even if there was more methane added but still without enough power available. In those circumstances, a carbon charge transfer reaction between positively charged carbon species and elements in the central channel of the plasma does not have the ideal conditions to take place. The only way to change the cooling methane effect in the central channel plasma, and to promote ionization yield, is to increase the power and/or reduce the nebulizer gas flow, as shown earlier (Fig. 2a).The effect of power on the response of polyatomic interferents (Fig. 4b–f) shows, once again, the dramatic influence on all polyatomic formation. The addition of methane reduces 90% of the BaO+ formation, 90% for ArCl+, 80% for ArO+, 74% for ArArH+ and 61% for ClO+. In fact, as more methane was added more polyatomic reductions were achieved. However, with increasing plasma energy, even without methane addition, polyatomic intensities and power are directly proportional. In general, the minimum values for polyatomic intensities and oxide formation were achieved with 3 mL min−1 CH4 addition at 1300 W, except for ClO+ whose minimum formation was achieved with 1600 W. These values are comparable to those found with the variation of the nebulizer gas flow, which shows the direct influence of the applied power as a determinant factor on the amounts of interferents present in plasma.
One of the reasons mentioned in the literature for these effects is the dissociation of polyatomic species.35,40 In our opinion such a reason is unlikely, since the lower plasma energy and the cooling effect in the central channel are not ideal for polyatomic dissociation. Unfortunately, it was not possible to collect temperature data in different parts of the plasma with and without methane addition to clarify this issue clearly. Therefore, we believe that this phenomenon may not be the dominant process with power below 1400 W. Nonetheless, above 1400 W, and especially for methane amounts greater than 1 mL min−1, a possible reason for intensity enhancement could be a competitive reaction between free metal and carbon for oxygen free atoms,39 leading to carbon oxide polyatomic ions. We can suggest that the carbon charge transfer effect, mentioned before as one of the possible reasons for the ionization yield of elements with high ionization potential, is not related to polyatomic reduction. In fact, polyatomic species involving atoms like Ar (15.76 eV) and Cl (12.97 eV), with considerable energy deviation (1.7–4.5 eV) compared to carbon (11.26 eV), need at least two electrons involved in the charge transfer process, and for that reason, the occurrence of this process is less favorable.41 Considering an easily ionizable element, such as Ba (5.21 eV), the charge transfer effect is even less important, since the element is already fully ionized in the plasma,42 so it is possible that polyatomic dissociation caused by carbon competition could be the reason for the reduction of BaO+.
According to nebulizer gas flow and power with different methane additions, and after checking polyatomic interferent and ionization element behaviors, it was possible to define two different instrumental conditions: II – instrumental conditions for element ionization maximization (power of 1700 W/1 mL min−1 CH4/0.8 L min−1 nebulizer gas flow); III – instrumental conditions for polyatomic interferent minimization (power of 1300 W/3 mL min−1 CH4/0.8 L min−1 nebulizer gas flow). Both of these will be compared to the normal plasma instrumental conditions: I – 1350 W/0 mL min−1 CH4/0.8 L min−1 nebulizer gas flow.
Accuracy
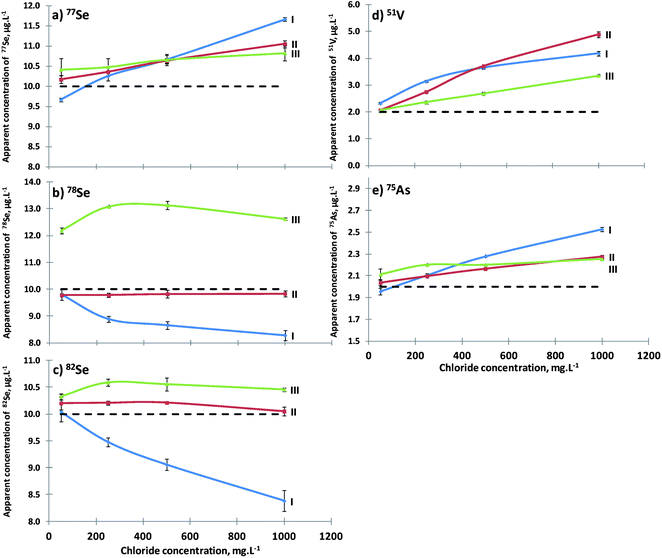
Carbon addition to the plasma has shown some benefits. Most of the elements with high ionization potential increase their sensitivities but also lower levels of interferents were achieved. However, such improvements must be evaluated in terms of accuracy. So the two instrumental conditions defined before (II and III) will be compared to the absence of methane (I). Fig. 5a–c show the behavior of three Se isotopes with a 10 μg L−1 solution with increasing chloride concentrations under three different instrumental conditions. The values reported for the three Se isotopes became more inaccurate as the chloride concentration increased when compared to the absence of methane (I). For the 77Se isotope, which felt direct influence of 77ArCl+ and with ideal instrument optimization for polyatomic minimization (III), it was possible to obtain a lower dispersion around the nominal value, with an addition of 3 mL min−1 CH4 and a power of 1300 W. However, the other two Se isotopes, 78Se and 82Se, since they are unaffected by any chloride polyatomic interferent, showed different behavior compared to the 77Se isotope, obtaining better accuracy with the addition of 1 mL min−1 CH4 and with a power of 1700 W, an ideal situation for the ionization improvement of elements with high ionization potential such as Se(II).The behavior of 51V and 75As (Fig. 5d and e) is similar to that of 77Se, since they had the same problem of polyatomic interferents. So the optimized instrumental conditions that need to be applied are number II. Despite the failure of complete polyatomic interferent elimination, the addition of methane improves not only the precision but also the accuracy of the results (Table 2).
| Instrumental conditions | Concentration, μg L−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 51V | %RSD | 75As | %RSD | 77Se | %RSD | 78Se | %RSD | 82Se | %RSD | |
| a Mean values with one standard deviation (1δ) for in-run statistics (n = 3). | ||||||||||
| I | 3.3 ± 0.8 | 23.7 | 2.2 ± 0.2 | 11.0 | 10.6 ± 0.8 | 8.0 | 8.9 ± 7.2 | 7.2 | 9.2 ± 0.7 | 7.6 |
| II | 3.4 ± 1.2 | 36.6 | 2.2 ± 0.1 | 4.7 | 10.6 ± 0.4 | 3.6 | 9.8 ± 0.03 | 0.3 | 10.2 ± 0.08 | 0.8 |
| III | 2.6 ± 0.6 | 21.2 | 2.2 ± 0.06 | 2.7 | 10.6 ± 0.2 | 1.8 | 12.8 ± 0.4 | 3.5 | 10.5 ± 0.12 | 1.1 |
The accuracy achieved for oxide minimization under the three instrumental conditions (I, II and III) was checked using the apparent concentration of Eu, Gd and Hg obtained with increments of Ba, Ce and W, respectively. It was found previously that instrumental condition III was the most suitable one for the reduction of BaO+ (Fig. 4b). Fig. 6a, c and e illustrate that only 3 mL min−1 CH4 causes significant Eu, Gd and Hg ionization yield reduction, unlike the addition of 1 mL min−1 CH4, which is innocuous to ionization elements. However, small methane additions, such as 1 mL min−1, could cause an immediate reduction of oxide formation, reaching residual values (Fig. 6b, d and f), when 3 mL min−1 CH4 was introduced in accordance with the instrumental conditions defined previously for polyatomic minimization (III).
Iron isotopes are affected severely by polyatomic interferents, such as ArO+ and ArOH+, leading to poor quantification of the element in ICP-MS. After optimized instrumental conditions were defined, it was checked if they could lead to better accuracy. Table 3 shows the performance achieved for Fe in the reference materials (SRM NIST 1643d) and five RMs of the proficiency testing scheme (LGC-Aquacheck). Only 54Fe could achieve a reasonable accuracy with the instrumental condition defined as I. Only an addition of 3 mL min−1 of CH4 allowed quantification of the 56Fe isotope, because of the drastic minimization of ArO+ polyatomic. However, the values obtained for 56Fe are still erroneous, except for NIST 1643d (Z-score of 2.4). In our opinion, despite the fact that addition of 3 mL min−1 CH4 allowed a significant reduction of ArO+ with 56Fe, it is more convenient to use the 54Fe isotope even with lower abundance for Fe quantification without methane addition.
| Ref. | Instrumental conditions | Concentration, μg L−1 | Certified values, μg L−1 | Z-score | ||
|---|---|---|---|---|---|---|
| 54Fe | 56Fe | 54Fe | 56Fe | |||
| a Mean values with one standard deviation (1δ) for in-run statistics (n = 3). | ||||||
| NIST 1643d | I | 119 ± 3.2 | — | 91.2 ± 3.9 | 5.5 | — |
| II | 251 ± 1.8 | — | 37.3 | — | ||
| III | 132 ± 33.1 | 116 ± 9.7 | 1.2 | 2.4 | ||
| AQ 380 | I | 450 ± 6.6 | — | 416 ± 42 | 0.79 | — |
| II | 1363 ± 1.0 | — | 22.5 | — | ||
| III | 1639 ± 94.0 | 715 ± 24.2 | 11.9 | 6.2 | ||
| AQ 392 | I | 199 ± 5.5 | — | 201 ± 15 | −0.1 | — |
| II | 510 ± 7.4 | — | 18.5 | — | ||
| III | 524 ± 75.5 | 343 ± 18.1 | 4.2 | 18.9 | ||
| AQ 396 | I | 314 ± 1.5 | — | 335 ± 25 | −0.9 | — |
| II | 1573 ± 3.2 | — | 49.1 | — | ||
| III | 1756 ± 181 | 685 ± 36.0 | 7.8 | 8.0 | ||
| AQ 400 | I | 393 ± 5.8 | — | 404 ± 30 | −0.4 | — |
| II | 1345 ± 2.1 | — | 31.3 | — | ||
| III | 1924 ± 285 | 776 ± 53.6 | 5.3 | 6.1 | ||
| AQ 404 | I | 674 ± 2.5 | — | 667 ± 50 | 0.1 | — |
| II | 690 ± 6.4 | — | 0.5 | — | ||
| III | 1889 ± 123 | 985 ± 23.8 | 9.2 | 5.7 | ||
Finally, methane addition improves the LOQ (LOQ = 10 × δ[blank concentration]) of elements with high ionization potential, such as V, As, Se, Br, and I. Using different approaches, and depending on the instrument set-up defined, minimizing polyatomic interferents, or by the ionization yield of elements with high ionization potential, LOQ improved from two to seven fold when compared with the absence of methane (Table 4).
| Instrumental conditions | Concentration, μg L−1 | ||||||
|---|---|---|---|---|---|---|---|
| 51V | 75As | 77Se | 78Se | 81Br | 82Se | 127I | |
| I | 2.6 | 0.57 | 2.8 | 2.4 | 12.1 | 1.7 | 2.7 |
| II | 1.9 | 0.12 | 0.53 | 0.72 | 7.9 | 0.35 | 1.2 |
| III | 0.38 | 0.71 | 4.7 | 7.1 | 8.3 | 0.15 | 6.3 |
Conclusions
This study demonstrates that polyatomic interferents, such as ClO+, ArO+, ArCl+, ArArH+ and BaO+, can be drastically reduced by adding methane to the nebulizer gas flow. It has been shown that the optimization of power and nebulizer gas flow is an efficient and rapid way to identify the best conditions for interference reduction. From a general point of view, the use of methane mixed with the nebulizer gas enabled the reduction of such interferents but also promoted the ionization of elements with high ionization potential, such as Be, Br and I, as well as the stability of the analytical signal. The success of this work was demonstrated by the reduction of LOQ for all elements tested; effective reduction of polyatomic interferents such as BaO+, CeO+ and WO+ led to accuracy and precision improvement for V and As in the presence of high concentrations of chloride.High methane flow of 3 mL min−1 caused a 25% signal reduction of analytes and enabled the quantification on 56Fe by the polyatomic ArO+ reduction. Unfortunately, it was not possible to reach the Fe values of the reference materials tested, leading us to consider that the best option to quantify Fe is by using the isotope 54Fe in the absence of methane.
This study has clearly demonstrated the analytical potential of methane addition mixed with nebulizer gas flow to the central channel of the Ar plasma as a way to enhance ion signals and to reduce polyatomic interferents in ICP-MS. These improvements are very important for quantifying elements with high ionization potential, like Br and I, or elements affected by seriously polyatomic interferents, such as ClO+ and ArCl+, or in a general way, to reduce all oxide/hydroxide formation in Ar plasma, without the need for using more expensive and time consuming methods to avoid some of the quadrupole disadvantages, especially for those who have an old ICP-MS without collision/reaction cells.
References
- A. L. Gray, Spectrochim. Acta, Part B, 1986, 41, 151–167 CrossRef.
- S. Munro, L. Ebdon and D. J. Mcweeny, J. Anal. At. Spectrom., 1986, 1, 211–219 RSC.
- S. H. Tan and G. Horlick, Appl. Spectrosc., 1986, 40, 445–460 CrossRef CAS.
- M. A. Vaughan and G. Horlick, Appl. Spectrosc., 1986, 40, 434–445 CrossRef CAS.
- A. L. Gray and J. G. Williams, J. Anal. At. Spectrom., 1987, 2, 599–606 RSC.
- R. C. Hutton and A. N. Eaton, J. Anal. At. Spectrom., 1987, 2, 595–598 RSC.
- S. Branch, W. T. Corns, L. Ebdon, S. Hill and P. Oneill, J. Anal. At. Spectrom., 1991, 6, 155–158 RSC.
- J. Sucharova, J. Anal. At. Spectrom., 2011, 26, 1756–1762 RSC.
- W. Guo, S. H. Hu, X. F. Li, J. Zhao, S. S. Jin, W. J. Liu and H. F. Zhang, Talanta, 2011, 84, 887–894 CrossRef CAS PubMed.
- D. Pick, M. Leiterer and J. W. Einax, Microchem. J., 2010, 95, 315–319 CrossRef CAS PubMed.
- N. Bradshaw, E. F. H. Hall and N. E. Sanderson, J. Anal. At. Spectrom., 1989, 4, 801–803 RSC.
- I. Rodushkin, P. Nordlund, E. Engstrom and D. C. Baxter, J. Anal. At. Spectrom., 2005, 20, 1250–1255 RSC.
- E. H. Larsen and S. Sturup, J. Anal. At. Spectrom., 1994, 9, 1099–1105 RSC.
- C. Agatemor and D. Beauchemin, Spectrochim. Acta, Part B, 2011, 66, 1–11 CrossRef PubMed.
- Z. C. Hu, S. Gao, Y. S. Liu, S. H. Hu, H. H. Chen and H. L. Yuan, J. Anal. At. Spectrom., 2008, 23, 1093–1101 RSC.
- M. Ohata, Y. Takaku, K. Inagaki, A. Hioki and K. Chiba, Anal. Sci., 2009, 25, 161–163 CrossRef CAS.
- M. Shaheen and B. J. Fryer, J. Anal. At. Spectrom., 2010, 25, 1006–1013 RSC.
- J. Kosler, H. P. Longerich and M. N. Tubrett, Anal. Bioanal. Chem., 2002, 374, 251–254 CrossRef CAS PubMed.
- M. Guillong and C. A. Heinrich, J. Anal. At. Spectrom., 2007, 22, 1488–1494 RSC.
- B. S. Sheppard, J. A. Caruso, K. A. Wolnik and F. L. Fricke, Appl. Spectrosc., 1990, 44, 712–715 CrossRef CAS.
- B. S. Sheppard, W. L. Shen and J. A. Caruso, J. Am. Soc. Mass Spectrom., 1991, 2, 355–361 CrossRef CAS.
- B. S. Sheppard, W. L. Shen, T. M. Davidson and J. A. Caruso, J. Anal. At. Spectrom., 1990, 5, 697–700 RSC.
- E. H. Evans and L. Ebdon, J. Anal. At. Spectrom., 1989, 4, 299–300 RSC.
- E. H. Evans and L. Ebdon, J. Anal. At. Spectrom., 1990, 5, 425–429 RSC.
- J. W. H. Lam and G. Horlick, Spectrochim. Acta, Part B, 1990, 45, 1313–1325 CrossRef.
- J. W. Lam and J. W. Mclaren, J. Anal. At. Spectrom., 1990, 5, 419–424 RSC.
- S. J. Hill, M. J. Ford and L. Ebdon, J. Anal. At. Spectrom., 1992, 7, 719–725 RSC.
- F. G. Smith, D. R. Wiederin and R. S. Houk, Anal. Chem., 1991, 63, 1458–1462 CrossRef CAS.
- P. Allain, L. Jaunault, Y. Mauras, J. M. Mermet and T. Delaporte, Anal. Chem., 1991, 63, 1497–1498 CrossRef CAS.
- D. Fliegel, C. Frei, G. Fontaine, Z. Hu, S. Gao and D. Gunther, Analyst, 2011, 136, 4925–4934 RSC.
- R. I. McCrindle and C. J. Rademeyer, J. Anal. At. Spectrom., 1996, 11, 437–444 RSC.
- R. I. Mccrindle and C. J. Rademeyer, J. Anal. At. Spectrom., 1994, 9, 1087–1091 RSC.
- R. I. Mccrindle and C. J. Rademeyer, J. Anal. At. Spectrom., 1995, 10, 399–404 RSC.
- R. I. McCrindle and C. J. Rademeyer, Fresenius. J. Anal. Chem., 1996, 355, 264–266 CAS.
- S. J. Hill, M. J. Ford and L. Ebdon, J. Anal. At. Spectrom., 1992, 7, 1157–1165 RSC.
- K. S. Park, S. T. Kim, Y. M. Kim, Y. Kim and W. Lee, Bull. Korean Chem. Soc., 2003, 24, 285–290 CrossRef CAS.
- F. Vanhaecke, R. Dams and C. Vandecastelle, J. Anal. At. Spectrom., 1993, 8, 433–438 RSC.
- A. S. Al-Ammar, E. Reitznerova and R. M. Barnes, Spectrochim. Acta, Part B, 1999, 54, 1813–1820 CrossRef.
- Z. C. Hu, S. H. Hu, S. Gao, Y. S. Liu and S. L. Lin, Spectrochim. Acta, Part B, 2004, 59, 1463–1470 CrossRef PubMed.
- G. H. Floor, R. Millot, M. Iglesias and P. Negrel, J. Mass Spectrom., 2011, 46, 182–188 CrossRef CAS PubMed.
- G. C. Y. Chan and G. M. Hieftje, Spectrochim. Acta, Part B, 2004, 59, 1007–1020 CrossRef PubMed.
- M. Kovacevic and W. Goessler, Spectrochim. Acta, Part B, 2005, 60, 1357–1362 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |