Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-term culture and functionality of pancreatic islets monitored using microelectrode arrays
Sven
Schönecker
a,
Udo
Kraushaar
a,
Martina
Düfer
b,
Anika
Sahr
c,
Carmen
Härdtner
c,
Elke
Guenther
a,
Reinhard
Walther
c,
Uwe
Lendeckel
c,
Winfried
Barthlen
d,
Peter
Krippeit-Drews
e and
Gisela
Drews
*e
aNMI Natural and Medical Sciences Institute at the University of Tübingen, Department of Electrophysiology, Markwiesenstraße 55, D-72770 Reutlingen, Germany
bInstitute of Pharmaceutical and Medical Chemistry, University of Münster, Corrensstraße 48, D-48149 Münster, Germany
cInstitute of Medical Biochemistry and Molecular Biology, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, 17475 Greifswald, Germany
dDepartment of Pediatric Surgery, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, 17475 Greifswald, Germany
eInstitute of Pharmacy, Department of Pharmacology, University of Tübingen, Auf der Morgenstelle 8, D-72076 Tübingen, Germany. E-mail: gisela.drews@uni-tuebingen.de; Fax: +49 7071 29 5382; Tel: +49 7071 29 77559
First published on 7th March 2014
Abstract
Extracellular recording of the glucose-induced electrical activity of mouse islets of Langerhans on microelectrode arrays (MEAs) is an innovative and powerful tool to address beta-cell (patho-)physiology. In a dual approach we tested whether this technique can detect concentration-dependent drug effects as well as characterize alterations in beta-cell activity during prolonged culture. First we established conditions that allow long-term investigation of beta-cell function by recording electrical activity. The results provide the first measurements of beta-cell membrane potential oscillations of individual murine islets during long-term culture. Oscillations were recorded for up to 34 days after islet isolation. Importantly, the glucose dependence of electrical activity did not change over a period of one month. Thus we can follow electrophysiological changes of individual islets induced by alterations in the beta-cell environment over weeks. Second, we used the MEA technique to assay beta-cell damage induced by oxidative stress and to evaluate appropriate protection mechanisms. Oxidative stress plays a key role in the development of type 2 diabetes mellitus (T2DM). Examination of the acute effects of H2O2 on electrical activity showed that the oxidant reduced the electrical activity in a concentration-dependent manner. The superoxide dismutase mimetic, tempol, protected against the detrimental effects of H2O2. In conclusion, we demonstrated that MEA recordings can be used to address disease-related mechanisms and protective interventions in beta-cells. In the future, this fundamental work should enable the monitoring of the electrical activity of islets of Langerhans under controlled ex vivo conditions including long-term exposure to oxidative stress, glucolipotoxicity, and other diabetes-inducing agents.
Insight, innovation, integrationType-2 diabetes mellitus (T2DM) is a tremendous health problem worldwide. Our work demonstrates that the microelectrode array (MEA) technique is an excellent tool to study in vitro the molecular basis of functional changes in beta-cells occurring during the development of type 2 diabetes mellitus. First, we established an in vitro model that allows long-term investigation of beta-cell function by registration of the electrical activity of isolated islets using the MEA technique. In the second step we applied oxidative stress to the islets which is crucial in the development of T2DM and evaluated appropriate protection mechanisms. This is a fundamental study that enables monitoring the electrical activity of murine islets in controlled ex vivo situations during long-term exposure to defined diabetes-associated, e.g. oxidative stress, glucolipotoxicity, and diabetes-inducing agents. |
Introduction
Insulin secretion of pancreatic beta-cells is determined by the degree of electrical activity, i.e. phases of depolarized membrane potential with action potentials and repolarized interbursts. Recently, we succeeded in recording the electrical activity of isolated mouse islets of Langerhans using planar, extracellular electrodes arranged in a microelectrode array (MEA).1 This approach was confirmed by Raoux and coworkers.2 The MEA system allows quantifying the electrical activity of beta-cells by calculating the fraction of the plateau phase (FOPP, the percentage of time with spike activity). We demonstrated that the glucose dependence of the FOPP measured using a MEA equals that measured using sharp intracellular microelectrodes and is almost identical with the glucose dependence of insulin secretion measured with isolated mouse islets.3 In addition, the MEA technology enables detection of the first phase of electrical activity elicited by an increase in glucose from a sub-stimulatory to a stimulatory concentration and allows simultaneous measurement of fluctuations in electrical activity and cytosolic Ca2+ concentration.1These results convincingly demonstrate that MEAs are excellent and unique tools for reliable measurements of the FOPP. MEAs are superior to conventional electrophysiological methods since they are (1) non-invasive, (2) use whole islets that – in contrast to single cells – consistently display oscillations of electrical activity, and (3) easy to handle, thus increasing the experimental throughput enormously.
In humans, T2DM develops over many years. Current in vitro models to investigate alterations in the oscillatory activity of pancreatic islets seldom exceed a time period of several hours and are thus inadequate to study long-term changes. We demonstrate, for the first time, that the MEA approach allows monitoring of electrical activity and glucose-responsiveness of cultivated islets over a period of weeks. Increased oxidative stress within beta-cells due to excessive fuel intake or increased hormone levels (e.g. angiotensin II, endothelin) is a key event in the development of T2DM.4 Since antioxidant defence mechanisms are low in beta-cells the characterization of strategies to prevent oxidative stress is a promising approach to identify beta-cell protective drugs. It is well known that oxidative stress reduces or even completely prevents glucose-mediated alterations in electrical activity,5 thus the ability to make extended electrical measurements is attractive. We show that the MEA technology is very sensitive, allows detection of small changes in electrical activity caused by acute treatment of islets with an oxidant and can be used to determine the protective effect of a drug mimicking superoxide dismutase.
Taken together, we demonstrate the suitability of the MEA-based approach (1) to develop in vitro models for the study of long-term changes in islet function, (2) to investigate effects of diabetes-promoting conditions, i.e. oxidative stress, and (3) to test strategies for protection of islets against diabetes-associated functional cell damage.
Research design and methods
Islet preparation
Experiments were performed using intact islets of Langerhans isolated from adult C57Bl/6N mice (Janvier, France). The principles of laboratory animal care were followed according to German laws. Mice were euthanized by CO2. Islets were isolated by collagenase digestion of the pancreas and cultured up to 34 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin.Solution and chemicals
Measurements of extracellular membrane potential were performed at 37 °C in a solution containing in mM: 140 NaCl, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 HEPES, pH 7.4, and glucose as indicated. The incubation medium for determining insulin secretion contained (in mM): 122 NaCl, 4.8 KCl, 2.5 CaCl2, 1.1 MgCl2, 10 HEPES, pH 7.4. RPMI 1640, fetal calf serum, and penicillin/streptomycin were provided by Invitrogen (Karlsruhe, Germany). All other chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany), Roth (Karlsruhe, Germany) or Merck (Darmstadt, Germany).Recording setup for measuring membrane potential using extracellular electrodes
Extracellular membrane potential recordings were obtained with the MEA technique6 using a MEA 1060-inv-standard amplifier system and software MC-Rack (Multi Channel Systems (MCS), Reutlingen, Germany). Data were low-pass filtered at 25 Hz and sampled at 1 kHz. Titanium-nitride electrodes had a diameter of 30 μm (200/30-Ti; MCS). In experiments with acute application of drugs one islet was placed on one of the 64 electrodes by means of a glass holding pipette with a tip angle of 30° (Reproline, Rheinbach, Germany) and a micromanipulator (Eppendorf, Hamburg, Germany). Extracellular voltage changes were only recorded from the electrode where the islet was placed using the grounded bath electrode as in ref. 1. In long-term culture experiments several islets were placed on different electrodes within the array.Experimental procedure for measuring extracellular membrane potential
For acute drug application islets were used after 1–3 days of culture. Islets were incubated at 3 mM glucose for 10 to 20 min prior to experiments. Single islets were then transferred to the MEA bath chamber using a holding pipette and fixed to an electrode with gentle mechanical pressure. The islet was continuously perifused with bath solution throughout the experiment. Recordings always started in 3 mM glucose which gave a base-line with no electrical activity. Oscillatory activity was usually followed for 30–45 min for each condition and evaluated at periods of steady-state oscillations for the last 5–10 min before a new manoeuvre started. For long-term culture experiments the islets were kept in culture medium (RPMI, 11.1 mM glucose) on the MEA for the indicated time period. The culture medium was changed every second day. Each experiment started in culture medium before changing to the bath solution. For quantification of electrical activity the fraction of the plateau phase (FOPP) was determined by dividing a distinct time interval by the time with bursting activity within this distinct time interval.Measurement of insulin secretion
After preparation islets were kept overnight in medium supplemented with 11.1 mM glucose. To determine insulin secretion, batches of 5 islets were incubated for 60 min at 37 °C with the indicated substances. Insulin was determined by radioimmunoassay using rat insulin (Crystal Chem. Inc., USA) as the standard.Presentation of results
The recordings are representative of results from different islets; islets from at least 3 different mice were used for each series of experiments. Values are given as means ± SEM for the indicated number of experiments. Statistical significance was assessed by ANOVA followed by the Student–Newman–Keuls post-test. A value of p ≤ 0.05 was considered to be significant.Results and discussion
Long-term measurements of beta-cell electrical activity
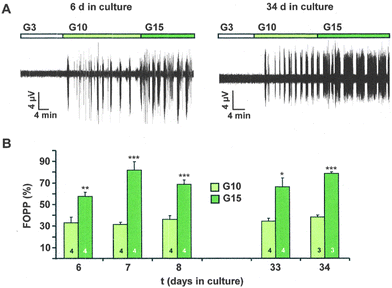
To study the effects of a diabetes-promoting environment, e.g. ROS, on the electrical activity of mouse beta-cells in more detail, it would be of great advantage to record changes over several days. However, all current electrophysiological techniques used in beta-cell research provide only short-term measurements of electrical activity, i.e. a range of minutes, or in exceptional cases, hours. In experiments with conventional microelectrodes or patch-clamp experiments the membrane potential measurement is regularly disturbed by leakage currents. Moreover, it is not possible to study the electrical activity of the same islet on different days. Therefore, we developed a system allowing long-term culture of isolated islets and, most importantly, regular measurements of the electrical activity of individual islets during culture. We validated physiological dynamics, i.e. the glucose responsiveness of the islets cultured on the MEA in the presence of 11.1 mM glucose and the appearance of oscillations over several weeks. As shown in Fig. 1A islets maintain their ability to respond adequately to glucose with oscillations up to 34 days. It was possible to record independent electrical activity simultaneously from several islets in contact with different electrodes on the MEA. Thus, the rate of successful experiments was increased compared to conventional electrophysiological methods.As observed in acute experiments, at 3 mM glucose no oscillations were detected even after 34 days of culture. Differences in the oscillations induced by 10 or 15 mM glucose, respectively, could be distinguished over time in the culture showing that the glucose dependence of electrical activity was preserved over several weeks (Fig. 1B). The data indicate that glucose metabolism is intact after long-term culture. The FOPP at 10 and 15 mM glucose, respectively, did not change over time. To evaluate possible culture time-dependent changes in electrical activity, the FOPP was summarized for experiments measured in the first week of culture (day 6 and 7) and compared with experiments performed in the last culture week (day 33 and 34). The FOPP amounted to 32 ± 3% (n = 8) and 36 ± 2% (n = 7) at 10 mM glucose and to 70 ± 6% (n = 8) and 72 ± 5% (n = 7) at 15 mM glucose at day 6/7 and day 33/34, respectively. The data clearly demonstrate that the FOPP at 10 and 15 mM glucose, respectively, did not change over time in culture. Notably, the number of non-responding islets is negligible with the MEA technology in contrast to microelectrode impaling. This paves the way to perform long-term experiments of electrical activity with low concentrations of redox-active compounds or diabetes-inducing agents and to study progressive changes evoked by gluco-, lipo- or glucolipotoxicity. Raoux and coworkers reported reduced action potential frequency of clonal INS-1E cells kept on a MEA after 3 days at elevated glucose concentration.2 Moreover, the method permits co-culture of islets with other tissues to investigate their influence(s) on electrical activity.
In summary, the data presented show that MEA technology offers numerous novel and interesting long-term applications in beta-cell research, e.g. investigation of chronic effects of diabetes-inducing environments on electrical activity or the development of an in vitro model for testing short and long-term effects of potential antidiabetic drugs.
MEA technology detects protection against oxidative stress-induced pathophysiological changes of beta-cell electrical activity
Oxidative stress is an important pathogenic factor that contributes to the development of T2DM.4a We have shown that up-regulation of the antioxidant enzymes catalase (Cat), glutathione peroxidase (Gpx) and superoxide dismutase (SOD) in primary mouse beta-cells protects against apoptosis and loss of beta-cell function, respectively, that was induced by oxidative stress5a This is in agreement with observations of Lortz and co-workers who showed that overexpression of antioxidant enzymes reduces cytokine-induced cytotoxicity in insulin-secreting RINm5F cells.7 H2O2 is one of the reactive oxygen species markedly contributing to oxidative stress in pancreatic islets. It is known to interact with several parameters of beta-cell stimulus-secretion coupling.8 In the present study we investigated H2O2-evoked changes in electrical activity of mouse beta-cells and tested whether the membrane-permeable SOD mimetic, tempol,9 protects beta-cells against H2O2-induced insult. First, we applied different concentrations of H2O2 in the presence of 10 mM glucose resulting in concentration-dependent inhibition of the FOPP that was well resolved by the MEA technology (Fig. 2A and B). Second, islets pre-incubated at 1 mM tempol prior to the addition of 300 μM H2O2 were compared to those exposed solely to 300 μM H2O2. To avoid direct interaction of tempol and H2O2, tempol was removed immediately before addition of H2O2. H2O2 significantly reduced the FOPP in the presence of 10 mM glucose but was without effect after 30 min of pre-incubation with 1 mM tempol (Fig. 2C and D). The SOD mimetic exerted a protective effect against the reduction of insulin secretion by 100 μM H2O2. In experiments without pre-treatment insulin secretion was reduced, but was unchanged after 15 min pre-incubation with the SOD mimetic (Fig. 2E). Tempol alone did not influence insulin secretion (2.91 ± 0.36 ng insulin per islet per h in the presence of 15 mM glucose vs. 3.18 ± 0.29 ng insulin per islet per h in the presence of 15 mM glucose and tempol; n = 4; n.s., not shown). The different efficacy of H2O2 on insulin secretion and electrical activity is most likely due to differences in diffusion in the two measurement systems (1 h steady-state incubation for insulin secretion vs. perifusion for the measurement of electrical activity). The results clearly demonstrate that tempol preserves insulin secretion by circumventing the detrimental effect of H2O2 on stimulus-secretion-coupling. This agrees with our previous observations that up-regulation of antioxidant enzymes protects beta-cells against oxidative stress.5a The new data also show that the SOD plays a crucial role in the antioxidant defence of beta-cells and seems to be more important than Cat and Gpx since an SOD mimetic alone is sufficient to protect against an oxidative insult. At first glance it seems astonishing that an SOD mimetic protects electrical activity and insulin secretion against H2O2 since SOD does not degrade H2O2. It is assumed that tempol-dependent protection of the islets is due to detoxification of H2O2-induced ROS production including O2−. First, H2O2 leads to a partial depolarization of the mitochondria5b and a decrease of the mitochondrial membrane potential is assumed to increase ROS production. Second, ROS, including H2O2, can induce mitochondrial ROS release.10 This mechanism has been extensively studied in cardiomyocytes and involves the mitochondrial permeability transition pore and/or the anion channel of the inner mitochondrial membrane. In both cases formation of superoxide anions in the mitochondria would rise and potentiate ROS generation and action. We suggest that this vicious circle is stopped by the SOD mimetic.We have shown that a SOD mimetic protects beta-cells against acute oxidative stress pointing to the central role of SOD in antioxidant defence of pancreatic islets. Our experiments demonstrate that glucose-responsiveness of isolated islets cultured on MEAs is stable for up to 34 days. These data indicate that MEA technology is a valuable tool to investigate long-term effects of drugs influencing beta-cells via membrane potential-dependent pathways. The results suggest the feasibility of developing MEA-based in vitro disease models where islets are chronically challenged by glucolipotoxicity or stress-inducing agents thus enabling evaluation of intervention strategies.
Acknowledgements
We acknowledge the skilful technical assistance of Isolde Breuning. The work was supported by grants from the Deutsche Forschungsgemeinschaft (G.D., M.D.) and the Deutsche Diabetes-Stiftung (G.D.).References
- T. Pfeiffer, U. Kraushaar, M. Düfer, S. Schönecker, D. Haspel, E. Guenther, G. Drews and P. Krippeit-Drews, Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays, Pfluegers Arch., 2011, 462, 835–840, DOI:10.1007/s00424-011-1029-z.
- M. Raoux, Y. Bornat, A. Quotb, B. Catargi, S. Renaud and J. Lang, Non-invasive long-term and real-time analysis of endocrine cells on micro-electrode arrays, J. Physiol., 2012, 590, 1085–1091, DOI:10.1113/jphysiol.2011.220038.
- (a) H. P. Meissner and H. Schmelz, Membrane potential of beta-cells in pancreatic islets, Pfluegers Arch., 1974, 351, 195–206 CrossRef CAS; (b) J. C. Henquin, Regulation of insulin secretion: a matter of phase control and amplitude modulation, Diabetologia, 2009, 52, 739–751, DOI:10.1007/s00125-009-1314-y.
- (a) V. Poitout and R. P. Robertson, Glucolipotoxicity: fuel excess and beta-cell dysfunction, Endocr. Rev., 2008, 29, 351–366, DOI:10.1210/er.2007-0023; (b) K. Y. Chu and P. S. Leung, Angiotensin II Type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet beta-cell function in young Type 2 diabetic mice, Antioxid. Redox Signaling, 2007, 9, 869–878, DOI:10.1089/ars.2007.1590.
- (a) B. Gier, P. Krippeit-Drews, T. Sheiko, L. Aguilar-Bryan, J. Bryan, M. Düfer and G. Drews, Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress, J. Clin. Invest., 2009, 119, 3246–3256, DOI:10.1172/JCI38817; (b) G. Drews, C. Krämer, M. Düfer and P. Krippeit-Drews, Contrasting effects of alloxan on islets and single mouse pancreatic beta-cells, Biochem. J., 2000, 352(2 Pt), 389–397 CrossRef CAS PubMed; (c) M. Nakazaki, M. Kakei, N. Koriyama and H. Tanaka, Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic beta-cells, Diabetes, 1995, 44, 878–883 CrossRef CAS PubMed; (d) P. S. Herson, K. Lee, R. D. Pinnock, J. Hughes and M. L. Ashford, Hydrogen peroxide induces intracellular calcium overload by activation of a non-selective cation channel in an insulin-secreting cell line, J. Biol. Chem., 1999, 274, 833–841 CrossRef CAS PubMed.
- A. Stett, U. Egert, E. Guenther, F. Hofmann, T. Meyer, W. Nisch and H. Haemmerle, Biological application of microelectrode arrays in drug discovery and basic research, Anal. Bioanal. Chem., 2003, 377, 486–495, DOI:10.1007/s00216-003-2149-x.
- S. Lortz, M. Tiedge, T. Nachtwey, A. E. Karlsen, J. Nerup and S. Lenzen, Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes, Diabetes, 2000, 49, 1123–1130 CrossRef CAS PubMed.
- G. Drews, P. Krippeit-Drews and M. Düfer, Oxidative stress and beta-cell dysfunction, Pfluegers Arch., 2010, 460, 703–718, DOI:10.1007/s00424-010-0862-9.
- B. S. Fleenor, D. R. Seals, M. L. Zigler and A. L. Sindler, Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice, Aging Cell, 2012, 11, 269–276, DOI:10.1111/j.1474-9726.2011.00783.x.
- (a) D. B. Zorov, M. Juhaszova and S. J. Sollott, Mitochondrial ROS-induced ROS release: an update and review, Biochim. Biophys. Acta, 2006, 1757, 509–517, DOI:10.1016/j.bbabio.2006.04.029; (b) N. R. Brady, S. P. Elmore, J. J. van Beek, K. Krab, P. J. Courtoy, L. Hue and H. V. Westerhoff, Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization, Biophys. J., 2004, 87, 2022–2034, DOI:10.1529/biophysj.103.035097.
| This journal is © The Royal Society of Chemistry 2014 |