Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The environmental profile of bioethanol produced from current and potential future poplar feedstocks in the EU†
Miao
Guo
*ab,
Jade
Littlewood‡
a,
James
Joyce§‡
a and
Richard
Murphy
*ac
aDepartment of Life Sciences, Imperial College London, SW7 2AZ, UK
bDepartment of Chemical Engineering, Imperial College London, SW7 2AZ, UK
cCentre for Environmental Strategy, University of Surrey, Guildford, Surrey GU2 7XH, UK. E-mail: miao.guo06@imperial.ac.uk; rj.murphy@surrey.ac.uk; Tel: +44 (0)7726479669
First published on 31st July 2014
Abstract
Although biofuels have the potential for mitigating climate change and enhancing energy security, controversy regarding their overall environmental sustainability is considered a significant bottleneck in their development at both global and EU levels. Life Cycle Assessment (LCA) was applied to model the current and prospective environmental profiles for poplar-derived bioethanol across various potential EU supply chains (different poplar plantation management, different pretreatment technologies for bioethanol production, five EU locations). LCA modelling indicated that E100 (100% bioethanol) and E85 (85% bioethanol, 15% petrol) fuels derived from Poplar from various locations in the EU had environmental impact scores some 10% to 90% lower than petrol in global warming potential, abiotic depletion potential, ozone depletion potential and photochemical oxidation potential depending upon the exact poplar supply chain and conversion technology modelled. Hybrid poplar clones with higher biomass yields, modified composition and improved cell wall accessibility had a clear potential to deliver a more environmentally sustainable lignocellulosic biorefining industry with environmental scores some 50% lower than with conventional poplar feedstocks. A particular aspect of the present study that warrants further research is the contribution that soil carbon accumulation can make to achieving low-GHG fuels in the future.
Introduction
Transport accounted for one-third of the total energy consumption in the EU-27 in 2010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2 and is responsible for approximately 25% of greenhouse gas (GHG) emissions, thereby representing the second largest source of GHG emissions in the EU.3 Over two thirds of transport-related GHG emissions are derived from road transport alone3 and the development of a biofuel market has been recognised by the European Commission (EC) as a component of its strategy to mitigate climate change.4 The Directive 2009/28/EC (the Renewable Energy Directive (RED)), implemented in December 2010, mandates that the EU reach a 10% share of renewables in the transport sector by 2020
1,2 and is responsible for approximately 25% of greenhouse gas (GHG) emissions, thereby representing the second largest source of GHG emissions in the EU.3 Over two thirds of transport-related GHG emissions are derived from road transport alone3 and the development of a biofuel market has been recognised by the European Commission (EC) as a component of its strategy to mitigate climate change.4 The Directive 2009/28/EC (the Renewable Energy Directive (RED)), implemented in December 2010, mandates that the EU reach a 10% share of renewables in the transport sector by 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,5 and that biofuels from waste, agricultural or forestry residues, and lignocellulosic material will count twice towards this EU target.6 Although biofuels have the potential for climate change mitigation and enhancing energy security, controversy regarding their overall environmental sustainability is considered as a significant bottleneck in their development in the EU and globally.
1,5 and that biofuels from waste, agricultural or forestry residues, and lignocellulosic material will count twice towards this EU target.6 Although biofuels have the potential for climate change mitigation and enhancing energy security, controversy regarding their overall environmental sustainability is considered as a significant bottleneck in their development in the EU and globally.
Life cycle assessment (LCA) is a cradle-to-grave approach used to evaluate the environmental impacts of products and services. The LCA method has been formalised by the International Organization for Standardization (ISO)7 and is becoming widely used to evaluate the holistic environmental aspects of various products and services derived from renewable resources on a life-cycle basis. Several studies on biofuels have used LCA as a basis for their overall assessment approach but the majority have tended to have a focus on GHGs and energy balance with less attention paid to the wider range of environmental impact categories typical of broader LCAs. Research and development continue to be necessary to develop holistic and forward-looking LCA models for lignocellulosic biofuels derived from emerging plant-based feedstocks and technologies.
Poplar (Populus spp.) is a fast-growing and genetically diverse hardwood species widely distributed across Eurasia and North America. Poplar has been utilised for many years as a source for pulp as well as for wood products, plywood and pallets due to its reasonably fast growth properties, including relatively low nutrient demand and potential for cultivation on marginal lands amongst many other attributes. Recently, poplar has attracted significant interest as an energy crop grown under Short Rotation Coppice or Short Rotation Forestry regimes to produce chip or pelletized wood fuel or feedstock for lignocellulosic bioethanol production.8 The ability to breed new clones is a strong advantage for poplar in such applications and poplars are well suited to genetic manipulation with the availability of a full genome sequence of Populus trichocarpa.9 Poplar is regarded as a model hardwood species for breeding “advanced” genotypes for these purposes. Relatively few LCA studies have been carried out on poplar-derived bioethanol10–12 and these have tended to focus on the comparison of different feedstocks and alternative bioenergy production systems. No LCAs have been found publically available on the comparisons of poplar-based bioethanol production under different processing technologies and also taking into account of feedstock production in different regions. Literature review also suggests that no research has yet been carried out on the implications for poplar feedstock optimization (e.g. genetic modification and advanced breeding programme) in an LCA context.
In this study, an attributional LCA approach (aLCA) was applied to model the current and projected environmental profiles for poplar-derived bioethanol fuels produced at various locations in the EU. The study was conducted as part of the EC Seventh Framework Programme (FP7) project ENERGYPOPLAR (FP7-211917) and aimed to provide scientific insight into the potential that current and future poplars have for delivering the so-called second generation (2G) bioethanol supplies offering more favourable environmental profiles than conventional petrol.
Methods
To evaluate the environmental viability of current and future (2020 and 2030) bioethanol derived from poplar in the EU, scenarios were used to explore:(1) bioethanol derived from poplar biomass grown under short- or very-short-rotation coppice (SRC or VSRC) management,
(2) bioethanol produced via two pretreatment processing technologies,
(3) different EU regions with various climatic and soil characteristics – Northern (Sweden), Southern (Italy, Spain), Western (France) and Eastern (Slovakia) Europe,
(4) prospective scenarios for year 2020 and 2030 with optimised poplar feedstock.
The cradle-to-grave aLCA approach was used to identify the major contributors to the environmental profiles of poplar-derived bioethanol in the five EU countries and to assess the overall environmental sustainability of bioethanol compared with the transport fuel petrol.
Functional unit
Bioethanol was modelled as a vehicle fuel used in three forms – 100% bioethanol (E100), a blend of 85% (v/v) bioethanol and 15% petrol (E85) and a blend of 10% (v/v) bioethanol and 90% petrol (E10). The functional unit was defined as “100 km distance driven in a Flex Fuel Vehicle (FFV) using various fuels compared on an equivalent energy basis”.Product system modelled
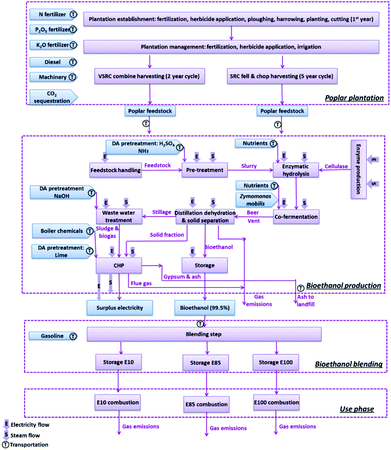
The product system for the poplar-derived bioethanol is illustrated in Fig. 1. The following subsystems were included in the system boundary – poplar plantation management and harvesting, bioethanol production, distribution and blending with petrol and final use in a vehicle. Soil carbon stock changes under poplar cultivation were taken into account in the analysis. The environmental burdens associated with human labour were excluded from the study scope.Allocation approach
A ‘system expansion’ allocation approach was applied for the bioethanol production stage to account for the multi-product nature of the system i.e. bioethanol plus surplus electrical power generated from the CHP system. The electricity co-product was assumed to displace an equivalent amount of electrical power generation from the average national grid mix of the corresponding country in each scenario. This allocation approach therefore awards the bioethanol production process with an ‘avoided burdens’ credit for the avoided fossil fuel consumption and emissions for the equivalent amount of electrical power generation from the national grid.18–20 An alternative allocation approach recommended by EU Renewable Energy Directive21 – energy allocation, where the environmental burdens were allocated among the co-products (bioethanol and surplus electricity) based on their energy contents – was applied in sensitivity analysis.A stoichiometric carbon-counting approach was used to ‘track’ the biogenic carbon flows from poplar biomass into bioethanol and its use as a fuel over the life cycle. As stated earlier, other biogenic carbon flows e.g. due to litter biodegradation, fermentation emissions etc. were assumed to be as CO2 and were therefore treated as carbon-neutral. This C-counting approach with regard to the bioethanol was applied to determine (1) carbon ‘sequestration’ into the bioethanol (from the poplar cultivation phase of the life cycle) and, (2) downstream release of this carbon during the subsequent processing and use stages of the bioethanol life cycle, and (3) mid-term soil carbon accumulation in the poplar plantation due to leaf litter and fine root inputs. The sequestration of carbon into biomass during the poplar growth phase of the life cycle thus represents a ‘negative’ GHG emission at this stage of the life cycle but this carbon is then returned to the environment in various ways depending upon the subsequent fate of the bioethanol products (mainly combustion of the fuel in vehicle).
Life cycle inventory, impact assessments and data quality analysis
Complete inventories for the life cycle of poplar-derived bioethanol were developed by combining simulation results from the process engineering model AspenPlus™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and literature data representing poplar plantations in the EU and advanced processing technology for poplar-derived bioethanol production.
22 and literature data representing poplar plantations in the EU and advanced processing technology for poplar-derived bioethanol production.
A problem oriented (midpoint) approach – CML 2 baseline 2000 (v2.05)23 – was applied in the current study as the ‘default’ life cycle impact assessment (LCIA) method. A second damage-oriented approach LCIA method – Eco Indicator 99 hierarchist version (EI 99 H) defining impact categories at the endpoint level – was also applied to analyse the sensitivity of the LCIA results to the LCIA methodological choice. The comparison in ESI Table S1† indicates that although the impact categories evaluated in the two methods are not identical, most overlapped. The LCA modelling was performed in Simapro 7.3.3 (PRé Consultants).
A scenario sensitivity analysis method was applied in this study, which involves calculating different scenarios, to analyse the influences of input parameters on either LCIA output results or rankings.24 A reversal of the rank order of counterparts for LCA comparisons and an arbitrary level of a 10% change in the characterized LCIA profiles for a single product system were chosen as the sensitivity threshold above which the influence of allocation approach, characterization model choice or variation in soil carbon accumulation was considered to be significant.
Life cycle inventory (LCI) analysis
Poplar plantation
To reflect variation in the country-specific agro-ecosystems and plantation management characteristics, literature data representing current country-level average fertilizer inputs and compositions, fertilizer-induced field emissions, poplar plantation management practices and average poplar biomass yields in different EU regions were used to develop the LCA inventory (see Table 1). The cycle length modelled for VSRC and SRC in different EU regions reflects longer growing seasons in Southern Europe. The data development for fertilizer application and the N fertilizer-induced field emissions are discussed in ESI Method S1;† total NPK inputs and emission factors (EFs) are given in Table 1. It was assumed that irrigation is only applied in Southern Europe and that precipitation during the poplar growing season in the other parts of the Europe is greater than the water required for growth. Maximum biomass yields are achieved early in densely planted poplar VSRC plantation, whereas SRC management tends to have higher long-term biomass yields than VSRC.25,26 Thus, the baseline current (SRC) biomass yields were derived from empirical data reported for the average yield in a given country, and a 10% lower biomass yield was assumed for VSRC plantation.27 The main differences between SRC and VSRC plantation management is their harvesting method (Table 1). The inventory for field operations and agrochemicals production were derived from the Ecoinvent database (v2.2).| Input parameters and N emissions factors for poplar plantation | ||||||
|---|---|---|---|---|---|---|
| N.EU | S.EU | S.EU | E.EU | W.EU | ||
| Sweden | Italy | Spain | Slovakia | France | ||
| SRCa (harvesting cycle in years) | 7 year | 5 year | 5 year | 7 year | 7 year | |
| VSRCa (harvesting cycle in years) | 3 year | 2 year | 2 year | 3 year | 3 year | |
| Carbon sequestration (kg C/oven dry (OD) kg above-ground woody biomass harvested) | Carbon in above-ground biomass | 0.5h | ||||
| Soil carbon accumulation | 0.12 as ‘mid-point’ value for baseline and prospective scenarios (data range 0.06–0.24)i | |||||
| N fertilizer (kg per cycle ha−1)b | 86.5 | 53.9 | 45.7 | 57.1 | 80.0 | |
| K2O fertilizer (kg per cycle ha−1)c | 9.8 | 19.2 | 6.1 | 22.7 | 15.7 | |
| P2O5 fertilizer (kg per cycle ha−1)c | 12.3 | 10.8 | 7.2 | 19.3 | 16.1 | |
| Herbicide & insecticide (kg per cycle ha−1)d | 10 | 10 | 10 | 10 | 10 | |
| Irrigation (m3 per year ha−1)e | 0 | 1350 | 1750 | 0 | 0 | |
| N loss (% total N fertilizer applied)f | NH3-N | 1.0% | 1.4% | 1.3% | 0.4% | 1.1% |
| N2O-N | 5.6% | 1.4% | 5.1% | 0.6% | 3.0% | |
| NOx-N | 1.1% | 0.1% | 0.3% | 0.0% | 0.3% | |
| N2-N | 27.2% | 14.1% | 31.5% | 10.8% | 16.8% | |
| N Leaching | 3.8% | 10.2% | 11.9% | 7.2% | 9.8% | |
| Field operations (pass per cycle) | SRC | Plantation establishment = 1 (1st cycle); fertilization = 1; agrochemical application = 1; harvesting (cutting & chipping) = 1j | ||||
| VSRC | Plantation establishment = 1 (1st cycle); fertilization = 1; agrochemical application = 1; combine harvesting = 1j | |||||
| Biomass yield (OD tonne ha−1 per year)g | ||||
|---|---|---|---|---|
| Current | 2020 | 2030 | References | |
| a Where the data were not available in literature, the yield for 2020 and 2030 scenarios were estimated to be 1.5 and 2 times the current biomass yield respectively.37 b The N fertilizer input for France was derived from expert estimation,37 the N fertilizer input for other countries was estimated based on their country-level average N application rate;41 the data represents the amount of fertilizer applied per harvesting cycle. c K and P fertilizer inputs were estimated based on the country-specific NPK consumption data derived from International Fertilizer Industry Association (IFA) online statistics;42 the data represents the amount of fertilizer applied per harvesting cycle per ha of cultivation land. d Assumption based on unpublished work.43 e Irrigation data for Italy and Spain were derived from unpublished work43 and ref. 37 and 38, respectively. f Country-specific emissions factors were calculated based on EU country-level N budget balances.44,45 g Based on data derived from Italian poplar commercial clone trial,27,43 the biomass yield of VSRC plantation was assumed as 10% lower than SRC. h Estimated based on literature data34,46,47 and experimental data.27,43 i Estimated based on the literature data of annual soil carbon sequestration rate.15,34,47–49 j Combine harvesting is more energy-efficient compared with cutting and chipping method, where fixed energy was modelled for per unit harvested SRC biomass (data from Ecoinvent database (V2.2)). | ||||
| N.EU | SRC 7 | SRC 11 | SRC 14 | Ref. 33, 34 and assumptionsa |
| Sweden | VSRC 6.3 | VSRC 9.9 | VSRC 12.6 | |
| S.EU | SRC 14 | SRC 20 | SRC 25 | Ref. 35–37 |
| Italy | VSRC 12.6 | VSRC 18 | VSRC 22.5 | |
| S.EU | SRC 14.4 | SRC 21 | SRC 28 | Ref. 38 and assumptionsa |
| Spain | VSRC 12.9 | VSRC 18.9 | VSRC 25.2 | |
| E.EU | SRC 8.4 | SRC 13.1 | SRC 18.1 | Ref. 39 |
| Slovakia | VSRC 7.6 | VSRC 11.8 | VSRC 16.3 | |
| W.EU | SRC 10 | SRC 15 | SRC 20 | Ref. 37, 40 and assumptionsa |
| France | VSRC 9 | VSRC 13.5 | VSRC 18 | |
Prospective scenarios for the years 2020 and 2030 were developed, where the underlying assumption was that screening new and improved hybrid poplar clones via advanced breeding programmes would lead to a genetic gain giving higher yield (under current management practice) over the current clones. Thus, the modelled plantation management parameters in the future scenarios were the same as in the current scenario (field operations, agrochemical applications and irrigations). Data from previous studies representing the best performing new poplar clone under suboptimal and optimal conditions were used to estimate biomass yields in the 2020 and 2030 scenarios respectively.
The carbon sequestration into above-ground biomass and the soil carbon accumulation were estimated based on the carbon content in poplar woody biomass and annual soil carbon accumulation rates reported in previous studies (see ESI Method S1† and data given in Table 1). The effects of including this soil carbon accumulation on the environmental profiles of poplar-based bioethanol were investigated via sensitivity analysis.
Bioethanol production process
The harvested poplar biomass (with bark) is delivered to the biorefinery plant to be processed to bioethanol. The chemical composition of baseline poplar biomass and the genetically modified low-lignin poplar biomass under future scenarios (2020 and 2030) are given in Table 2.| % of oven dry weight ODW | Baseline poplara | GM Poplarb |
|---|---|---|
| a The composition of poplar whole tree (with bark) derived from the NREL on-line database were obtained from the NREL standard protocol for composition analysis.50 b The compositional data reported for low-lignin transgenic poplar stem in previous studies29,30 were used for the 2020 and 2030 scenarios. | ||
| Glucan | 45.27 | 55.09 |
| Xylan | 15.50 | 22.77 |
| Galactan | 0.96 | 1.00 |
| Arabinan | 0.96 | 0.45 |
| Mannan | 2.09 | 1.79 |
| Lignin | 28.19 | 11.33 |
| Extractives | 5.04 | 5.41 |
| Ash | 1.99 | 2.15 |
The key parameters and inventory data for the poplar-to-bioethanol production processes under the different processing technologies simulated using AspenPlus™ software22 are given in Table 3. The process design was mainly adapted from the NREL model.17 DA and LHW pretreatment technologies were modelled under current scenarios based on the research data reported by the Consortium for Applied Fundamentals and Innovation (CAFI).8,28 The transgenic poplar lines and bioethanol production potentials described in previous studies29,30 were used in modelling the prospective 2020 and 2030 scenarios.22 As indicated in Table 3, the GM low-lignin poplar in the prospective scenario achieved high sugar release (80%) without pretreatment after 72 hours of saccharification with an enzyme loading of 10 filter paper units (FPU, a measure of cellulase activity) per g glucan. The cellulolytic enzyme complex, Cellic Ctec 1, was assumed to be used for enzymatic saccharification and the site-specific dataset for Cellic Ctec 1 production provided by Novozymes A/S was used in the LCA model. The inventories for other chemicals were derived from the Ecoinvent database (v2.2).
| Baseline poplar | Baseline poplar | GM poplar (prospective scenario) | |
|---|---|---|---|
| DA pretreatment | LHW pretreatment | ||
| Key parameters | |||
| Pretreatment technologyb | 190 °C, 1.1 min, 2.0% sulphuric acid | 200 °C, 10 min, water | No pretreatment |
| Saccharificationb | Enzyme loading 15 FPU g−1 glucan | Enzyme loading 15 FPU g−1 glucan | Enzyme loading 10 FPU g−1 glucan |
| 50 °C, 72 hours | 50 °C, 72 hours | 50 °C, 72 hours | |
| Conversion efficiency of glucan to glucose | 86.63% | 56.0% | 79.9% |
| Conversion efficiency of xylan to xylose | 71.78% | 95.83% | 80% |
| Fermentationc | Co-fermentation by recombinant Zymomonas mobilis, 32 °C, 1.5 days | ||
| Conversion of glucose and mannose to ethanol 95% | |||
| Conversion of xylose and arabinose to ethanol 85% | |||
| WWTc,d | Biogas composition (dry molar basis) CH4 51% CO2 49% | ||
| Total COD removal 99.6% (86% converted to biogas) | |||
| CHPc | Boiler efficiency (feedstock heating value/steam heat) 80% | ||
| Flue gas treatmentc | Desulphurisation by adding lime | None | None |
| Inputs | |||
|---|---|---|---|
| Poplar (OD kg) | 1.00 | 1.00 | 1.00 |
| Sulphuric acid (93%) (kg) | 2.01 × 10−2 | 0.00 × 10 | 0.00 × 10 |
| Ammonia (kg) | 7.87 × 10−3 | 0.00 × 10 | 0.00 × 10 |
| Enzyme Cellic Ctec 1 (kg) | 1.34 × 10−1 | 1.41 × 10−1 | 1.00 × 10−1 |
| Corn steep liquor (kg) | 1.44 × 10−2 | 1.38 × 10−2 | 1.38 × 10−2 |
| Diammonium phosphate (kg) | 1.91 × 10−3 | 1.82 × 10−3 | 1.82 × 10−3 |
| Sorbitol (kg) | 5.79 × 10−5 | 5.47 × 10−5 | 5.47 × 10−5 |
| Caustic (kg) | 6.72 × 10−2 | 0.00 × 10 | 0.00 × 10 |
| Boiler chemicals (kg) | 5.47 × 10−6 | 4.48 × 10−6 | 4.48 × 10−6 |
| Lime (kg) | 1.77 × 10−3 | 0.00 × 10 | 0.00 × 10 |
| Cooling tower chemicals (kg) | 6.11 × 10−5 | 6.98 × 10−5 | 6.98 × 10−5 |
| Makeup watere (kg) | 3.28 | 3.47 | 3.13 |
| Output | |||
|---|---|---|---|
| Ethanol production (kg) | 2.57 × 10−1 | 2.01 × 10−1 | 3.27 × 10−1 |
| Exported electricity (kWh) | 3.05 × 10−1 | 4.18 × 10−1 | 1.13 × 10−1 |
| Emissions and waste disposal | |||
|---|---|---|---|
| a Ref. 22. b Based on results reported by Wyman et al.8 c Based on previous study carried out by National Renewable Energy Laboratory (NREL).17 d WWT includes anaerobic digestion (AD) followed by aerobic treatment. During AD, organic compound (chemical oxygen demand (COD)) removal was assumed as 91% (86% converted to biogas, 5% to cell mass); during aerobic treatment, COD removal was assumed to be 96% (74% converted to water and CO2, and 22% to cell mass). e Water assumed as natural origin. | |||
| Ethanol (kg) | 3.25 × 10−5 | 1.97 × 10−5 | 4.42 × 10−5 |
| CH4 (kg) | 1.77 × 10−4 | 2.85 × 10−5 | 2.29 × 10−5 |
| N2O (kg) | 5.52 × 10−7 | 5.52 × 10−7 | 5.52 × 10−7 |
| NH3 (kg) | 7.20 × 10−5 | 0.00 × 10 | 0.00 × 10 |
| SO2 (kg) | 1.33 × 10−3 | 5.36 × 10−4 | 4.15 × 10−4 |
| CO (kg) | 3.36 × 10−8 | 3.36 × 10−8 | 3.36 × 10−8 |
| HNO3 (kg) | 1.14 × 10−5 | 0.00 × 10 | 0.00 × 10 |
| Landfill disposal of ash (kg) | 2.73 × 10−2 | 2.43 × 10−2 | 2.42 × 10−2 |
Transport
The transport involved in the poplar-derived bioethanol supply chains is given in Table 4. On-site transport is the transport of harvested poplar wood from field to plantation gate.| Transport | Distance | Mode |
|---|---|---|
| a Tractor assumed to drive alongside the harvester to collect harvested chips; the transport distance was estimated for a field with row spacing of 3 m as 5.5 km; during transportation it was assumed a linear loading-weight increase from empty to full capacity. b The transport distance was assumed as 1 km from field to gate; loaded with a full capacity. c Default value for transport from field to bioethanol plant derived from farmed wood was given by the Department for Transport.51 d Personal communication with BP biofuels.37 | ||
| On-site transport for VSRC plantationa | 5.5 km | Tractor and trailer |
| On-site transport for SRC plantationb | 1 km | Tractor and trailer |
| Poplar to bioethanol plant | 50 kmc | 32-tonne lorry |
| Bioethanol from bio-refinery plant to storage | 160 kmd | 32-tonne lorry |
| Bioethanol from storage to forecourt | 160 kmd | 32-tonne lorry |
Petrol production, distribution and use phase
The dataset for unleaded petrol derived from Ecoinvent database (v2.2) was used to represent the average EU refinery industry for petrol production including extraction, transportation and refining of crude oil to unleaded petrol. The same distribution distances and transport modes as bioethanol were assumed for petrol (160 km, 32-tonne lorry). The depletion of easily extractable oil reserves, and a consequent shift to more environmentally damaging sources of crude oil (such as oil sands) is possible by 2030, but modelling this was deemed beyond the scope of this study and the EU unleaded petrol production profile was held the same as for the current scenario for both the 2020 and 2030 scenarios.The quantity of E100 (100% bioethanol) and petrol required to travel the functional unit of 100 km in a FFV is 9.9 kg and 6.6 kg based on their respective energy densities. The combustion emissions (CO2, CH4, N2O, CO, NMVOC, SOx, NOx, NH3 and PM) from ethanol and petrol in the FFV were estimated based on Intergovernmental Panel on Climate Change (IPCC) Tier 1 approach31 and EMEP-EEA Tier 1 approach.32
LCIA results
The results for all LCA impact categories and normalised comparisons (%) are presented in Fig. 2–6. The LCIA scores for each individual impact category and scenarios are given in ESI Tables S4–S32.†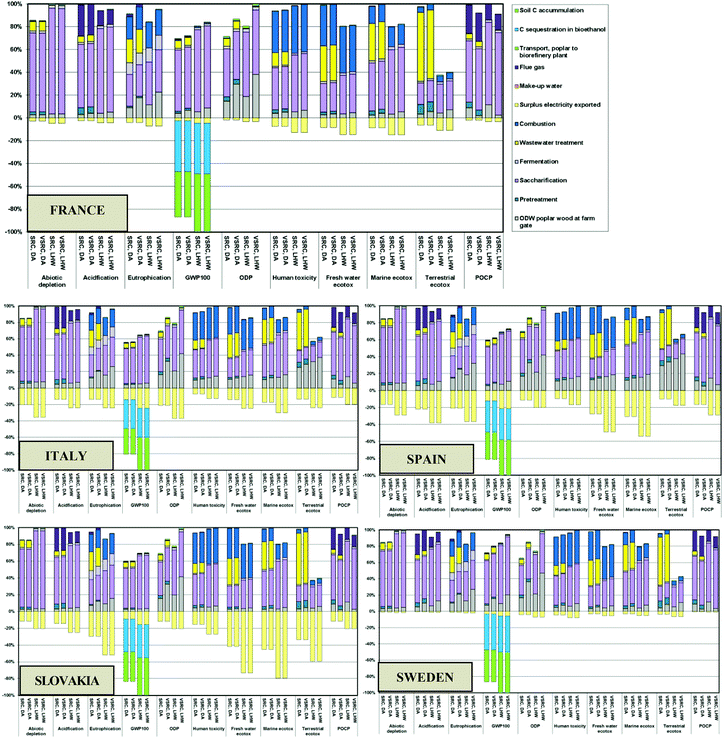 | ||
| Fig. 3 Characterized LCIA profiles of poplar-derived bioethanol at the biorefinery factory gate (unit: 1 kg poplar-derived bioethanol; method: CML 2 baseline 2000). | ||
 | ||
| Fig. 5 Characterized LCIA profiles of VSRC poplar-derived E100 bioethanol over the whole life cycle in current vs. future scenarios (unit: driving FFV for 100 km; method: CML 2 baseline 2000). | ||
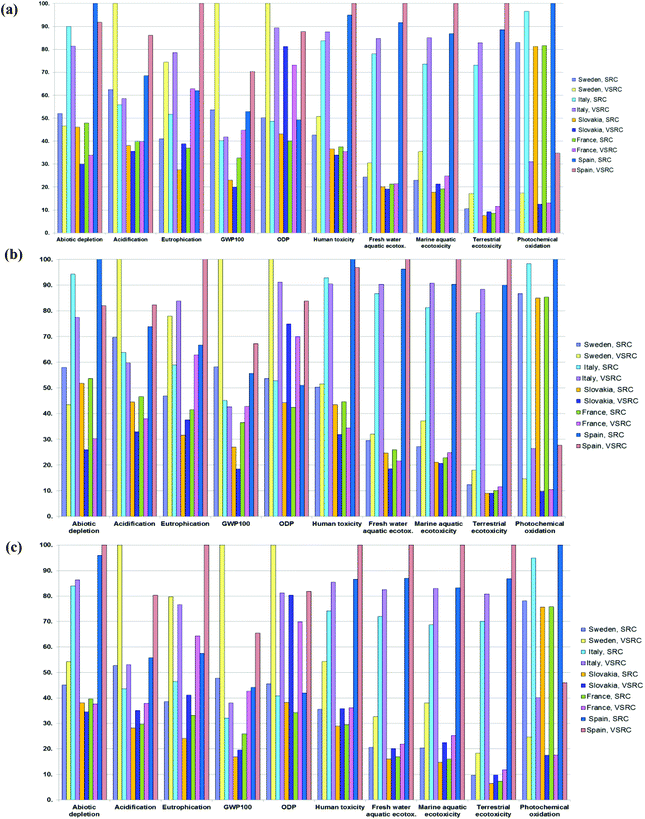
Cradle-to-farm-gate LCIA profiles for poplar biomass feedstock production
The environmental burdens caused by poplar SRC/VSRC plantations in five EU countries, are given in Fig. 2. For simplification, C-sequestration into the poplar biomass and soil C accumulation from pre-Poplar plantation levels are not represented in the global warming potential (GWP100) results shown here, but are accounted for in the results given in ESI Tables S4–S6.†The results between plantation management options vary with the countries and impact categories investigated. Generally, SRC plantation management showed environmental advantages over VSRC in most cases due to the higher biomass yields and lower agrochemical inputs per unit of harvested poplar. Particularly on ODP and eutrophication, where the environmental burdens are mainly caused by the production of agrochemical (herbicides, N/P fertilizers) and the induced field emissions, SRC delivers less impact. For abiotic depletion and photochemical oxidation (POCP), combine harvesting applied in the VSRC management consumes less diesel fuel than SRC harvesting (cutting and chipping), therefore giving lower POCP emissions (e.g. SO2, CH4 and NOx release from diesel consumption). In the remaining impact categories, the comparisons between SRC and VSRC vary with countries and time horizons, depending on the relative share of two main contributors (agrochemicals vs. harvesting method). With the increasing biomass yield over time moving from 2010 to 2030, the environmental burdens caused by the cutting and chipping remains stable per unit of harvested SRC poplar basis whereas the impacts from combine harvesting and agrochemical inputs decrease per unit of harvested VSRC basis. Thus, in GWP100 and acidification, where approximately 50–85% of the environmental burdens are attributed to N fertilizer inputs and the induced field emissions (N2O, NH3, NO3−) as well as emissions (CO2, CH4, SOx, and NOx) released from fuel combustion during field operations, VSRC turns from being environmentally inferior to superior to SRC in Slovakia and Italy with expended time horizon (harvesting method is the dominant factor accounting for 40–65% impacts); whereas in Sweden, SRC delivers better GWP100 and acidification performance than VSRC over all time horizons (field emission is the determining factor for their comparison on GWP100 and acidification).
Irrigation and agrochemical inputs are important drivers of differences between the environmental impact profiles between the five EU countries. Although Spain and Italy were modelled as having the highest biomass yields, the additional energy required for irrigation results in higher environmental burdens compared with the other EU regions across all impact categories. Slovakia benefited from its lower fertilizer inputs, and this feature in the current study is the main reason for it being the environmentally favourable location for poplar cultivation amongst those modelled.
Cumulative cradle-to-factory-gate LCIA profiles for bioethanol produced
The ‘cradle-to-factory gate’ LCIA profiles for the current scenarios of poplar-derived bioethanol produced via alternative pretreatment technologies in five EU countries are presented in Fig. 3. The main drivers of environmental impacts are the cellulase enzyme and chemical inputs, as well as emissions involved in the bioethanol production process. The poplar farming stage accounted for 5–40% of the environmental impacts of the bioethanol across all impact categories due to the diesel and agrochemicals consumed in plantation management and the field emissions released from agricultural land (e.g. N leaching).Generally, DA pretreatment caused higher environmental impacts than LHW pretreatment on acidification, eutrophication and eco-toxicity due to the additional chemical inputs and induced emissions in DA process e.g. sulphuric acid input and consequential SO2 emissions, ammonia input (for neutralisation) and induced NH3 emissions, lime (for flue gas desulphurisation). DA showed environmental advantages over LHW pretreatment in abiotic depletion, GWP100 and ODP impact categories where the higher enzyme (Cellic Ctec 1) loading for LHW was the dominant factor. Regardless of different pretreatment technologies, the positive scores in abiotic depletion, GWP100, acidification, ODP and POCP up to the factory gate were dominated by enzyme loading (60–90% of impacts) due to the energy-intensive enzyme production process. Cellic Ctec 1 also contributed 20–40% of environmental burdens in toxicity and eutrophication due to the emissions involved in its production system (e.g. field emissions from agricultural land due to the carbon substrates required for enzyme production). Caustic soda addition in WWT for neutralisation of nitric acid (HNO3 converted from NH4+via nitrification during aerobic WWT) was an important contributor to environmental impacts of the DA pretreated bioethanol product system, accounting for 20–50% of burdens on eutrophication and toxicity. 20–30% of the impacts on POCP and eutrophication burdens were attributed to flue gas emitted to the atmosphere during bioethanol production e.g. NH3 emissions induced by ammonia neutralisation in the DA process, as well as SO2, CO and CH4 released during combustion. Landfilling of ash generated at combustion caused 10–40% of impacts on eutrophication and toxicity impact categories.
Biogenic carbon sequestered into bioethanol and soil carbon accumulation in the poplar plantation brought significant ‘negative’ impacts on GWP100, acting to ‘offset’ the positive emissions incurred from the bioethanol production and leading to bioethanol with a net negative GHG balance at the factory gate. Environmental ‘savings’ (see below the line in Fig. 3) across all impact categories also derived from the ‘avoided burden’ credit from exported surplus electricity. The LHW pretreated bioethanol product system had greater export of surplus electricity compared to DA due to its lower carbohydrate conversion efficiencies and this resulted in more biomass residues being sent to combustion for electricity generation (Table 3). However, these benefits were overridden by environmental burdens in most cases, except for LHW bioethanol modelled for Slovakia, which delivered a bioethanol product with negative terrestrial eco-toxicity scores.
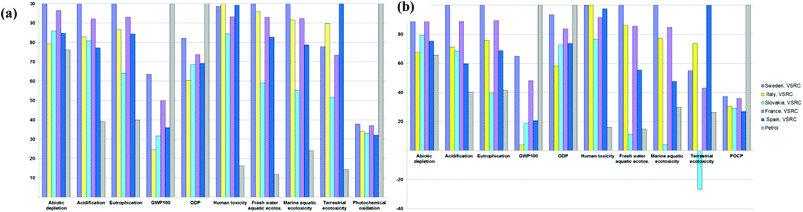
Cumulative whole life cycle impacts for E100 bioethanol use as FFV fuel¶
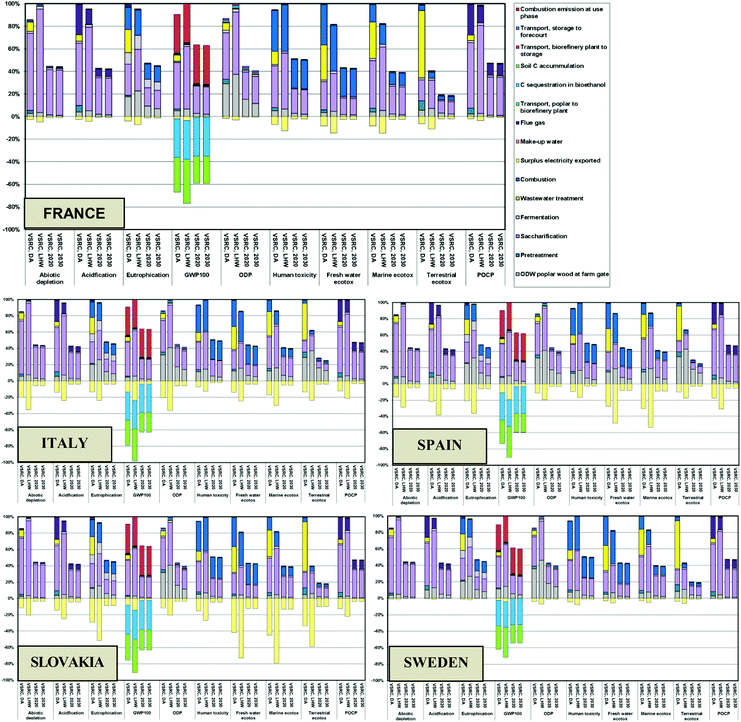
The environmental impacts of poplar-derived E100 bioethanol over its whole life cycle from cradle (Poplar plantation) to grave (combustion in an engine) were dominated by the poplar farming and bioethanol conversion processes. The transportation involved in the bioethanol supply chain contributed less than 5% (Fig. 5 and S2†). The GHG balance of bioethanol turned from negative (at factory-gate) into positive at the use phase. This can be explained by the GWP100 burdens resulting from the fuel combustion in the vehicle engine, which along with other GHGs emitted from bioethanol production override the ‘negative’ GWP100 scores contributed by carbon sequestration (into biomass and soil) and the avoided emissions credit from surplus electricity export.Bioethanol produced in Italy delivered the lowest whole life cycle environmental scores amongst the five EU countries in abiotic depletion, GWP100 and ODP (Fig. 4 and S3, Tables S7–S8†). For all other impact categories, Slovakia represented the lowest impact location for producing bioethanol. These outcomes were driven by the different fossil resources for national grid electricity (‘avoided burdens’ credit) in EU countries. The system expansion allocation approach credited the bioethanol with ‘avoided burdens’ credits for the electrical energy exported from the biorefinery and substitution for the equivalent amount of electricity generated from the respective national grids. In Italy, coal, natural gas and crude oil are the major fuel resources (over 70%) for grid electricity generation, whereas in Slovakia grid electricity is highly dependent on nuclear (55%), lignite and hard coal (nearly 20%) (see country-specific energy sources in ESI Table S3†). A greater amount of “green” electricity is generated in Sweden (40% derived from hydropower), resulting in lower ‘avoided burden’ credits allocated to bioethanol produced, which explains why the ethanol in Sweden tends to have higher impacts than modelled for the other EU countries modelled.
Regardless of different pretreatment technologies and poplar plantation management options, the results in Fig. 4 and Tables S7–S8† show poplar-derived bioethanol produced under the current scenario in all five EU countries to be overall environmentally superior to petrol in GWP100, ODP and POCP impact categories. However, higher impact scores than petrol are found in the other impact categories (except for eutrophication and ecotoxicity scores of E100 produced under LHW in Slovakia).
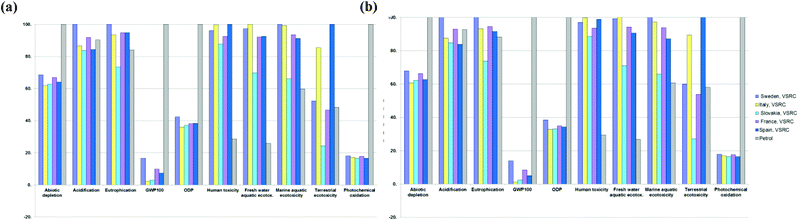
Prospective scenarios for 2020 and 2030
The modified low-lignin poplar showed enhanced environmental performance for E100 bioethanol over conventional clones with approximately 50% environmental savings being achieved in most impact categories (except for eco-toxicity). These significantly reduced environmental impacts over the life cycle were associated with reduced bioethanol production impacts due to removal of the pretreatment stage and the reduction in enzyme loading (see Fig. 5 and S2†). Bioethanol life cycles approaching net-zero GHGs were delivered as a result of this advanced plant breeding in combination with the soil carbon sequestration from poplar cultivation and avoided emissions credits for electricity exports from the biorefinery. The effects of the soil carbon factor and allocation approach on the overall GHG balance were analysed via sensitivity analysis.On eco-toxicity, E100 bioethanol produced in Slovakia under the prospective scenarios incurred higher environmental impacts than current scenarios. This is explained by the lower lignin level in the improved poplar feedstock reducing the amount of surplus electricity export thereby leading to a reduction in the ‘avoided burden’ credits allocated to the bioethanol produced in Slovakia. The environmental savings achieved from increasing biomass yields in future scenarios (2020 vs. 2030 scenarios) were negligible (Fig. 5). As illustrated in Fig. 6 (also see ESI Fig S3†), the environmental advantages of Slovakia over the other EU countries shown in the current scenario (Fig. 4) remained under the prospective scenarios. However, the gaps between different EU countries diminished in the prospective scenarios due to the high carbohydrate conversion efficiencies and low lignin levels achieved by genetic modification of poplar – lower surplus electricity exports (‘avoided burdens’ credits to bioethanol product) were therefore modelled for 2020/2030 scenarios compared to the current scenario (see Table 3). Under 2020 and 2030 scenarios, E100 bioethanol was an environmentally advantageous or equivalent product system to petrol in most impact categories except for human and eco-toxicity (Fig. 6, ESI Fig. S3 and Tables S9–S10†). Significant environmental savings (40–98% lower impacts) could be achieved in abiotic depletion, GWP100, ODP and POCP by switching from petrol to E100 bioethanol from advanced poplar feedstocks.
Bioethanol blends (E85 and E10) over whole life cycle
Under the current and future scenarios, the petrol component in E10 bioethanol blends was the dominant factor driving the environmental profiles across all impact categories. E10 delivered marginal environmental advantages (1–5%) over petrol in GWP100 and POCP across all E10 scenarios, and only achieved small environmental savings compared with petrol on abiotic depletion and ODP (approximately 2% and 4%, respectively) in future scenarios. E85 bioethanol exhibited a similar environmental profile to E100. With differences in the LCIA profiles of five EU countries driven by the ‘avoided burdens’ credits allocated to the E85 from energy substitution by exporting the surplus electricity, all E85 bioethanol products showed great environmental advantages over petrol in GWP100 (30–80% savings) and POCP (50–65% savings) under both current and future scenarios. Full data for these blends are given in ESI (Fig. S4–S5, Tables S11–S18†).Sensitivity analysis on soil carbon accumulation
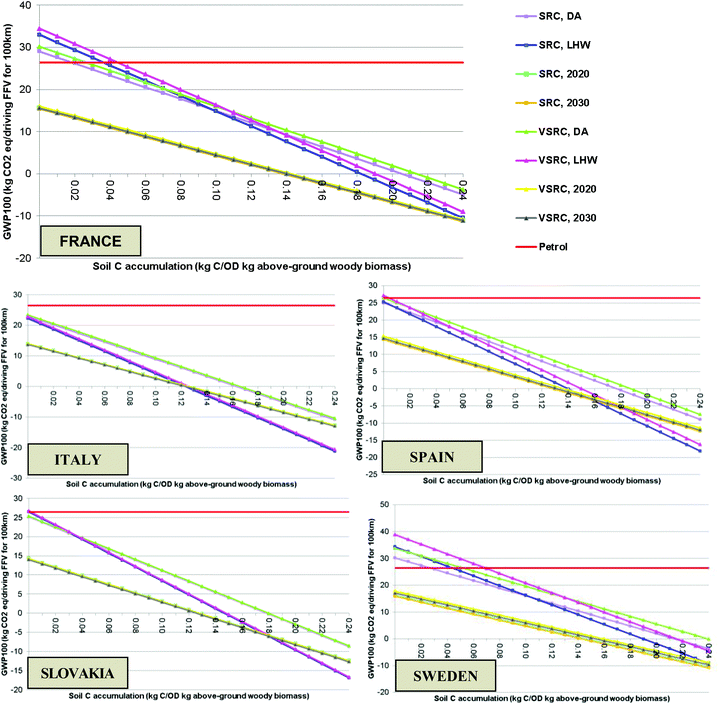
The soil carbon accumulation range given in Table 1 (up to 0.24 kg C kg−1 OD above-ground woody biomass) was investigated in sensitivity analysis. As shown in Fig. 7, with an assumption of the higher level of soil carbon accumulation, the GWP100 profiles of the current poplar-derived bioethanol life cycle moved from being positive (some net addition of GHG to atmosphere) to negative values (net GHG removed from atmosphere), which is above our chosen 10% sensitivity threshold. With an assumption of a zero soil carbon accumulation, bioethanol E100 produced in Spain, Italy and Slovakia remained environmentally competitive, in GWP100 terms, compared with petrol. However, current bioethanol E100 in Sweden and France moved to a disadvantageous GWP100 position regarding petrol in the absence of soil carbon accumulation. The GWP100 saving of bioethanol over petrol is 33% to 48% under the prospective scenarios with a zero soil carbon accumulation assumption as compared with an 80% to 98% saving for future E100 modelled with the default value for soil carbon accumulation. It is clear that the GWP100 impacts for poplar-derived bioethanol are very sensitive to the inclusion of soil carbon accumulation and that this affects the scale of the GWP100 savings shown for the bioethanol over petrol.Sensitivity analysis on characterisation model and allocation approach
As an alternative to the mid-point method CML 2 Baseline 2000, the damage-oriented method Eco-Indicator 99 H (Hierarchist version 2.08, land use excluded) was also applied to the LCA model. Detailed discussion and data are presented in ESI, Method S2, Fig. S6, S7 and Tables S19–S22.† The results based on EI 99 broadly agree with the outcomes based on the CML method in most comparable impact categories except for abiotic depletion, acidification and eutrophication (see ESI Method S2†). Overall, the LCIA comparisons of E100 and petrol counterparts were not sensitive to the characterisation models adopted. Similar findings also occurred in the LCIA comparisons between bioethanol blends (E10/E85) and petrol examined under the two different characterization methods.Sensitivity analyses on allocation approach (see ESI Method S2†) indicated that the influences of allocation choice on LCIA profiles of bioethanol vary with the countries and scenarios modelled and the impact categories investigated. GWP100 was the impact category most sensitive to the allocation approach. Switching from system expansion to the energy allocation approach led to significantly increased GWP100 scores for current E100 bioethanol modelled for Spain, Italy and Slovakia, whereas a decline in GWP100 impacts of E100 bioethanol was observed in the case of France and Sweden. The allocation approach was not a sensitivity factor in terms of the LCIA comparisons between E100 bioethanol and petrol (further detailed breakdown of the sensitivity analyses is given in the ESI, Method S2, Fig. S8, S9 and Tables S23–S32†).
Discussion and conclusion
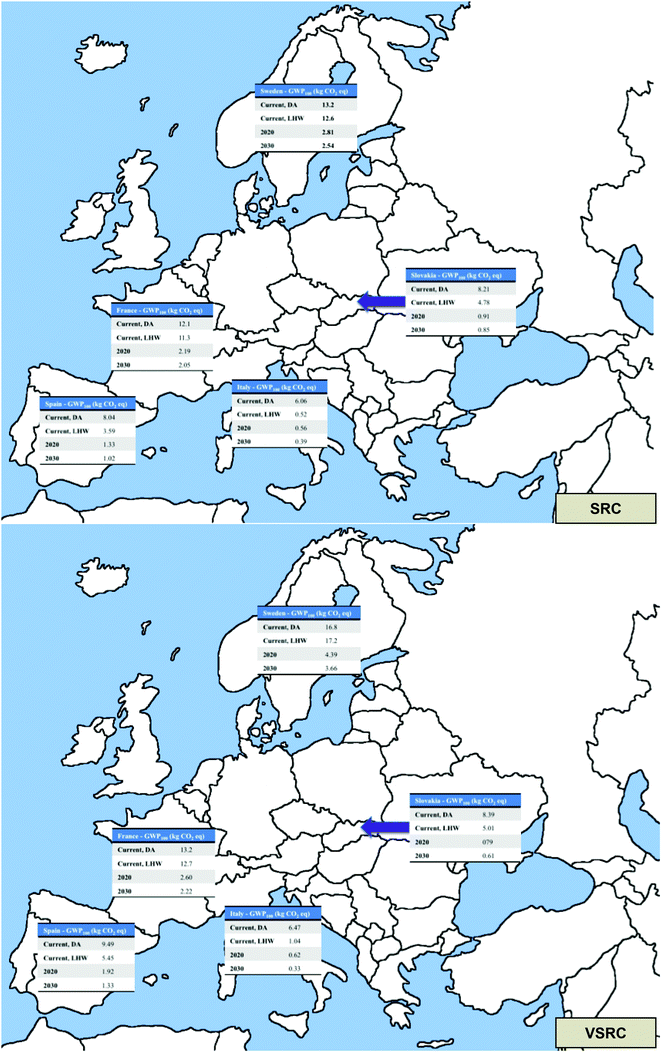
The overview of EU potential bioethanol supply chains modelled and their GWP100 profiles are shown in map form in Fig. 8. LCA modelling has demonstrated that hypothetical bioethanol production from poplar via leading processing technology in the five EU countries examined can have environmental profiles offering substantial GWP100 benefits over petrol and that these are expected to increase significantly in prospective scenarios with advanced poplar clones. Environmental impacts in a variety of other impact categories for current poplar bioethanol production present a mixed picture in comparison with petrol with higher scores occurring in impact categories associated with agricultural activity and bioethanol conversion processes. Prospective scenarios for 2020 and 2030 showed improvements in environmental profiles with the introduction of advanced poplar clones leading to bioethanol products with substantial environmental savings (e.g. 30 to 95%) over petrol in GWP100, abiotic depletion, POCP, ODP and parity in categories such as acidification and eutrophication.Poplar cultivation accounted for up to 40% of the environmental impacts of the bioethanol product systems. Our analysis further suggests that there is additional potential for advances in poplar management (e.g. harvesting techniques) to play an important role in minimising the environmental impact from the whole life cycles of poplar-derived bioethanol. At the biorefinery stage, cellulase enzymes dominated the environmental burdens of E100 in abiotic depletion, GWP100, acidification, ODP and POCP. Our modelling was conducted on an early variant of the Cellic Ctech production series (Cellic Ctech 1) and advances have been made more recently in this series. However we consider that our level of enzyme requirement in the saccharification process are modest, likely to apply also for more advanced cellulases usage and that the activity and production of cellulase enzymes will remain an important element contributing to the environmental impact of 2G bioethanol production. Undoubtedly, future technology advances (e.g. genetic improvement in the Z. mobilis strain with metabolic pathways to convert all available hexose and pentose sugars to bioethanol, development of low-cost enzymes) will further the development of 2G bioethanol markets, which could be explored in future LCA research. Comparisons between the two pretreatment technologies in this study indicate that the beneficial effects of lowering enzyme loadings can be offset by environmental burdens brought by additional chemical inputs in a more severe pretreatment (e.g. DA). This suggests that achieving higher ethanol yields per unit of enzyme consumed without introducing chemically-intensive pretreatments will continue to be essential to reducing the overall environmental profile of this stage of the 2G biofuel life cycle. However, only biochemical processes have been modeled in the current study. Alternative conversion pathways for 2G biofuel production e.g. thermochemical processes will be investigated in further research.
A key aspect of the comparative analyses presented here for bioethanol production across various potential EU supply chains has been to highlight the importance of the following main factors on the resulting biofuel profiles:
• Feedstock quality and processability (e.g. significant advantage are conferred by advanced poplar clones).
• Inclusion of mid-term soil carbon accumulation is a substantial factor in the overall GWP100 balance of the biofuel. The soil carbon accumulation expressed in this study is a direct Land Use Change (dLUC) occurring by the poplar cultivation on set-aside, marginal, degraded or no longer cultivated lands. The effects of indirect land use change due to poplar plantation were not considered here due to the land types being evaluated (neither was foregone sequestrations associated with a potential land reversion to forest). Such wider potential land use issues could be explored in future work.
• The specific agricultural system being used (e.g. advantage from low nutrient inputs; disadvantage of mechanical irrigation) and processing technology.
• Importance of co-product(s) and emissions profiling methodology applied in the LCA methodology (e.g. system expansion vs. energy allocation approach).
A broad review of the literature on LCAs of biofuel products (to be presented in a separate publication) indicates that the key factors identified here (e.g. dLUC) are generally also confirmed by previous LCA-type studies (on other biofuel feedstocks).52–54
By modelling prospective hybrid poplar clones with higher biomass yields, modified composition and improved cell wall accessibility, this work indicates that genetic improvements and advanced breeding programmes have a clear potential to advance the environmental profile of poplar-derived bioethanol and other products to deliver a more environmentally sustainable lignocellulosic biorefining industry. Under current and future scenarios, E100 and E85 show substantial environmental advantages as transport fuels over petrol in abiotic depletion, GWP100, ODP and POCP. Advanced poplar feedstocks are shown in our modelling to offer life cycle GWP100 savings over petrol of 80% or more, placing them well within the most desirable categories being targeted by policymakers internationally (e.g. the EU Renewable Energy Directive,21 the USA Renewable Fuel Standard). A particular aspect of the present study that warrants further attention and new ‘before and after’ research is the contribution that soil carbon accumulation under feedstocks can make to achieving low GHG fuels and biorefinery products in the future.
Acknowledgements
This study is based on the research supported by the European Commission 7th Framework for Research, Food Agriculture and Fisheries, and Biotechnology, within the project ENERGYPOPLAR, FP7-211917. We thank all the participants in ENERGYPOPLAR led by the French National Institute for Agricultural Research (INRA). We also wish to acknowledge Novozymes A/S, Demark and Dr Gianni Facciotto and Sara Berganate at the Agriculture Research Council, Italy for their valuable support respectively with the inventory development on cellulase enzymes production and the poplar plantation.Notes and references
- European Commission, Consumption of energy: Eurostat. http://epp.eurostat.ec.europa.eu/portal/page/portal/energy/introduction [accessed April 2014].
- European Union, Energy Markets in the European Union in 2011, Publications Office of the European Union, Luxembourg, 2012 Search PubMed.
- European Commission, Reducing emissions from transport. http://ec.europa.eu/clima/policies/transport/index_en.htm [accessed May 2014].
- L. Pelkmans, L. Govaerts and K. Kessels, Inventory of biofuel policy measures and their impact on the market, Report D2.1 in ELOBIO, European Commission, Brussels, Belgium, 2008 Search PubMed.
- European Commission, Renewable energy targets by 2020. URL: http://ec.europa.eu/energy/renewables/targets_en.htm [accessed Aug 2012].
- EBTP, Biofuels Policy and Legislation, CPL Press, Newbury, UK, 2012 Search PubMed.
- ISO, Environmental management —Life cycle assessment —Principles and framework, British Standards Institution (BSI Group), London, UK, 2006 Search PubMed.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch, Y. Y. Lee, C. Mitchinson and J. N. Saddler, Biotechnol. Prog., 2009, 25, 333–339 CrossRef CAS PubMed.
- G. A. Tuskan, S. DiFazio, S. Jansson, J. Bohlmann, I. Grigoriev, U. Hellsten, N. Putnam, S. Ralph, S. Rombauts, A. Salamov, J. Schein, L. Sterck, A. Aerts, R. R. Bhalerao, R. P. Bhalerao, D. Blaudez, W. Boerjan, A. Brun, A. Brunner, V. Busov, M. Campbell, J. Carlson, M. Chalot, J. Chapman, G.-L. Chen, D. Cooper, P. M. Coutinho, J. Couturier, S. Covert, Q. Cronk, R. Cunningham, J. Davis, S. Degroeve, A. Déjardin, C. dePamphilis, J. Detter, B. Dirks, I. Dubchak, S. Duplessis, J. Ehlting, B. Ellis, K. Gendler, D. Goodstein, M. Gribskov, J. Grimwood, A. Groover, L. Gunter, B. Hamberger, B. Heinze, Y. Helariutta, B. Henrissat, D. Holligan, R. Holt, W. Huang, N. Islam-Faridi, S. Jones, M. Jones-Rhoades, R. Jorgensen, C. Joshi, J. Kangasjärvi, J. Karlsson, C. Kelleher, R. Kirkpatrick, M. Kirst, A. Kohler, U. Kalluri, F. Larimer, J. Leebens-Mack, J.-C. Leplé, P. Locascio, Y. Lou, S. Lucas, F. Martin, B. Montanini, C. Napoli, D. R. Nelson, C. Nelson, K. Nieminen, O. Nilsson, V. Pereda, G. Peter, R. Philippe, G. Pilate, A. Poliakov, J. Razumovskaya, P. Richardson, C. Rinaldi, K. Ritland, P. Rouzé, D. Ryaboy, J. Schmutz, J. Schrader, B. Segerman, H. Shin, A. Siddiqui, F. Sterky, A. Terry, C.-J. Tsai, E. Uberbacher, P. Unneberg, J. Vahala, K. Wall, S. Wessler, G. Yang, T. Yin, C. Douglas, M. Marra, G. Sandberg, Y. Van de Peer and D. Rokhsar, Science, 2006, 313, 1596–1604 CrossRef CAS PubMed.
- S. Gonzalez-Garcia, M. T. Moreira, G. Feijoo and R. J. Murphy, Biomass Bioenergy, 2012, 39, 378–388 CrossRef CAS.
- H. J. Huang, S. Ramaswamy, W. Al-Dajani, U. Tschirner and R. A. Cairncross, Biomass Bioenergy, 2009, 33, 234–246 CrossRef CAS.
- J. S. Daystar, R. A. Venditti, R. Gonzalez, H. Jameel, M. Jett and C. W. Reeb, BioResources, 2013, 8, 5261–5278 CrossRef.
- D. Tonini, L. Hamelin, H. Wenzel and T. Astrup, Environ. Sci. Technol., 2012, 46, 13521–13530 CrossRef PubMed.
- D. Arrouays, A. Freibauer, M. D. A. Rounsevell, P. Smith and J. Verhagen, Carbon sequestration in soils: estimates for Europe and France, uncertainties and research and references needs, 2004, URL: http://eusoils.jrc.ec.europa.eu/projects/scape/uploads/8/Arrouays_etal.pdf.
- A. Freibauer, M. D. A. Rounsevell, P. Smith and J. Verhagen, Geoderma, 2004, 122, 1–23 CrossRef CAS.
- H. Blanco-Canqui, Agron. J., 2010, 102, 403–419 CrossRef CAS.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, D. Sexton and D. Dudgeon, Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: National Renewable Energy Laboratory, National Renewable Energy Laboratory, Colorado, USA, 2011 Search PubMed.
- ISO, ISO 14041 Environmental management — Life cycle assessment — Goal and scope definition and inventory analysis, British Standards Institution (BSI Group), London, UK, 1998 Search PubMed.
- J. E. A. Seabra, I. C. Macedo, H. L. Chum, C. E. Faroni and C. A. Sarto, Biofuels, Bioprod. Biorefin., 2011, 5, 519–532 CrossRef CAS.
- ISO Environmental management, Life cycle assessment Requirements and guidelines, British Standard Institution (BSI Group), London, UK, 2006 Search PubMed.
- European Union, Directive 2009/28/EC of the European Parliament and of the Council, The European Parliament and the Council of the European Union, Brussels, Belgium, 2009 Search PubMed.
- J. Littlewood, M. Guo, W. Boerjan and R. J. Murphy, Biotechnol. Biofuels, 2014, 7 CAS , 113.
- J. B. Guinée, M. Gorree, R. Heijungs, G. Huppes, R. Kleijn, A. Koning, L. V. Oers, A. W. Sleeswijk, S. Suh, H. A. U. Haes, H. Bruijn, R. V. Duin and M. A. J. Huijbregts, Life Cycle Assessment An Operational Guide to the ISO Standards Final report (part 1, 2, 3)Ministry of Housing, Spatial Planning and the Environment & Center of Environmental Science – Leiden University, 2001.
- M. Guo and R. J. Murphy, Sci. Total Environ., 2012, 435, 230–243 CrossRef PubMed.
- N. O. Di Nasso, W. Guidi, G. Ragaglini, C. Tozzini and E. Bonari, GCB Bioenergy, 2010, 2, 89–97 CrossRef.
- S. Gonzalez-Garcia, J. Bacenetti, R. J. Murphy and M. Fiala, J. Cleaner Prod., 2012, 26, 56–66 CrossRef.
- M. Guo, G. Facciotto, C. Li, S. Bergante and R. Murphy, in 24th Session of International Poplar Commission, International Poplar Commission, Dehradun, India, 2012, pp. 177–178 Search PubMed.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CrossRef CAS PubMed.
- S. D. Mansfield, K. Y. Kang and C. Chapple, New Phytol., 2012, 194, 91–101 CrossRef CAS PubMed.
- H. D. Coleman, J. Yan and S. D. Mansfield, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13118–13123 CrossRef CAS PubMed.
- IPCC, 2006 IPCC Guidelines for National Greenhouse Gas Inventories, ed. H. S. Eggleston, L. Buendia, K. Miwa, T. Ngara and K. Tanabe, IGES, Prepared by the National Greenhouse Gas Inventories Programme, Hayama, Kanagawa, Japan, 2006 Search PubMed.
- EEA, The EMEP/EEA Air Pollutant Emission Inventory Guidebook-2009, European Environment Agency, Copenhagen, Denmark, 2009 Search PubMed.
- T. Johansson and A. Karačić, Biomass Bioenergy, 2011, 35, 1925–1934 CrossRef.
- R.-M. Rytter, Biomass Bioenergy, 2012, 36, 86–95 CrossRef CAS.
- P. Paris, L. Mareschi, M. Sabatti, A. Pisanelli, A. Ecosse, F. Nardin and G. Scarascia-Mugnozza, Biomass Bioenergy, 2011, 35, 1524–1532 CrossRef.
- R. Spinelli, Short Rotation Coppice (SRC) Production in Italy, National Research Council of Italy Trees and Timber Institute, Sesto Fiorentino, Italy, 2011 Search PubMed.
- P. J. Joyce, Using scenario-based Life Cycle Assessment (LCA) to evaluate the present and future environmental impact of bioethanol production from genetically improved poplar wood in the EU, Imperial College London, London, UK, 2010 Search PubMed.
- S. González-García, C. M. Gasol, X. Gabarrell, J. Rieradevall, M. T. Moreira and G. Feijoo, Renewable Energy, 2010, 35, 1014–1023 CrossRef.
- G. Fischer, S. Prieler and H. van Velthuizen, Biomass Bioenergy, 2005, 28, 119–132 CrossRef.
- S. Nonhebel, Harvesting the sun's energy using agro-ecosystems, NRP Programme Office, Illinois, USA, 1997 Search PubMed.
- European Commission, Fertiliser consumption and nutrient balance statistic, European Commission Eurostat. URL: http://epp.eurostat.ec.europa.eu/statistics_explained/index.php/Fertiliser_consumption_and_nutrient_balance_statistics Search PubMed [accessed Aug 2012].
- IFA, 2011 IFA statistics, International Fertilizer Industry Association, Paris, France. URL: http://www.fertilizer.org/ifa/ifadata/search [Accessed Aug 2012] Search PubMed.
- M. Guo, G. Facciotto, C. Li, S. Bergante and R. Murphy, in 20th International Symposium on Alcohol Fuels, Cape Town, 2013.
- G. L. Velthof, D. Oudendag, H. P. Witzke, W. A. H. Asman, Z. Klimont and O. Oenema, J. Environ. Qual., 2009, 38, 402–417 CrossRef CAS PubMed.
- W. De Vries, A. Leip, G. J. Reinds, J. Kros, J. P. Lesschen, A. F. Bouwman, B. Grizzetti, F. Bouraoui, K. Butterbach-Bahl, P. Bergamaschi and W. Winiwarter, Geographical variation in terrestrial nitrogen budgets across Europe – The European Nitrogen Assessment, Cambridge University Press, Cambridge, UK, 2011 Search PubMed.
- B. Gielen, C. Calfapietra, M. Lukac, V. E. Wittig, P. De Angelis, I. A. Janssens, M. C. Moscatelli, S. Grego, M. F. Cotrufo, D. L. Godbold, M. R. Hoosbeek, S. P. Long, F. Miglietta, A. Polle, C. J. Bernacchi, P. A. Davey, R. Ceulemans and G. E. Scarascia-Mugnozza, Tree Physiol., 2005, 25, 1399–1408 CrossRef CAS PubMed.
- E. A. Hansen, Biomass Bioenergy, 1993, 5, 431–436 CrossRef CAS.
- C. T. Garten, S. D. Wullschleger and A. T. Classen, Biomass Bioenergy, 2011, 35, 214–226 CrossRef CAS.
- N. Gupta, S. S. Kukal, S. S. Bawa and G. S. Dhaliwal, Agrofor. Syst., 2009, 76, 27–35 CrossRef.
- NREL, Biomass Feedstock Composition and Property Database, National Renewable Energy Laboratory, Colorado, USA. http://www.afdc.energy.gov/biomass/progs/search1.cgi [accessed Aug 2012] Search PubMed.
- Department for Transport, Biofuels Carbon Calculator, Department for Transport, London, UK. http://www.dft.gov.uk/publications/carbon-calculator/ [accessed Aug 2012] Search PubMed.
- S. Gonzalez-Garcia, D. Iribarren, A. Susmozas, J. Dufour and R. J. Murphy, Life cycle assessment of two alternative bioenergy systems involving Salix spp. biomass: Bioethanol production and power generation, Appl. Energy, 2012, 95, 111–122 CrossRef CAS.
- J. Lindorfer, K. Fazeni and H. Steinmüller, Life cycle analysis and soil organic carbon balance as methods for assessing the ecological sustainability of 2nd generation biofuel feedstock, Sustainable Energy Technol. Assess., 2014, 5, 95–105 CrossRef.
- J. Malça and F. Freire, Addressing land use change and uncertainty in the life-cycle assessment of wheat-based bioethanol, Energy, 2012, 45(1), 519–527 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Inventory development, sensitivity analysis and detailed LCIA data. See DOI: 10.1039/c4gc01124d |
| ‡ Equivalent contributions. |
| § Present address: Environmental Resources Management Ltd., London, EC3A 8AA, UK. |
| ¶ For simplicity of presentation, hereafter only results for VSRC poplar feedstock are given in the paper. Full results for both SRC and VSRC poplar feedstock are given in ESI.† |
| This journal is © The Royal Society of Chemistry 2014 |