Open Access Article
Open Access ArticleSustainability assessment of organic solvent nanofiltration: from fabrication to application
Gyorgy
Szekely
,
Maria F.
Jimenez-Solomon
,
Patrizia
Marchetti
,
Jeong F.
Kim
and
Andrew G.
Livingston
*
Department of Chemical Engineering, Imperial College London, Exhibition Road, SW7 2AZ, UK. E-mail: a.livingston@imperial.ac.uk; Tel: +44 (0)207594 5582
First published on 10th July 2014
Abstract
Can Organic Solvent Nanofiltration (OSN) be considered green? Is OSN greener than other downstream processing technologies? These are the two main questions addressed critically in the present review. Further questions dealt with in the review are as follows: What is the carbon footprint associated with the fabrication and disposal of membrane modules? How much solvent has to be processed by OSN before the environmental burden of OSN is less than the environmental burden of alternative technologies? What are the main challenges for improving the sustainability of OSN? How can the concept of Quality by Design (QbD) improve and assist the progress of the OSN field? Does the scale have an effect on the sustainability of membrane processes? The green aspects of OSN membrane fabrication, processes development and scale-up as well as the supporting concept of QbD, and solvent recovery technologies are critically assessed and future research directions are given, in this review.
1 Introduction
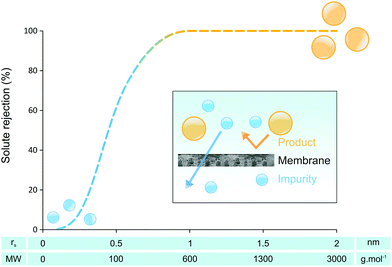
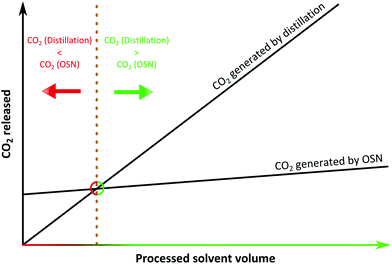
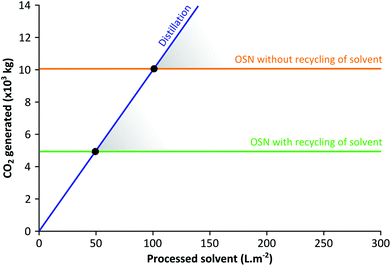
Although various approaches are used to eliminate or reduce solvent consumption within chemical processes, solvents are often used in substantial amounts to carry out reactions in dilute environments because of solubility and product selectivity issues. Even the crude products of neat, ball mill or microwave assisted reactions inevitably require organic solvents at the purification stage. Organic Solvent Nanofiltration (OSN) is a relatively new technology that allows size-exclusion based separation of solutes between 50 and 2000 g mol−1, solvent exchange or solvent recovery, all in organic media (Fig. 1) simply by applying a pressure gradient. OSN requires solvent-resistant membranes that preserve their separation characteristics while processing a large range of solvents with defect-free morphology and controlled molecular weight cut off. The main focus of research in OSN has been proving the stability of OSN membranes in a wide range of solvents, and improving solvent permeance. Unlike the water industry, the OSN market is fragmented across many different industrial sectors. Membrane technology is now recognized as a key factor for sustainable growth in many solvent using industrial segments,1,2 and several reported lab/pilot-scale applications in oil,3 food,4 pharmaceutical5 and the fine chemicals industry,6 already proved the potential of OSN technology. In addition to applications where OSN can be employed as a stand-alone approach, hybrid approaches that advantageously combine membranes with adsorption,7 imprinting technology,8 distillation,9 and crystallisation and chromatography,10 all of which routinely run in solvents, are likely to become significant as well.There are two main questions to be addressed regarding the greenness of OSN technology. First, how much solvent has to be processed by OSN before the environmental burden of OSN is less than the environmental burden of alternative technologies, i.e. carbon footprint of the fabrication and disposal of the membranes as well as OSN processing versus carbon footprint of distillation (Fig. 2). Secondly, is OSN greener than other downstream processing technologies? These questions will be addressed in sections 2 and 4, respectively. The carbon footprint of OSN is comprised of the CO2 generated by (i) the membrane formation and disposal, and (ii) OSN process operation which will be reviewed in sections 2 and 3, respectively. On the other hand, the carbon footprint of distillation is comprised of the CO2 generated by heating and evaporating the solvent and condensing the vapours, and this will be used for comparison.
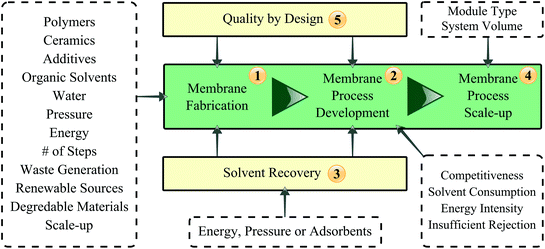
The present review addresses the green aspects of OSN membrane fabrication, processes development and scale-up as well as the supporting concept of quality by design (QbD) and solvent recovery technologies (Fig. 3). OSN starts with the fabrication of membranes stable in organic media. The first section of the review discusses the types of polymers, ceramics and additives; the water, organic solvents and energy consumption as well as the number of steps during the membrane fabrication process from a sustainability point of view. The second section assesses the competitiveness of OSN compared to conventional downstream processes in terms of solvent and energy consumption, and discusses its main drawback, insufficient rejection, and how it can be overcome. The third section gives an insight into solvent recovery which is crucial for sustainable OSN processes. The fourth section compares the CO2 generation and saving throughout the lifetime of OSN membranes from fabrication to disposal in comparison to distillation. The fifth section examines the effect of scale on the productivity and sustainability of membrane processes considering membrane types and system volume. Finally, the sixth section addresses the concept of QbD and how it can speed progress of the OSN field.
1.1 Greener organic solvent nanofiltration membranes
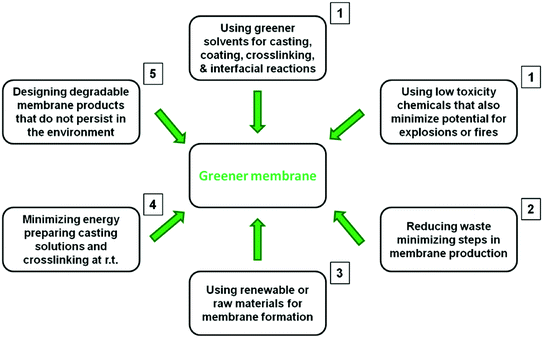
Now that OSN is becoming a mature technology, different strategies to make OSN greener, including aspects related to membrane fabrication should be considered. Ideally one should be able to produce a greener membrane without compromising its performance following some of the principles of green chemistry as shown in Fig. 4.11 We have ranked these principles in order of priority according to their contribution to making a membrane fabrication process greener. The first priority is tied between two principles: (a) substituting conventional solvents by greener solvents as they account for the majority of liquid waste generated during the membrane fabrication process and; (b) using low toxicity chemicals that also minimize potential for explosions or fires, reducing their environmental impact and making the process safer. Secondly, when possible, one should reduce the number of steps involved to produce a membrane, which could considerably minimize toxic waste, energy consumption and costs. The third place goes for using renewable or raw materials for membrane formation, making the membrane fabrication process more sustainable. The fourth place goes for dissolving polymers and crosslinking at room temperature to reduce the energy consumption. Finally, designing degradable membrane products that do not persist in the environment can also contribute to making a greener membrane. However, the mass of membrane disposed after the lifetime of a membrane product is small compared to the liquid waste generated during the membrane fabrication process, which will be exemplified and calculated in section 4.Compared to other traditional separation techniques OSN has several advantages, including lower energy consumption, easy up-scaling and flexibility. However, manufacturing OSN membranes involves a number of stages where hazardous chemicals are discharged as waste, and the membranes themselves are discharged eventually.12 Thus the environmental advantages of employing OSN are being compromised to some extent by the waste released during OSN membrane production and discharge.12 We will calculate the kg of CO2 saved when using OSN and the kg of CO2 produced during membrane fabrication and discharge and propose a strategy to reduce the amount of CO2 during membrane formation (see section 4). In the following sections several strategies to achieve greener membrane products and minimize the environmental impact during the formation of OSN membranes will be discussed.
1.1.1.1 Polymeric OSN membranes. Polymeric membranes can be made in flat sheet or hollow fibre configurations. Hollow fibre membranes are a greener option as no non-woven backing material or spacers are required in the membrane module. Most polymeric flat sheet membranes are formed on a non-woven backing material to provide mechanical stability. However, in OSN the non-woven backing material must be solvent resistant. Polymeric membranes must be solvent stable and preserve their separation characteristics in organic solvents. This requires stable polymers that are difficult to dissolve, sometimes requiring high temperatures or the use of aggressive toxic solvents to prepare the desired casting solution, resulting in a negative impact to the environment. Membranes made of less solvent stable polymers can be made stable via crosslinking, which can generate extra steps during the manufacturing process, resulting in more chemical waste. Several strategies to make polymeric membranes greener will be discussed in the following sections.
Integrally skinned asymmetric (ISA) membranes
Integrally skinned asymmetric membranes are formed by phase inversion, developed by Loeb and Sourirajan,13 which involves the precipitation of a casting solution by immersion in a nonsolvent bath (usually water). These membranes possess a skin-layer on top of a more porous sublayer with the same chemical composition (Fig. 5a).
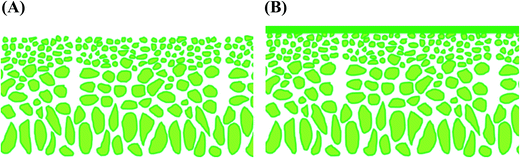 | ||
| Fig. 5 Schematic representation of polymeric membranes: (A) Integrally skinned asymmetric (ISA) membrane; (B) thin film composite (TFC) membrane. | ||
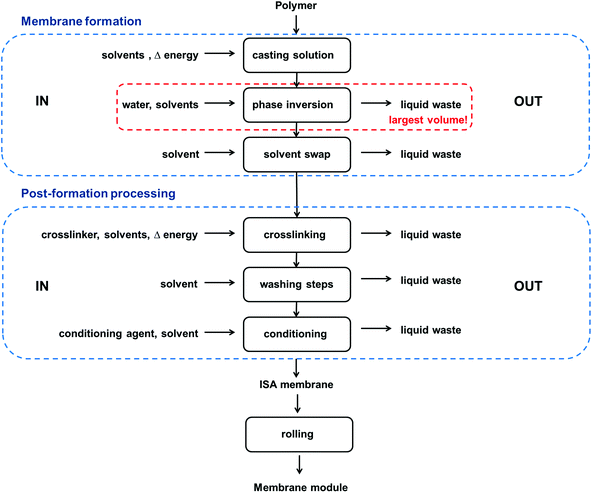
The key for high performance is the thin skin-layer, which makes higher selectivity and permeability possible. After phase inversion, the solvents and toxic additives such as plasticisers in the casting solution (usually between 75–85 wt%) will remain in the nonsolvent bath, generating a large amount of liquid waste. Replacing these solvents for greener solvents can reduce the waste impact to the environment substantially. A process diagram for the fabrication of ISA membranes, including energy use and waste generated on each step of the process is shown in Fig. 6.
In order to increase the long term stability of ISA membranes and to enhance their separation performance, various conditioning or post-treatment methods can be used, such as crosslinking and wet or dry annealing.14 Crosslinking is used to enhance chemical stability and rejection properties of ISA membranes. Different crosslinking methods have been used for polymeric membranes, including thermal crosslinking, UV crosslinking and chemical crosslinking. The previous review by Vanherck et al.15 discusses crosslinking polyimide, the dominant type of OSN membranes, for different applications. During chemical crosslinking the membrane is usually immersed in a crosslinking medium comprised of a crosslinker and a solvent. After the crosslinking reaction is complete, the crosslinking medium can be recycled or disposed of, producing toxic liquid waste. Alternative crosslinking chemistries or using greener solvents in the crosslinking medium could make the crosslinking process greener. Thermal and UV crosslinking are greener alternatives to chemical crosslinking as they do not produce solvent waste during the crosslinking step and the membranes do not need to go through a washing step after crosslinking. However, when membranes are crosslinked thermally or by UV irradiation they are exposed to high temperatures and safety measurements must be considered to avoid the possibility of a fire or an explosion. Different aspects of how to make the crosslinking process greener by reducing the use of solvents or the number of steps will be discussed in section 2.4 and 2.5, respectively. After crosslinking the membrane is usually conditioned to store the membrane dry while preserving its porous structure. The membrane is placed in a bath comprised of a conditioning agent and a solvent that will be disposed as liquid waste once the membrane is removed and put to dry.
Thin film composite (TFC) membranes
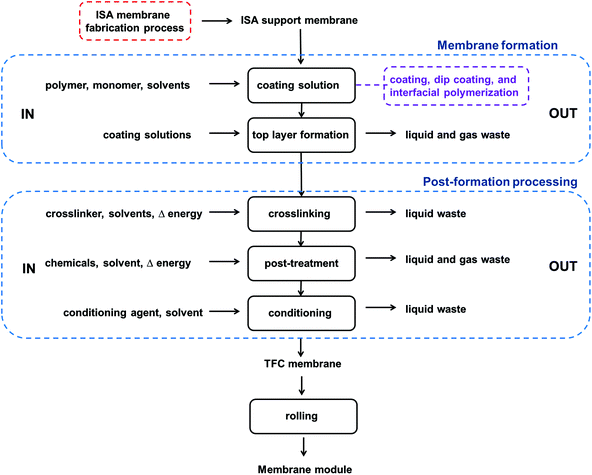
These membranes consist of an ultra-thin “separating top layer” on top of a chemically different porous support (Fig. 5b), which gives more freedom to design a better membrane for a specific application. Solvent flux is proportional to the top layer thickness. If the support does not create a resistance to solvent flux, in most cases the thinner the top layer, the higher the flux. High fluxes result in greener separation processes as less membrane area is required and less time to achieve the desired separation. Most of the top layer fabrication techniques,†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 involve the evaporation of the solvent used in the casting solution or an interfacial reaction in an organic solvent, which is then subjected to high temperature post-treatments. Thus, the solvent used should not possess a risk to the ozone layer, should be of low toxicity, should not be flammable and should also have high flashpoint to prevent fires or explosions. Fig. 7 shows a process diagram for the fabrication of TFC membranes, including the energy use and waste generated. TFC membranes are less green than ISA membranes as two fabrication processes take place. First, the preparation of the polymeric ISA UF support membrane, and then the fabrication of the top layer.
16 involve the evaporation of the solvent used in the casting solution or an interfacial reaction in an organic solvent, which is then subjected to high temperature post-treatments. Thus, the solvent used should not possess a risk to the ozone layer, should be of low toxicity, should not be flammable and should also have high flashpoint to prevent fires or explosions. Fig. 7 shows a process diagram for the fabrication of TFC membranes, including the energy use and waste generated. TFC membranes are less green than ISA membranes as two fabrication processes take place. First, the preparation of the polymeric ISA UF support membrane, and then the fabrication of the top layer.
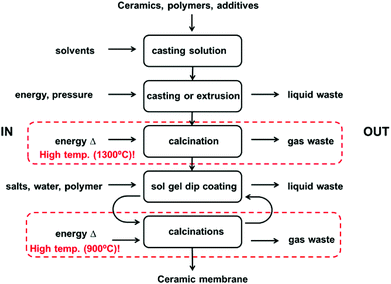
1.1.1.2 Ceramic membranes. Compared to polymers, fewer studies have been published on the green synthesis of inorganic materials, mainly because ceramics and glasses are normally obtained at high pressure and high temperature, leaving little room to improve their synthesis from a green point of view (Fig. 8). However, in the case of nanocomposite materials, the inorganic phase is formed in mild conditions via the sol–gel process, providing plenty of room to design alternative greener processes. Ceramic membranes present an asymmetric structure composed of two or more porous layers. Ceramic materials including silicium carbide and Zr-, Ti- and Al-oxides are stable in organic solvents and at high temperatures, making them excellent materials for the development of ceramic membranes for OSN applications. However, a disadvantage is that these metal alkoxides are obtained by organometallic chemistry involving the use of solvents and release of toxic by-products.17 To produce ceramic membranes within the NF range the pore size is reduced even further by applying an additional defect free layer via the sol–gel process. This process involves the hydrolysis and condensation of alkoxides or salts dissolved in water or organic solvents. Finally, the gel is dried and after controlled calcinations the NF membrane is obtained. One way to make ceramic membrane formation greener is to replace the metal alkoxides used in the sol–gel process by aqueous salts. In the case of silica these aqueous precursors consist of silicate solutions, also called ‘waterglass’. Their toxicity is low and they have low impact on the environment.17 Therefore, we believe the use of aqueous silicates as an alternative to silicon alkoxides to make the sol–gel process to form the top layer of ceramic NF membranes is a greener way to make NF ceramic membranes.
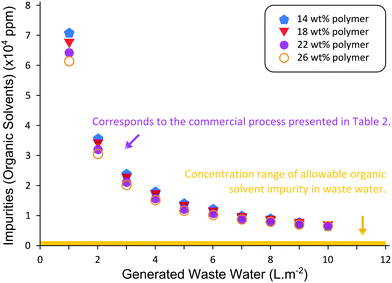
We have picked a specific concentration of the polymer in the dope solution as an example. However, concentration of the polymer in the dope usually varies between 14 and 26 wt%. In this particular example, for the preparation of a P84 polyimide membrane, 22 wt% of P84 polymer is dissolved at room temperature in a mixture of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF–1,4-dioxane, which corresponds to 58.5 wt% and 19.5 wt% of DMF and 1,4-dioxane respectively.12 Preparing the casting solution of polyimide P84 at room temperature already represents an environmental advantage compared to other polymers that have to be dissolved at high temperatures. After casting the dope solution on a non-woven fabric, the membrane is immersed in a water bath where phase inversion occurs. In our research group each bench cast membrane is made by casting 30 g of polyimide P84 dope solution on a non-woven backing material and immersed in a 20 L water bath. After phase inversion, the membrane is rinsed for 3 h in 5 L of water and later stored in isopropanol. In the large scale membrane fabrication process, 83.3 kg of polyimide P84 dope solution are cast on a non-woven backing material and immersed in a 10 m3 water bath. After phase inversion, the membrane is rinsed for 3 h in 500 L of water and later washed with IPA.
1 DMF–1,4-dioxane, which corresponds to 58.5 wt% and 19.5 wt% of DMF and 1,4-dioxane respectively.12 Preparing the casting solution of polyimide P84 at room temperature already represents an environmental advantage compared to other polymers that have to be dissolved at high temperatures. After casting the dope solution on a non-woven fabric, the membrane is immersed in a water bath where phase inversion occurs. In our research group each bench cast membrane is made by casting 30 g of polyimide P84 dope solution on a non-woven backing material and immersed in a 20 L water bath. After phase inversion, the membrane is rinsed for 3 h in 5 L of water and later stored in isopropanol. In the large scale membrane fabrication process, 83.3 kg of polyimide P84 dope solution are cast on a non-woven backing material and immersed in a 10 m3 water bath. After phase inversion, the membrane is rinsed for 3 h in 500 L of water and later washed with IPA.
As shown in Table 2, both the mass intensity and the solvent intensity of the membranes prepared at a large scale are lower, suggesting that working at larger scales is greener than fabricating membranes at a smaller scale.‡ Design of experiments is very important to reduce waste and minimize the amount of times a membrane must be prepared to be optimized and will be discussed in section 5 in more detail. Particular attention must be paid to controlling as many parameters as possible (e.g. temperature and humidity in the room, temperature of the water bath, purity of raw materials used) during membrane development to achieve repeatability. The inability to reproduce key product properties both at lab and industrial scale will invariably increase waste and require greater materials and energy use.
| Process | Dope (kg) | Membrane (kg) | P84 (kg) | DMF (kg) | Dioxane (kg) | Water (kg) | Mass intensity (MI) | Solvent intensity (SI) |
|---|---|---|---|---|---|---|---|---|
| Bench | 0.03 | 0.0066 | 0.0066 | 0.0175 | 0.0058 | 25 | 3792 | 3.54 |
| Commercial | 83.3 | 18.33 | 18.33 | 48.7 | 16.2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
550.1 | 3.54 |
Usually, polyimide P84 membranes are chemically crosslinked after membrane formation to make them solvent stable. This step involves the use of a crosslinking medium usually a diamine (e.g. 1,6-hexamethylenediamine) dissolved in a solvent (0.8 kg HDA kg−1 of dope in our research group). 1,6-Hexamethylenediamine is toxic (acute dermal and oral toxicity category 4; organ toxicity category 3) and the crosslinking medium must be disposed as liquid waste, costing £7.50 per 25 L of chlorinated or non-chlorinated solvent. If this step is carried out at room temperature, the only environmental concern will be the solvent waste generated after crosslinking, which is small (in our research group: 0.2 kg of crosslinking medium per 0.03 kg of dope at the bench scale and 7 kg of crosslinking medium per 1 kg of dope) compared to the waste water volume generated during phase inversion. It seems that the environmental problem lies in the phase inversion step.
As seen in Table 3, the solvent ppm increases at a large scale, making the waste water treatment a necessary step. DMF is particularly difficult to remove from water by evaporation due to its high boiling point. If DMF and dioxane are replaced by greener solvents, the ppm allowance would be higher and the water would not need special treatment, reducing the mass intensity considerably. There is no published work on the scale-up optimization in terms of greenness to produce ISA membranes by phase inversion, and attention should be paid when developing greener membrane preparation processes at bench scale, as at larger scale the green metrics may change.
| Process | DMF in waste water (ppm) | Dioxane in waste water (ppm) | Solvent waste treatment cost (£ kg−1 membrane) |
|---|---|---|---|
| Bench | 702 | 234 | 1136 |
| Commercial | 4870 | 1620 | 163 |
Fig. 9 shows the ppm concentration of organic solvent impurity in the water coagulation bath for different concentrations of polymer considering 83.3 kg of dope and different volumes of water in the bath. Increasing the amount of water can greatly decrease the ppm of solvent as shown in Fig. 9. However, this would only be recommended if one wants to lower the ppm to be below the acceptable threshold allowed by regulatory authorities in order not to dispose the water as chemical waste. If the solvent used has a very low ppm threshold, then one should use as little water as possible to reduce the amount of chemical waste. Alternatively water recovery could be sought. The relatively high boiling point of water makes its recovery by distillation highly energy intensive. Hence, future research should focus on high capacity adsorbents for water recovery.
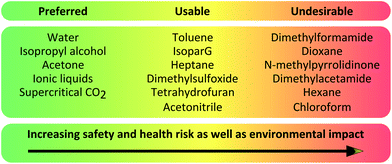
Taking into account GSK's and Pfizer's solvent guidance,18,19 we have selected those used for the membrane fabrication process and classified them in Table 1. Unfortunately, most of the solvents used for membrane fabrication fall in the red category, which are undesirable and should be avoided and replaced by solvents in the green category preferably, and if not possible by solvents in the yellow category.
UV crosslinking can reduce chemical waste considerably by removing the use of solvents and toxic chemicals present in the crosslinking medium and the washing steps required after crosslinking. Vanherck et al. recently published a review where they discuss the different methods used for crosslinking polyimides for membrane applications, including UV crosslinking.15 UV crosslinking on PI membranes was previously used to prepare membranes for the pervaporation of an acetone–cyclohexane mixture.20 Lee et al. controlled the membrane morphology and gas transport properties of PI membranes by UV crosslinking.21 They irradiated the freshly cast PI membrane with UV before immersing it in the coagulation bath and non-photoinitiator was added. In principle, UV irradiation could also be implemented for the formation of OSN membranes as a milder crosslinking alternative to chemical or thermal crosslinking.
Soroko et al.12 have successfully replaced the solvents used in the dope solution to form polyimide P84 ISA OSN membranes by an environmentally friendly solvent system without compromising membrane performance. They replaced DMF and 1,4-dioxane, which are toxic, carcinogenic and hazardous airborne pollutants18,19 with dimethyl sulfoxide (DMSO) and acetone, both of which are considered greener alternatives.18,19
In the past decade, ionic liquids have gained attention worldwide as green solvents.22,23 They are organic salts that keep their liquid states at room temperature, are thermally and chemically stable, non-volatile, non-flammable, non-toxic, and have negligible vapour pressure. However, their greenness is debatable24 as it is important to look at their entire life cycle, from how they are made all the way through to recycling and disposal. They are also expensive and better ways to recycle them need to be developed. However, ionic liquids could be used for certain new advances and still be environmentally friendly.24 Therefore, ionic liquids provide new insights into the development of greener membrane formation processes, minimizing chemical waste. In polymer science, ionic liquids are being used as the media for polymerization and also in the preparation of functional polymer materials.25 Ding Yu Xing et al.25 were the first ones to explore the use of ionic liquids as a new generation of solvents to replace traditional organic solvents for the fabrication of flat sheet and hollow fibre membranes. They used the ionic liquid 1-butyl-3-methylimidazolium thiocyanate ([BMIM]SCN) as the solvent for the formation of cellulose acetate flat sheet and hollow fibre membranes. They have also demonstrated and achieved the recovery of [BMIM]SCN from the coagulation bath by water evaporation. The recycled [BMIM]SCN was reused to prepare CA flat sheet membranes. Membranes made from recovered [BMIM]SCN showed morphological and performance characteristics similar to those made from fresh [BMIM]SCN. Their cellulose acetate membrane fabrication process is greener than the conventional process, which uses N-methyl-2-pyrrolidinone NMP, an undesirable toxic solvent.18,19 Furthermore, in their process no waste is generated as the ionic liquid is recovered by distillation and reused in the membrane fabrication process, making the process even greener and more economic. However, even though no chemical waste is produced, distillation requires high energy, compromising waste reduction. In order to evaluate whether producing chemical waste in this particular case is worse than distillation, one most look at the whole life cycle analysis of the membrane production including the recovery of ionic liquids.
Polybenzimidazole is a good candidate for OSN membranes as it has high thermal and chemical stability. However, one of the main drawbacks is its poor solubility in common solvents. PBI has limited solubility in certain solvents including, N,N-dimethylacetamide (DMAc) and DMF, both of which are toxic and hazardous to humans and the environment.18,19,26 Moreover, PBI can only be dissolved in these solvents under high temperatures27,28 (165–240 °C) and high pressures (15–100 psig), resulting in high energy consumption and environmental pollution. Recently, Ding Yu Xing et al.26 replaced DMAc, a toxic solvent used to prepare ISA PBI membranes in the casting solution, by an ionic liquid. They also suggest that the use of ionic liquids can minimize chemical waste and losses during chemical processes as they can be recycled and reused repeatedly.26 They used the ionic liquid 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc), which exhibits superior efficiency in dissolving PBI under much lower temperatures and pressures compared to DMAc. They suggest that this ionic liquid is not only ideal to dissolve PBI, but also has excellent miscibility with water so that phase inversion can occur and the ionic liquid can be leached out from the membrane, recovered by evaporating the water, and then recycled.26 Their PBI UF ISA membrane was further crosslinked with dichloro-p-xylene and successfully used for the separation of proteins. They suggest that future work will focus on utilizing ionic liquids to prepare PBI membranes for pharmaceutical separation and organic solvent recovery. Their work could definitely be implemented for the formation of greener PBI OSN membranes using ionic liquids as a greener alternative to the conventional toxic solvents.
Ionic liquids have also been employed as environmentally friendly solvents for the fabrication of membranes composed of PBI and P84 blends.29 The incorporation of P84 into the PBI system reduced the dope viscosity and water permeability was 50% higher than the plain PBI asymmetric membranes. These new membranes could also be implemented for OSN due to their outstanding chemical and thermal stabilities.
US patent 2010/0224555 A130 suggests a greener alternative for the manufacturing of RO polyamide nanocomposite membranes to avoid explosions, fires and the use of toxic solvents, such as hexane. For the interfacial polymerization step they suggest use of solvents for the organic phase that do not pose a threat to the ozone layer and yet are sufficiently safe in terms of their flashpoints and flammability to undergo routine processing without having to undertake extreme precautions. The selected organic solvent should be a high boiling point hydrocarbon, with boiling points greater than 90 °C, such as C8–C24 hydrocarbons and mixtures thereof, which have more suitable flashpoints than their C5–C7 counterparts. In their interfacial polymerization process they used Isopar G as the solvent for the organic phase (an isoparaffin based hydrocarbon oil from ExxonMobil), which is a greener alternative than hexane,18 reducing possible fires or explosions and toxicity to humans. The use of Isopar G as the organic solvent for the organic phase could also be implemented in the formation of TFC OSN membranes by interfacial polymerization to make the fabrication process safer. Ionic liquids have also been studied for the fabrication of porous materials by interfacial polymerization31,32 and could in principle be used as green solvents for the formation of OSN TFC membranes prepared by interfacial polymerization.
Replacing solvents used in the membrane casting solution by water to prepare dense TFC membranes via coating for OSN requires water soluble polymers that can become resistant in organic solvents after certain post-treatments. US patent 3992495/197633 discloses the formation of a RO TFC membrane by coating a suitable porous support with an aqueous solution of a polymer (polyethylene oxide, polyvinylamine, or polyacrylamine), the cast membrane is then exposed to a plasma to crosslink only the surface layer and remove the uncrosslinked portions by dissolving in water. Since the plasma only penetrates trough a very small thickness of the layer, it is suitable for crosslinking only the surface layer of the water-soluble polymer film to yield a water-insoluble ultrathin membrane. Their membrane exhibited up to 99% salt rejection and 100% rejection of methylene blue in water. This green membrane made from water soluble polymers and crosslinked with plasma could potentially be applied in OSN.
Polydimethylsiloxane (PDMS) membranes are widely used in OSN. However, solvents like n-hexane and n-heptane are used in large amounts during its traditional preparation process. After the polymer solution is cast on a UF support, the solvent is evaporated to form a dense membrane, causing threats to the environment and to process operators. These solvents should ideally be replaced by greener organic solvents, and replacing them with water represents an even greater challenge. A recent study34 provides a low-pollution and high efficiency preparation method using water as a solvent in the presence of surfactant (dodecylbenzene sulfonic acid) for the preparation of PDMS membranes used in the pervaporation of butanol–water mixtures. Comparisons between the PDMS membranes prepared separately with the traditional method and the green method show that the PDMS membranes prepared using the green method increased their separation performance by 30–53% with only a small loss in flux.34 Their green method is not only environmentally friendly and economically competitive but also led to enhanced pervaporation performance. This method holds enormous potential for the preparation of greener PDMS OSN membranes using water as an alternative to hexane or heptane.
Soroko et al.12 have significantly reduced the amount of isopropanol (IPA) used during the formation of OSN P84 membranes by using water as the crosslinking medium instead of IPA and by removing the isopropanol washing steps before and after membrane crosslinking. The total IPA reduction was 60% reduction, making the membrane formation process greener. In contrast to Vanherck et al.15 crosslinking was carried out in batch after phase inversion.12
Organic solvent nanofiltration requires solvent-resistant membranes that preserve their separation characteristics under a large range of solvents. In order to prepare ISA membranes, the polymer must be soluble in a solvent to form a casting solution, which means that the membrane could then solubilize in the solvent which it was cast from. Crosslinking is often used to enhance chemical stability and rejection of ISA membranes in organic solvents. Different crosslinking methods have been used for polymeric membranes, including thermal crosslinking, UV crosslinking, and chemical crosslinking. A recent review by Vanherck et al.15 discusses in detail the work that has been published on crosslinking polyimide membranes. One way to make the membrane fabrication process greener would be to use polymer materials that do not need crosslinking and avoid that extra step.
Very stable polymers that do not require further crosslinking are available. However, in terms of membrane formation they present a disadvantage as they are very hard to dissolve to form the casting solution (e.g. Torlon, PEEK). PEEK for instance, is stable in most organic solvents but prepared from a casting solution containing sulfuric and methanesulfonic acid. Using PEEK as a polymer to prepare OSN membranes avoids the crosslinking step. However, the acids used in the casting solution represent a high hazard for people working in the membrane fabrication process. In terms of environmental impact, the acidic aqueous solution left after phase immersion should not be a problem if neutralized prior to disposal.
A greener alternative for membrane formation would be to use cellulose as raw material, instead of using one of its derivative forms. Membranes prepared using cellulose as raw material will keep the cellulose native characteristics, including remarkable hydrophilic properties and good solvent resistance, making them potential membranes for OSN applications. To use cellulose as raw material, a simple high temperature dissolution process to prepare the dope solution can be carried out, using aqueous N-methylmorpholine-N-oxide (NMMO) as the solvent. NMMO can dissolve cellulose directly without the formation of the cellulose complex or its derivatives, avoiding the use of chemicals for its degradation, making the membrane formation process environmentally friendly.36 The use of NMMO as the new organic solvent for cellulose has opened up new perspectives for cellulose membrane development and shows potential for OSN.
Cellulose membranes for water applications were successfully prepared through a simple and environmentally friendly process by dissolving cellulose in an aqueous NMMO solution.36 Zhang et al.36 have studied the influence of different parameters on the formation and characterization of cellulose flat sheet membranes. H. J. Li et al.35 have developed a hydrophilic cellulose hollow fibre UF membrane for oil–water separation by casting a membrane from a dope solution containing cellulose from wood pulp, NMMO as the organic solvent, and polyethylene glycol 400 as an additive. Treatment of the oily water with their UF cellulose membrane was feasible, showing resistance in a wide range of PH.
Mao et al.37 developed a novel cellulose membrane used for isopropanol dehydration. The membrane was prepared using NMMO as the solvent for the casting solution. They showed that the prepared cellulose membrane had much higher crystallization degree and better mechanical strength compared to traditional cellulose acetate membranes. Their membrane exhibited acceptable fluxes of 349 g m−2 h−1 and much higher separation factors than other pure polymer membranes, such as chitosan and poly(vinyl alcohol).
As cellulose is difficult to dissolve in common organic solvents, to make full use of cellulose resources, it is necessary to develop a cellulose dissolution method to develop cellulose membranes ideally using non-toxic solvents.38 The use of ionic liquids has opened new avenues for the efficient utilization of lignocellulosic materials.39 Li et al.38 established an environmentally friendly method to prepare NF cellulose membranes with a MWCO§ of 700 Da and high water flux, using the ionic liquid 1-allyl-3-methylimidazolium chloride (AMIMCl) as the solvent. The cellulose was completely dissolved at 90 °C. Their work is the first reported study on the formation of NF membranes from a cellulose/ionic liquid dope solution. These NF cellulose membranes could in principle be also applied for OSN.
Another ionic liquid has been used as solvent for the development of cellulose membranes by phase inversion. H. Z. Chen et al.38 have prepared a cellulose membrane dissolving wheat straw cellulose in ionic liquid 1-butyl-2-methylimidazolium chloride [BMIM]Cl to form the casting solution. Because ionic liquids have extremely low vapour pressure, they can be recovered by distilling to remove precipitators under reduced pressure to reduce cost and avoid chemical waste generation. However, distillation requires a lot of energy and this should be considered when assessing the greenness of the overall process. After the cellulose membrane was prepared, the residual [BMIM]Cl in the coagulation bath was recovered by vacuum distillation to remove water, and subsequently dried for 24 h in a vacuum drying oven. The recovery ratio was 95.2% and the recovered ionic liquid was recycled to prepare other cellulose membranes. Cellulose membranes prepared using ionic liquids as the solvent have great potential for OSN applications.
2. The role of OSN in downstream processing
Downstream process development is one of the most important stages of Active Pharmaceutical Ingredient (API) manufacturing due to its crucial effect both on the lifecycle and performance of the final manufacturing process.40 Moreover, downstream processing is the major contributor to the production costs of APIs.41 Conventional API purification steps include recrystallization, precipitation, solvent extraction, distillation, chromatography, adsorbents and resins. The pharmaceutical industry has been pioneering in process development with regard to (i) the optimization of API purification42 and (ii) the introduction of green metrics43–46 in order to stimulate process effectiveness minimizing environmental impacts.47 Membrane technologies are listed among the technologies expected to contribute to the reduction of environmental impact.48 Besides solvent recovery OSN has also been repeatedly proposed for API purification by both academic and industrial research groups.7,8,49–51A comparative study of OSN, chromatography and recrystallization demonstrated the performance efficiency and sustainability of these downstream processes through an API purification case study (Fig. 10).52 API losses were 5–6% for OSN, 6–12% for chromatography and 15–16% for recrystallization during the removal of 4-dimethylaminopyridine (DMAP) and methyl mesylate (MeMS) impurities, respectively. In terms of generating solid waste the processes can be ranked as chromatography (330 kg silica gel per API batch), recrystallization (6 kg charcoal per API batch) and OSN (<1 kg membrane per API batch). The study shows that – considering process performance, solid waste generation, energy consumption and batch time – OSN is highly competitive to conventional chromatography and recrystallization (Fig. 10). Furthermore, the advantage of size-exclusion based OSN over affinity based separation techniques (e.g. adsorbents) was demonstrated by the simultaneous removal of two impurities, N,N-dimethylaminopyridine and p-toluenesulfonic acid ethyl ester, representing different chemical classes, namely amine and ester.53 The removal of these impurities solely by adsorption would require two chemically different adsorbents, and API matrix effects should be investigated.54
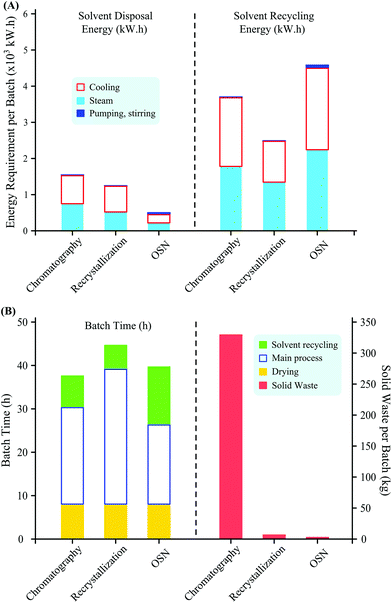 | ||
| Fig. 10 Processes comparison concerning (A) energy consumption with solvent disposal or solvent recovery as well as (B) batch operation times and solid waste generated. | ||
Despite high competitiveness of OSN demonstrated by Szekely et al.,50 two main drawbacks were identified in many reports: high solvent consumption and low product yield. Solvent consumption and recovery are discussed in details in section 3. The second, but not secondary, limiting factor of OSN, namely low product yield or insufficient rejection, has been addressed by means of process chemistry and process engineering. For instance, even with a seemingly high rejection of 98%, the product yield can be unacceptably low after diafiltration. In order to achieve a sustainable separation, Keraani et al.,55 gradually enlarged homogeneous catalysts for olefin metathesis from 627 to 2195 g mol−1. Up to five cycles were performed before a deterioration in the performance of the process was observed with the Hoveyda II type catalyst in toluene and dimethyl carbonate employing StarMem228. It is worth mentioning that the decrease in performance was due to the slow degradation of the catalyst and not because of a reduction in catalyst rejection. Similarly, Siew et al.56 developed a highly enantioselective quinidine-based organocatalyst for homogeneous catalyst recycling through OSN. Enlarging the organocatalysts through polyalkylation allowed easier recycling as well as higher enantioselectivities of >92% ee. However, permanent chemical modification of the desired product in order to obtain sufficient rejection is rarely acceptable. Hence, in other cases temporary chemical modification was used. So et al.57 developed a new technology platform that advantageously combines OSN with solution phase peptide synthesis where polyethylene glycol is used as an anchor to increase the rejection of the products. Liquid-phase synthesis on polyethylene glycol has been extensively investigated in the past and successfully applied in the field of oligonucleotides and oligosaccharides production as well as for combinatorial library synthesis.58,59 More recently Kim et al.53 proposed an efficient purification methodology employing a simplified two-stage cascade configuration for diafiltration which significantly increases product yield (Fig. 11). A mathematical model to predict the cascade performance was developed.60 The membrane cascade process overcomes previous control problems by operating with a single high-pressure pump and without any buffer tank between membrane stages (Fig. 12). The process was demonstrated through the removal of genotoxic impurities from an API. By implementing the two-stage cascade, the product yield was increased from 58% to 95% while maintaining less than 5 ppm genotoxin in the final solution. Through this yield enhancement, the membrane process has been “revamped” from an unfeasible process to a highly competitive unit operation when compared to other traditional processes. In general, the two-stage diafiltration configuration achieves significant yield improvement, overcoming the membrane limitations inherent in the single stage diafiltration for rejections less than 99%. As long as the product rejection is above 90%, the process promises high product yield, making the process competitive to other traditional unit operations.
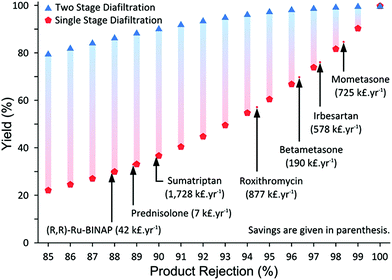 | ||
| Fig. 11 Predicted yield improvement with 2-stage cascade after 10 diavolumes for different product rejection. Annual cost savings are given in brackets, based on literature rejection data50,53,61 considering 100 kg annual API production. | ||
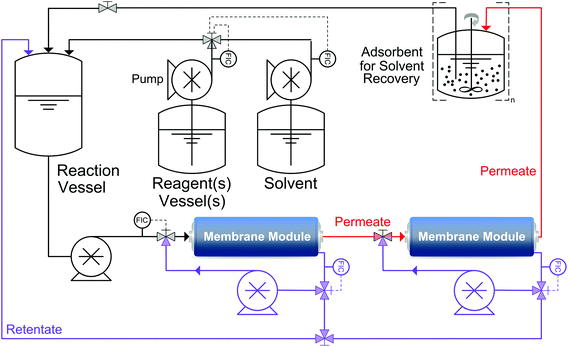 | ||
| Fig. 12 Schematic system diagram for a two-stage cascade diafiltration: the permeate from the first stage is directly connected to the feed of the second stage. | ||
Furthermore, process intensification using OSN can be achieved through the concept of hybrid processes. OSN has been synergistically coupled with distillation,9 chromatography,10 adsorbents7 and molecular imprinting,8 complementing each other to achieve a defined separation task. As an example, membrane distillation integrates the advantages of distillation (e.g. robustness and high capacity) with the advantages of membrane separations (e.g. high selectivity and separations that are able to surpass the limitations of distillation).62 In addition to integrating membrane distillations into new processes, they can also be used for flexible capacity increases due to their modular nature; they can easily be scaled-up and integrated into existing processes.63 One example of OSN-assisted distillation is the recycling of homogenous Rh catalysts during hydroformylation of octene and dodecene (Fig. 13) by Priske et al.64
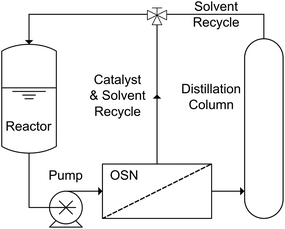 | ||
| Fig. 13 Schematic system diagram for an OSN-assisted distillation process for the separation of homogenous catalysts and recycling of solvent. | ||
Furthermore, the separation of heavy boiler (hexacosane, 5%) from low- and middle boiler (decane, 70% and dodecanal 25%) in a wide boiling mixture from hydroformylation (Fig. 14) was investigated by Micovic et al.9 Micovic et al.9 elaborated a four step design method for combination of organic solvent nanofiltration and distillation in a hybrid separation of wide boiling mixtures. The method is based on: generation of different processes, evaluation based on quantitative metrics using rigorous models, identification of the best membrane and process optimisation. Experimental effort was reduced by specifying parameters that have strong influence on the process and by narrowing down the experimental range. Optimization showed that at high temperatures, the OSN-process may be more economical than stand-alone distillation.
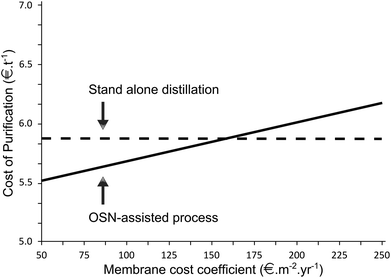 | ||
| Fig. 14 Economic process performance is evaluated in form of the cost for the purification of one ton of product. The reaction mixture (4500 [kg h−1]) consists of n-decane (solvent with MW of 142 g mol−1), n-dodecanal (product with MW of 198 g mol−1) and hexacosane (reference for heavy boiler with MW of 367 g mol−1) was investigated.9 | ||
Functionalised OSN membranes allow adsorption of specific molecules whilst retaining and permeating other species. Three-way separation of ternary mixtures using a single OSN membrane has been recently demonstrated using molecularly imprinted OSN membranes.65 This approach has the potential to increase the sustainability of downstream processing by integrating size exclusion and adsorption separation in a single unit operation.
3. Solvent recovery: an enabling technology
3.1 The need for solvent recovery and potential solutions
The pharmaceutical and fine chemical industries produce the majority of their products utilizing batch processes, which contain multiple reaction and purification steps. Most active APIs are produced carrying out reactions in organic solvents. Chemical reactions often require large quantities of different organic solvents. Furthermore, organic solvents are used to perform purifications, to clean process equipment and for different analytical instruments employed for process control and quality assurance. Both the type of solvent and the amounts required can vary widely, depending on the chemical reactions performed and the physico-chemical properties of the reactants and products.66 For a typical batch chemical process in the pharmaceutical industry, solvent use (including water) can account for as much as 80–90% of the total mass in the process.66 The consequence of this is that solvents make a major contribution to both the overall economy and toxicity potential associated with the process.Although the pharmaceutical industry has been known to be relatively solvent intensive for a long time, it is only over the last decade that public and industry demand for more sustainable processing has resulted in an increased interest to reduce solvent usage.10,52,67–69 In 2007, the ACS GCI Pharmaceutical Roundtable70 (ACS GCIPR) collected mass-efficiency data from seven pharmaceutical companies to illustrate typical materials and quantities used during the development and manufacture of API. Data indicated that for the production of 1 kg of commercially available API a median value of 45 kg of material was used (ACS website).70 Approximately 50% of that material and 60% of the overall energy used during API production was reported to be related to organic solvents. This indicates that solvent recovery could be of interest for improving energy-efficiency.67
The waste generated by pharmaceutical companies and the need to dispose of large amounts of solvent at the end of the processes has increased concerns about environmental and human safety. This has led to the implementation of many regulations and has created a widespread interest in Green Chemistry and Technology.66 Several metrics have been proposed to help assess the efficiency and “greenness” of existing and new processes such as the E factor, mass yield, atom economy, mass productivity and reaction mass efficiency.
When trying to establish what is “green”, another important aspect is the life cycle of a solvent, which includes production, transportation, use and disposal. Each kg of solvent that is not recycled or reused must in fact be disposed. This means that the solvent has to be manufactured. This process also generates waste and greenhouse gas emissions, and adds to the cumulative annual waste generation worldwide.66 A Life Cycle Inventory/Assessment (LCI/A) is used to determine the overall amounts of materials used, waste generated, and energy used during the manufacture of solvents, their use in pharmaceutical processes, and their eventual disposal. Fig. 15 identifies the major stages in a solvent's life cycle: production, transport, use, and disposal.
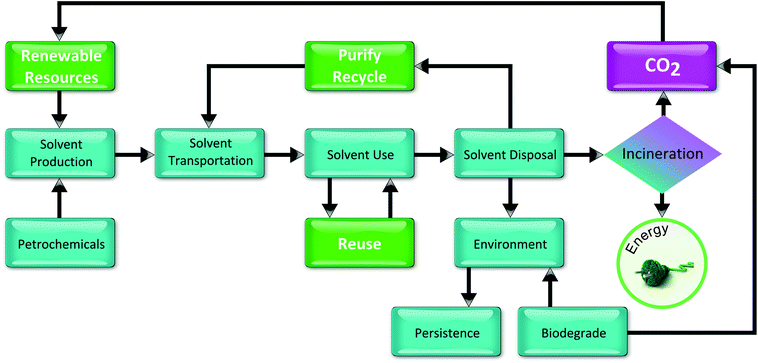 | ||
| Fig. 15 Life cycle flow chart for solvent usage adapted from Slater et al.66 | ||
Excessive solvent use is a major contributor to a chemical company's “carbon footprint”. The disposal of excessive solvent waste then further contributes to the release of greenhouse gases and other emissions. It has been estimated that incineration alone creates 6.7 kg CO2 kg−1 organic carbon treated.66 The most common waste disposal method in the chemical industry today is incineration. Recent introduction of stricter environmental legislation in combination with increasing pressure from regulatory agencies and expected price increases of virgin solvents is making solvent recovery a more competitive alternative to incineration. Solvent recovery can offer significant benefits with regards to reduced purchase, storage and waste costs, increased compliance with environmental legislation and reduced emission of greenhouse gases.
High volume recovery processes typically use distillation, which is a thermally driven process and though generating high purity solvent, the unit operation generally requires high energy input. Furthermore, it can be difficult and energy intensive, when it is required to separate solvent mixtures due to the closeness of boiling points and the formation of azeotropes. Nevertheless, from an industrial standpoint, distillation is the most commonly employed method for solvent recovery in the pharmaceutical industry and it is used for approximately 95% of all solvent separation processes.71 Batch adsorption on activated carbon (or other more efficient adsorbers) and fixed bed adsorbers are also largely used for impurity removal and solvent recovery.72 Recently, OSN has been proposed as a low energy alternative for solvent recovery or in combination with distillation in hybrid processes. One of the main drivers for using OSN as an alternative to distillation for solvent recovery is potential improvements to the overall energy-efficiency. Furthermore, solvent recycling distillation systems – due to their wide variety including atmospheric, vacuum, steam, azeotropic, extractive, pressure and membrane distillation – often have to be used off-site. However, solvent recycling by OSN can be easily implemented as a final stage of a downstream processing membrane cascade. On the other hand, the major drawback is related to the small size of impurities to be retained. Small size impurities are advantageous for purification by OSN but turn solvent recovery by OSN problematic. To purify solvents from impurities of small molecular size, virtually 100% rejection is required calling for tight membranes, which are generally characterised by low flux. Operating time depends on membrane permeance and filtration area, which makes modules more advantageous than flat sheet membranes at an industrial scale. Applications of solvent recovery by OSN in the pharmaceutical and in the oil industry are summarized in Table 4 and discussed in the following paragraphs.
| Industry | Solute/solvent | Membrane | Reference |
|---|---|---|---|
| Pharma | APIs/methanol | Starmem™120, Starmem™122, Puramem™228 | Geens et al.73 |
| Pharma | APIs/IPAc | Starmem™122, Starmem™240, Puramem™280 | Rundquist et al.68 |
| Pharma | APIs/crystallisation mother liquor (82% methanol, 15.9% methyl isobutyl ketone, 2.1% toluene) and ethyl acetate | Starmem™122 | Rundquist et al.10 |
| Pharma | APIs/methanol and DCM | GMT-oNF-2 | Szekely et al.52 |
| Pharma | Peptide/MeCN and water | Inopor Nano 450 and 750 | Marchetti et al.74 |
| Refining | Waxy oil stream/toluene and methyl ethyl ketone | Starmem 228 | White et al.75 Gould et al.76 |
| Refining | Free fatty acids/methanol | Nitto Denko, Film-Tec, Osmonics, Desalination Systems, Fluid Systems | Raman et al.77 |
| Refining | Oil/n-hexane | PDMS/PAN | Stafie et al.78 |
| Refining | Cotton oil/n-hexane, IPA, ethanol | Romicon, Osmonic, Paterson Candy International | Koseoglu et al.79 |
| Refining | Soybean oil/n-hexane | Whatman (ceramic membrane) | Wu and Lee80 |
| Refining | Free fatty acids/ soybean oil and n-hexane | Osmonics | Ribeiro et al.4 |
| Refining | Corn oil/ethanol | Koch, FilmTec, Osmonic-Desal, Hydranautics | Kwiatkowski et al.81 |
| Refining | Ethanol, isopropanol, acetone, cyclohexane, hexane | GE Osmonic, Nadir, Evonik MET and Solsep | Darvishmanesh et al.82 |
| Refining | Vegetable oils/hexane | NTGS-2200 | Manjula et al.83 |
| Refining | Soybean oil/hexane | PDMS/PVDF and Zeolite PDMS/PVDF | Weibin et al.84 |
| Refining | Free fatty acids/soybean oil and hexane | PDMS/PVDF and CA/PVDF | Firman et al.85 |
| Refining | Free fatty acids/soybean oil and hexane | Nitto Denko, Film-Tec, Osmonics, Desalination Systems, Fluid Systems | Raman et al.86 |
| Refining | Heavy boilers from a hydroformylation mixture | Puramem S 380, GMT ONF 1, GMT ONF 2, GMT NC 1 | Micovic et al.9 |
3.2. Solvent recovery by OSN in the pharmaceutical industry
Geens et al.73 evaluated the potential of nanofiltration as a separation tool in the chemical production process of APIs and compared the energy requirements for methanol recovery with OSN and distillation, respectively. The comparison for the energy consumption of the two processes was made based on a pressure pump for the nanofiltration system, and a total reboiler for the distillation.The energy requirement of the pressure pump required to provide a pressure difference ΔP for a feed flow Ff is given by eqn (1):
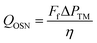 | (1) |
The energy required in a throughput distillation was calculated as the sum of three contributions, heating the fluid to the boiling point, and evaporation of the liquid at the boiling point, respectively:
| Qdistillation = Qheating + Qvaporisation + Qcondensation | (2) |
| Qheating = FmCΔT | (3) |
| Qvaporisation = Qcondensation = FmΔH | (4) |
As schematically shown in Fig. 16, for recovering 451 tons of methanol by distillation (which corresponds to a process time of 1371 h at a feed flow of 417 l h−1), the total energy consumption is 1123 GJ Geens et al. calculated 593 GJ, as they did not included the heat of condensation), whereas the nanofiltration set-up requires only 2.8 GJ (for an operating pressure of 15 bar), which is more than 200 times less. It is indeed clear that a membrane process is advantageous in terms of energy consumption, and might become a lucrative alternative for a traditional distillation.
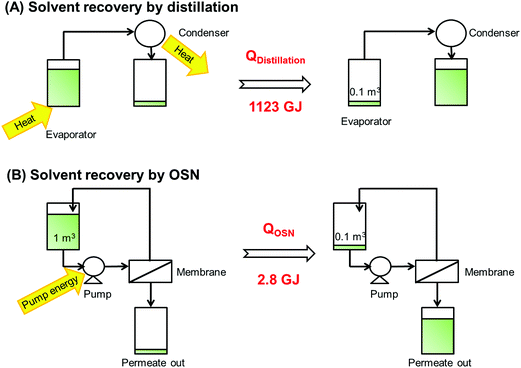 | ||
| Fig. 16 Schematic representation of solvent recovery by distillation and OSN and relative energy consumptions. | ||
Slater66 tabulated the top 20 chemical wastes generated by the pharmaceutical and medicinal/botanical sectors according to the United States EPA TRI from 1995 and 2006. For some of these solvents, the energy required to recover solvent by distillation and by OSN have been compared. They are shown in Fig. 17 and reported in Table 5, from which it is clear that the energy requirements become higher for distillation as a function of the boiling point, while they remain more or less constant for OSN.
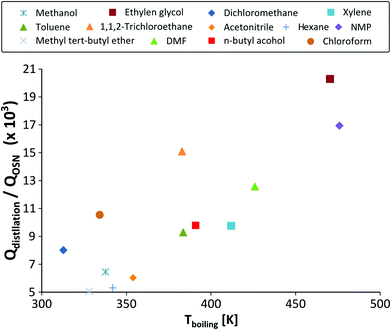 | ||
| Fig. 17 Comparison of the energy requirements for solvent recovery by distillation vs. solvent recovery by OSN, as a function of the solvent boiling point. T = 25 °C. P = 15 bar. | ||
| Solvent | Rank66 | Solvent generated [106 kg per year] | Q distillation [kWh] | Q OSN [kWh] | Q distllation/QOSN | CO2 footprint [106 kg per year] |
|---|---|---|---|---|---|---|
| Methanol | 1 | 44.8 | 150 | 0.023 | 6453 | 18 |
| Dichloromethane | 2 | 22.3 | 111 | 0.014 | 8010 | 3 |
| Toluene | 3 | 12.1 | 197 | 0.021 | 9278 | 12 |
| Acetonitrile | 4 | 7.9 | 141 | 0.023 | 6029 | 3 |
| Chloroform | 7 | 3.71 | 131 | 0.012 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543 543 |
0.4 |
| n-Hexane | 8 | 2.99 | 149 | 0.028 | 5300 | 3 |
| n-Butyl alcohol | 9 | 2.86 | 223 | 0.023 | 9788 | 2 |
| DMF | 10 | 2.79 | 244 | 0.019 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 569 |
2 |
| N-Methyl-2-pyrrolidone | 12 | 2.02 | 303 | 0.018 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
1 |
| Xylene | 13 | 1.47 | 208 | 0.021 | 9748 | 1 |
| 1,1,2-Trichloroethane | 15 | 1.23 | 194 | 0.013 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.2 |
| Methyl tert-butyl ether | 16 | 1.2 | 126 | 0.025 | 5062 | 1 |
| Ethylene glycol | 18 | 0.82 | 337 | 0.017 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
0.3 |
Rundquist et al.68 demonstrated the advantages of OSN to recover API from mother liquors after a crystallization process. The process flow diagram of API recrystallization with solvent recovery and recycle is shown in Fig. 18.
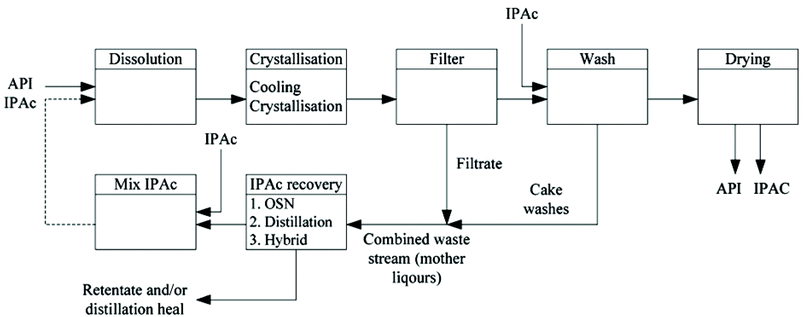 | ||
| Fig. 18 Process diagram of an API recrystallization combined with solvent recovery and recycle (source: reprinted from ref. 68. Copyright (2012), with permission from The Royal Society of Chemistry). | ||
Crystallisation is an important and commonly used operation in API purification; it can however generate large volumes of solute rich waste (mother liquors) containing the impurities removed during operation as well as dissolved API up to the saturation limit. API containing mother liquors are generally discarded as waste and, depending on the API, solubility yield losses can be significant. Further processing of mother liquors could be a lucrative way to boost mass-efficiency of a process through recovery of organic solvent as well as potential recovery of valuable API.68 In this work, energy comparisons between a pump-pressurised OSN system and a pilot-scale distillation unit were extended to include high concentration, multi-solute process liquors. For a batch distillation the overall power consumption was calculated through summation of individual contributions required to heat the liquid to the boiling point, vaporise the solvent and re-condensate in the overhead condenser.
Based on data from the distillation process used for isopropyl acetate, calculations assume a 90% recovery level and an overall processing time of 2 h for a 100 L batch. Assuming an ideal system where no heat is lost to the surroundings and a constant heat of vaporisation, the total power required for processing a 100 L batch was calculated using eqn (2) with Fm = 0.11 mol s−1, C = 199.4 J mol−1 K−1, ΔT = 64 K and ΔH = 37.2 kJ mol−1 giving a value of QDistillation = 9.3 kW for a 2 h operating time.
On a larger scale, OSN systems are commonly set up to operate in a feed-and-bleed configuration. Energy required for OSN processing can be calculated using eqn (5) where F is the pump flow rate of the feed (f) and recirculation (r), ΔP is transmembrane pressure (TM) and the pressure drop over the module (D) and η is the pump efficiency:
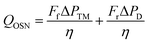 | (5) |
For OSN processing the maximum recovery level was based on solubility and for the OSN process presented by the authors the maximum volume recovery level was 80%. A membrane area of 1 m2 was assumed for calculations. The overall processing time for processing 80% of a 100 L batch equalled 1.8 h, assuming an average flux of 45 L m−2 h−1. Based on processing time, Ff = 56 L h−1 and Fr was selected to 278 L h−1 (5 × Ff), ΔPTM = 60 bar, ΔPD = 0.5 bar and η = 0.3 resulting in a total energy requirement of QOSN = 0.3 kW for a 1.8 h operating time. The overall energy consumption was 32 times lower for OSN compared to distillation.
However, for OSN the maximum amount of solvent recovered was limited by solubility and required that the concentration remain above the solubility limit to prevent solutes from precipitating. As reported by the authors, the maximum amount of solvent recovered by OSN was limited to 80% whereas the distillation could be continued until 90% of the original volume was recovered. An equivalent volume recovery of 90% could be reached by using OSN to recover 80% of the original solvent added, and distillation to recover an additional 10% up to a 90% total. In this case, the energy requirement would still be 9 times lower per L of recovered solvent compared to the use of distillation alone.
To fully evaluate the impact of waste minimisation on the process mass-efficiency, the full crystallisation process including potential solvent recovery stages was studied.68 For a crystallisation batch size generating a 100 L waste stream, the E factor¶ for the full crystallisation process was equal to 9.7 for no solvent recovery and 4.8 and 4.2 when OSN and distillation were used for solvent recovery respectively. Comparison of E factor values for the full crystallisation process indicated comparative values for OSN and distillation and methods offer similar mass-efficiency improvements of 43–49% compared to when no solvent recovery is used.
This study finally points out to the importance of considering potential improvements in waste reduction and energy-efficiency, capital investment cost and calculated payback period for membrane equipment when comparing distillation and OSN. Distillation equipment is usually readily available in most batch pharmaceutical plants, while a strong business case is needed to justify the initial investment required for OSN operation.68 Additionally, the membrane used is a consumable product and membrane modules have to be replaced on a regular basis, depending on the membrane lifetime. Membrane replacement can increase investment as well as maintenance cost for OSN operation.
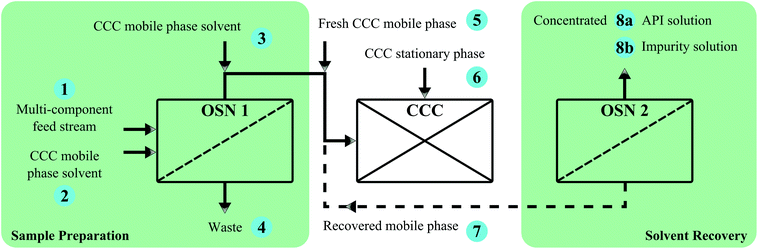
In another study, Rundquist et al.10 demonstrated the potential for using OSN to perform an initial solvent exchange for a crystallization mother liquor, supplied by GlaxoSmithKline (GSK), into a selected counter-current chromatography (CCC) mobile phase system for recovery of a GSK drug component from a complex impurity profile. The solvent exchange transferring the solute matrix to be separated from the process solvent to the solvent mixture selected for the mobile phase is usually required to avoid solvent contamination of the column. Thermal techniques, such as evaporation, commonly used for this purpose, can be time consuming, energy intensive and in certain cases cause product degradation. High mass intensity values can be seen as an additional limitation of CCC application. Rundquist et al.10 demonstrated how membranes are capable of reducing the typically high solvent burden of the CCC process through recovery and recycle of mobile phase solvent (Fig. 19). Eqn (2) was used to calculate the solvent mass-intensity for CCC operation with and without solvent recovery and OSN solvent exchange respectively. When combining CCC operation with solvent recovery the solvent mass-intensity is calculated to decrease by 60% for a solvent recovery level of 70%.
Siew et al.87 proposed a multistage membrane cascade for solvent recovery. Most commercial membranes do not retain APIs sufficiently to enable solvent recovery in a single stage membrane process. The 3-stage cascade was able to achieve an effective rejection of 80% compared to a single pass rejection of 55%.
Kim et al.53 exploited the high impurity uptake of activated charcoal at low concentrations for solvent recovery coupled to a two-stage OSN membrane cascade, as the permeate from the system mostly contains only impurities with negligible amount of API. Both the economic and environmental impacts of a solvent recovery unit depend on the solvent type as well as the recovery process itself (e.g. adsorption or distillation).18 Hence, the differences between distillation and an adsorption unit in terms of energy consumption and CO2 generation considering solvents used widely in the pharmaceutical industry was assessed by Kim et al.53 It was concluded that the energy required for the charcoal based adsorption process is 92–96% lower than that from distillation. Furthermore, the implementation of a solvent recovery unit achieved 70% and 73% reduction in mass intensity and solvent intensity, respectively. The associated carbon footprints are shown in Table 5.
Darvishmanesh et al.88 tested two types of commercially available polymeric OSN membranes (StarMem122 and DuraMem150) for their abilities to recover methanol, ethanol, isopropyl alcohol and ethyl acetate solvents from API mixtures. Furthermore, to evaluate the potential of OSN as a substitution for traditional solvent recovery, a pore flow mechanism based model was developed for single OSN membrane modules and implemented in the common Aspen Plus process simulation software.
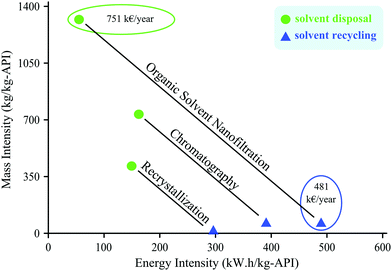
Szekely et al.52 discussed the effect of solvent recycling on the sustainability of three purification processes for degenotoxification of API post reaction streams: recrystallization, flash chromatography and OSN. In this particular case study 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 kg methylene chloride solvent was needed for the purification by OSN which is 2 and 4 times more than by chromatography and recrystallization, respectively.52 Hence, OSN has the highest mass intensity being 1312 kg kg−1 API. Fig. 20 compares the mass and energy intensity of the processes which change in accordance with the extent of solvent consumption. Recovering the solvent by distillation turns OSN from the least to the most energy intensive process with values of 55 and 491 kWh kg−1 API, respectively. On the other hand, the cost of solvent recovery was estimated to be 481 k€ per year allowing 36% cost reduction relative to solvent disposal. Solvent recycling allowed the reduction of mass intensity by two orders of magnitude (from 400–1300 to 14–63 kg kg−1 API, depending on the process) and narrowed down the process carbon intensity to the range of 100–200 kg CO2 kg−1 API. Furthermore, a significantly smaller amount of fresh solvent has to be purchased for the purification. Solvent recovery was demonstrated to be advantageous for OSN, as OSN requires the use of large diafiltration volumes, therefore its high performance is achieved at the cost of high solvent usage. Solvent recovery by other tools such as adsorbents53 and OSN itself68 were also assessed. The main obstacle to the use of OSN for solvent recovery lies in the fact that impurities in the permeate from OSN downstream processing by OSN should be fully rejected by nanofiltration membranes. Hence, even tighter membranes have to be developed for solvent recovery by OSN. Through a modelling study Abejón et al.89 also found that the treatment of the residual stream leaving the system is the major contributor to the overall cost of the process being more than 85% for dual membrane cascades, but the solvent recovery units can reduce the costs up to 77% depending on the required solvent quality.
200 kg methylene chloride solvent was needed for the purification by OSN which is 2 and 4 times more than by chromatography and recrystallization, respectively.52 Hence, OSN has the highest mass intensity being 1312 kg kg−1 API. Fig. 20 compares the mass and energy intensity of the processes which change in accordance with the extent of solvent consumption. Recovering the solvent by distillation turns OSN from the least to the most energy intensive process with values of 55 and 491 kWh kg−1 API, respectively. On the other hand, the cost of solvent recovery was estimated to be 481 k€ per year allowing 36% cost reduction relative to solvent disposal. Solvent recycling allowed the reduction of mass intensity by two orders of magnitude (from 400–1300 to 14–63 kg kg−1 API, depending on the process) and narrowed down the process carbon intensity to the range of 100–200 kg CO2 kg−1 API. Furthermore, a significantly smaller amount of fresh solvent has to be purchased for the purification. Solvent recovery was demonstrated to be advantageous for OSN, as OSN requires the use of large diafiltration volumes, therefore its high performance is achieved at the cost of high solvent usage. Solvent recovery by other tools such as adsorbents53 and OSN itself68 were also assessed. The main obstacle to the use of OSN for solvent recovery lies in the fact that impurities in the permeate from OSN downstream processing by OSN should be fully rejected by nanofiltration membranes. Hence, even tighter membranes have to be developed for solvent recovery by OSN. Through a modelling study Abejón et al.89 also found that the treatment of the residual stream leaving the system is the major contributor to the overall cost of the process being more than 85% for dual membrane cascades, but the solvent recovery units can reduce the costs up to 77% depending on the required solvent quality.
Marchetti et al.74 proposed an improved purification strategy for peptide fragment condensation, named Peptide Reactive Nanofiltration. This strategy is based on the incorporation of nanofiltration units into the reaction step, the separation of small side products from the reaction mixture, the solvent recycle after the nanofiltration unit, and the elimination of time-consuming steps, typical of the conventional strategy. Lower solvent consumption was achieved, thanks to the integration of membrane technology into the reaction step, and the recycle of the permeate to the reactor, after removal of the small side products permeated through the membrane. Evident advantages from technological, economic and environmental point of view were demonstrated by shorter production time and lower solvent consumption. Solvent costs were reported to be 10% and total costs 50% compared to the costs of the conventional strategy.
3.3 Solvent recovery by OSN in the refining industry
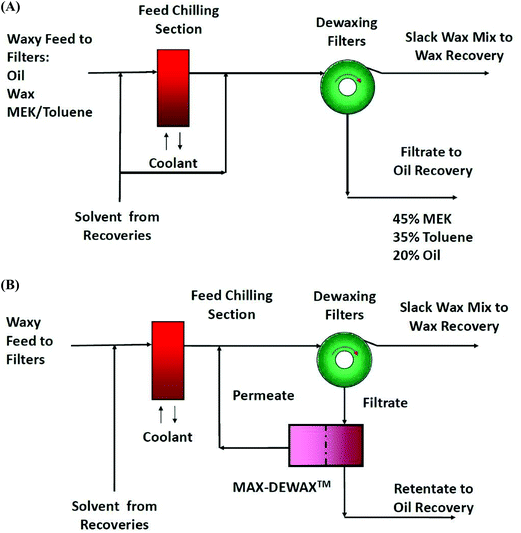
Apart from pharmaceuticals, increasing demand for more sustainable processing has led to the development of membrane-based chilled solvent recovery from lube oil filtrates75,90 and edible oil.82Solvent lube oil dewaxing processes are applied worldwide in refineries where a waxy oil stream is mixed with apolar solvents, such as toluene and methyl ethyl ketone. The wax component is precipitated by cooling the system followed by the filtration of the wax. Conventionally, the dewaxed solvent mixture is submitted to multi-stage flash and distillation operations in order to recover the solvent which has to be cooled prior to recycling in the dewaxing process (Fig. 21A).75
In order to reduce the energy consumption of the recovery unit, different kinds of OSN membranes and processes have been developed.75 The MAX-DEWAX process at a refinery in Beaumont, TX, shown in Fig. 21B, is a large-scale OSN application for solvent dewaxing.75
The chilled dewaxing process uses a waxy feed dissolved in methyl ethyl ketone (MEK) and toluene to precipitate the wax components. Filtration of the chilled wax leaves lube oil dissolved in MEK and toluene. A membrane process can recover the solvents for recycle to the wax precipitation step. Lube oils have a molecular weight in the range of 300 g mol−1 and higher, while the solvents are at 84 and 96 g mol−1. Therefore this can be considered as mostly a sized based separation suitable for OSN. Economic drivers for the installed membrane system include reduced energy consumption (20% per unit volume of product), increased yield of lube and wax products from a barrel of oil (3–5%), and increased product quality, while the overall solvent consumption is still kept low. The membrane-based solvent recovery unit of ExxonMobil's refinery allows the annual recovery of 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 of clean solvent in the lube processing area.76
000 m3 of clean solvent in the lube processing area.76
Crude edible oils need to be refined to obtain the properties required for their consumption.82 The extraction stage of edible oil in the oil industry is commonly performed by using toxic solvents (e.g. hexane) and processes with high energy consumption (e.g. distillation, evaporation) to recover the solvent, which represents around 70–75 wt% in the oil–solvent mixture.82 The conventional processes for solvent recovery feature a series of distillation columns, evaporators, stripping columns and condensers, which consume around 50% of the total energy in the edible oil processing. In addition, significant losses of solvent (via vapours) are typically produced even in efficient plants,82 with additional consequences for air pollution. Thus, the challenge in solvent extraction is to recover as much oil as possible while minimizing solvent losses.
Several studies focus on the development of new oil purification, deacidification, discolouration and solvent recovery methods. Raman et al.77 applied a commercial available NF membrane to recover n-hexane, observing that membrane technology can reduce 50% of the consumption energy compared to an evaporation unit. Stafie et al.78 achieved over 90% oil rejections in n-hexane with a hydrophobic polydimethylsiloxane (PDMS) membrane. The use of different commercial RO/NF membranes for separating cotton oil from hexane, ethanol, and isopropanol solvents was reported by Koseoglu et al.79 They showed that only the poly-amide material permeated hexane without being destroyed. Wu and Lee80 investigated hexane removal from a crude soybean oil–hexane mixture by using porous UF membranes in a continuous or discontinuous way and it allows combination with other processes. Ribeiro et al.4 investigated solvent recovery from soybean oil–hexane miscellas at bench-scale using flat sheet polymeric commercially available membranes made from polysulfone and polysulfone/polyamide under different operational conditions. They found higher temperatures showed positive effects on the permeate flux, retention of oil and free fatty acids permeation. A process combining solvent extraction with membrane technology to recover the oil was studied by Kwiatkowski et al.81 Darvishmanesh et al.82 proposed a nanofiltration-based extraction methodology to perform solvent recovery. Their study compared a wide range of membranes – from GE-osmonics, Nadir, Evonik MET and SolSep – in a wide range of solvents: ethanol, isopropyl alcohol, acetone, cyclohexane and hexane. Flux permeation and the rejection test confirmed the applicability of the new commercial OSN membranes for solvent recovery, promoting the use of renewable solvents (ethanol, isopropyl alcohol, acetone) instead of the traditionally used n-hexane. The flux behavior of a dense commercial polydimethylsiloxane membrane with various vegetable oils under undiluted and hexane diluted conditions was studied by Manjula et al.83 They found hexane dilution improved the permeate oil flux in all the vegetable oils. However the dense PDMS membrane did not show any oil–hexane selectivity over the experimental range studied. Weibin et al.84 prepared PDMS/PVDF and Zeolite PDMS/PVDF composite membrane to be used in hexane recovery from soybean oil–hexane miscella. Zeolite PDMS/PVDF membrane showed the best separation performance.
In the oil industry the deacidification process is important, not only for the final product quality, but also for its economic impact in the production process. The free fatty acids (FFA) deliver undesirable qualities to crude oil, such as unpleasant taste and smell. Furthermore, they can cause corrosion and contamination when the oils are industrially used.85 Physical, chemical and enzymatic processes can be carried out to perform deacidification. The main disadvantages of these processes are the high energetic demand and the volume of effluent generated. Membrane technology can be an alternative to these processes if membranes with the adequate characteristics for the simultaneous separation of oil-FFA in hexane are prepared. Raman et al.86 described a process for solvent recovery and partial solvent deacidification from miscella using different commercial membranes. Bhosle et al.91 used polymeric hydrophobic nonporous and hydrophilic nanofiltration (NF) membranes for the deacidification of model vegetable oils with and without addition of organic solvents. Firman et al.85 studied four tailor-made composite membranes of poly(vinylidenefluoride) (PVDF) as a support and polydimethylsiloxane (PDMS) or cellulose acetate (CA) as coating layer, and the commercially available SolSep 030306 composite membrane to remove n-hexane and free fatty acid from crude soybean oil–hexane mixture. The effects of trans-membrane pressure, temperature and feed oil concentration on membrane selectivity and permeation flux were determined. The best performing membranes in terms of flux and rejection were the PVDF membranes featuring 12 wt% Siloc paste.
4. Carbon footprint of organic solvent nanofiltration
As described in detail in section 1, the membrane fabrication process comprises preparing a polymer dope solution, casting the membrane on a polypropylene non-woven backing, followed by phase inversion in a water bath, washing the membrane with IPA, crosslinking the membrane in a crosslinking medium, followed by a second wash with IPA, and finally conditioning the membrane in a conditioning medium. Once the membrane module has lost its performance, it has to be disposed of, usually by incineration which generates 1917 kg of CO2. Although there is no data on OSN, a typical lifetime of five years for desalination membranes means having to dispose of almost 1 million modules per year, and the number is growing at about 10% annually.92 Hence, future research work should not only focus on improving the sustainability of membrane module fabrication but should consider recycling waste membrane modules. The carbon footprint calculations are based on a case study taking into account the following:✓ 1000 m2 crosslinked P84 polyimide membrane is prepared with a thickness of 160 μm,
✓ The polypropylene non-woven backing has a thickness of 160 μm,
✓ 83 kg of dope solution is needed,
✓ 22 wt% of P84 polyimide polymer dissolved in a mixture of DMF–1,4-dioxane (3/1),
✓ 10 m3 of water for the coagulation bath,
✓ The kg of CO2 for the waste water purification was calculated assuming the water is distilled,
✓ After phase inversion 7 kg of IPA are used per kg of dope solution prior to crosslinking,
✓ For the crosslinking step 7 kg of crosslinking medium are used per kg of dope,
✓ HDA was selected as the crosslinker (0.8 kg of HDA per kg of dope),
✓ After the crosslinking step 10 kg of IPA per kg of dope are used to remove excess crosslinker,
✓ 7 kg of conditioning media per kg of dope comprising PEG400/IPA (3/1),
✓ Polypropylene non-woven backing (92 kg) conditioned with PEG (108 kg),
✓ The spacer is made of polypropylene with a thickness of 0.800 mm and 0.17 kg m−2,
✓ The glue is an epoxy resin (D.E.N. 438 Novalac Epoxy) and its curing agent is tetraethylenepentamine (TEPA) with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and an average density of 1.1 g mL−1 and an area 20 times smaller than the membrane area,
1 and an average density of 1.1 g mL−1 and an area 20 times smaller than the membrane area,
✓ Disposal of the membrane module by incineration.
On the other hand, the calculations do not take into account any of the transportation of the membrane to the location of final use, the energy required to cure the glue, or the membrane housing. Comparison of the carbon footprint of OSN (including membrane module fabrication and disposal as well as OSN operation) versus distillation (including the heating of the liquid, evaporating the liquid and condensing the vapours) is shown in Fig. 22. Based on Table 5 methanol was chosen as a solvent for the calculations. The intersection of the curves gives the minimum amount of solvent that has to be processed so OSN becomes CO2 negative compared to distillation. In a worst case scenario all the solvents and chemicals used for the membrane fabrication are incinerated. However, in a more optimistic scenario the crosslinking and the conditioning media can be reused, and the IPA can be recovered by distillation. In this case the overall carbon footprint can be reduced by 50% (Fig. 22). It can be concluded that 50 and 100 L of solvent per m2 membrane has to be processed in order to surpass distillation, counting with and without recycling, respectively (Fig. 22). Notice that fabrication of ceramic membranes can be more energy intensive than that of polymeric membranes due to sintering at high temperature (see Fig. 8). Nevertheless, with a typical membrane module with 5 L m−2 h−1 flux, 100 L of solvent can be processed within the first 20 hours of its operation, which is less than a fraction of a module's lifetime.
5. The effect of scale on the sustainability of membrane processes
5.1. Advantages of membrane processes upon scale-up
When comparing different unit operations for purification, it is difficult to get a sensible comparison, as the scale and scalability of a particular process has a significant effect on the overall economics. Hence, scalability of a process can often be a deciding factor when making a process selection. Some applications that produce bulk chemicals with low margins only reach break-even point at large scale, requiring an inexpensive and straightforward technique for downstream separation. For instance, compared to the chromatography processes that are difficult to scale up due to their sensitivity of performance to the column dimensions (i.e. aspect ratio),93 scaling up a membrane process is linear, straightforward, and more robust to variations.94In fact, one of the well-known advantages of membrane processes is their straightforward scalability. Membrane processes do not suffer from loss of selectivity and increase in waste with scale, features commonly observed with other techniques.47 On the contrary, membrane scale-up brings benefits such as improvements in productivity and reduction in operation time. As a case study, an API purification application recently published by Kim et al.53 was taken as an example to illustrate the effect of membrane scale on the productivity and operation time. The paper discusses a membrane diafiltration process as a competitive platform for genotoxins removal.53 Standard module sizes for OSN membranes (spiral wound membrane module, SWMM) and the reported data have been employed for the following calculations shown in Table 6 and Fig. 23.
 | ||
| Fig. 23 Compare different process parameters at different membrane scale. Both productivity and operation time improves with membrane scale. | ||
| Typical module size (diameter vs. length, mm) | Lab scale | 2.5′′ | 4′′ | 8′′ |
|---|---|---|---|---|
| 64 × 1016 | 102 × 1016 | 203 × 1016 | ||
| a Membrane flux was 12 L m−2 h−1 at 10 bar, initial product concentration of 10 kg m−3 with the final yield of 95% was assumed.53 | ||||
| Module size (m3) | 0.001 | 0.003 | 0.008 | 0.033 |
| Membrane area (m2) | 0.0051 | 2 | 6 | 30 |
| Area to modulevolume (m−1) | 9 | 612 | 723 | 912 |
| System volume (m3) | 0.0003 | 0.1 | 0.1 | 0.1 |
| Area-to-system volume ratio (A/V, m−1) | 17 | 20 | 60 | 300 |
As it can be seen in Fig. 23, the productivity and operation time are all in favour of large scale. For commercially available OSN membrane modules, the productivity-to-size ratio (PS),95 one of process intensification (PI) metrics, improves drastically with larger modules. This is a direct consequence of higher membrane area-to-volume (A/V) ratio. As membrane units usually occupy smaller space compared to that of other conventional unit operations, they can also offer a particular advantage for offshore platforms and large cargo vessels. Following the same logic, the operation time required to process a diafiltration batch decreases significantly with scale, owing to the increasing A/V ratio. Although the system volume has been assumed as 0.1 m3 for module systems, the trend is quite clear that operation time decreases with larger scale. In addition, as expected, economies of scale apply to membrane processes including pumps, pipework, and labour. It has been reported that the cost-scale relationship is inverse-exponential and the scale exponents can be as low as 0.4,96–99 as shown in eqn (6).
| C = nS0.4 | (6) |
It should be noted that the mass transfer coefficients for all modules have been assumed to be the same,100 which may not be the case in real applications. In addition, in comparison to the lab-scale equipment, the pressure drop along the module becomes considerable in large-scale applications and it should also be carefully assessed. Further study is necessary to understand the effect of scale-up in flux and rejection over the long-term, as such literatures are scarce100 for OSN. Also, membrane modules typically require periodic cleaning and/or replacement, adding to the overall operating costs. However, these factors are mostly application-dependent. Regardless, the general trend is that scaling up a membrane process brings higher productivity at lower cost.
5.2. Economic feasibility and scale-up considerations
Membrane processes are often considered as expensive despite the well-known notion of easy-scalability and simplicity of the process.101 Although the cost of membrane processes may have been an inhibiting factor in the early days, steady improvements in material science, module fabrication technology, and process improvements have decreased the cost significantly.For instance, the cost of RO processes has gone down by 5 times in the past 20 years, mainly due to standardization of parts and energy-recovery devices.92 A typical RO plant now can purify 1000 L of seawater for approximately £0.3 (cheaper than a bottle of water), and purified freshwater using RO costs approximately 4 times less.92 On the other hand, the gas separation industry has expanded substantially since 1980s and now captures more than 50% of the N2 production market,102 implying that the economics are competitive. The field of pervaporation shares a considerable ethanol dehydration market, and it is expected to grow much bigger as the bioethanol industry grows.103 Also, the UF and MF fields have enjoyed a steady increase in market share since the 1970s and it is now a vital part in many fields ranging from wastewater treatment to food industry and biotechnology. Particularly, economics were so compelling in many UF applications such as recycling paint solution in automobile plants104 and cheese whey filtration in food industry,105 hundreds of similar systems came online after their introduction. Furthermore, the data compiled and reported by Judd et al.106 show that the price of submerged membrane modules have dropped 8-fold from $400 m−2 in 1992 to less than $50 m−2 in 2005, and dropping. Also, as shown in Fig. 24, the total operating cost of submerged membrane system had come down more than 10-fold owing to several process innovations such as constant-flux operation and efficient air sparging.106,107 In summary, all of these processes have gone through many breakthroughs to lower the costs, making the overall process competitive and feasible.
 | ||
| Fig. 24 Operation cost of submerged membrane systems.108 The price has come down drastically owing to process innovations and better membrane fabrication technology. | ||
As the field of OSN grows, the overall cost is expected to follow a similar trend as the other membrane industries. The parts will become standardized as the demand rises, which in turn will drive down the capital cost and the associated operating costs. Recent work by Szekely et al.52 has extensively compared OSN with other purification processes such as recrystallization and flash chromatography in terms of capital investment, operating costs, and environmental impact. It has concluded that the capital costs among the studied processes are competitive and the main advantages of OSN are: low labour intensity and easy scale-up. It also highlighted the main disadvantages of OSN: low process yield and high solvent consumption. A detailed analysis has shown that implementing a solvent-recovery step is more beneficial to OSN processes compared to other separation methods. Readers are referred to the work of Szekely et al.52 and Vanneste et al.51 for detailed calculations on capital costs.
Several OSN manufacturing companies already exist such as Evonik-MET,109 Solsep BV,110 AMS Technologies,111 and GMT MembranTechnik.112 Until now, however, only a few applications have already been successfully commercialized due to several interacting reasons.
A notable application success is the MAX-DEWAX process developed by ExxonMobil that recovers organic solvent (methyl ethyl ketone) from lube dewaxing process76,113 as shown in Fig. 21B. The process scale was up to 5800 m3 per day (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrels per day).114 The installation cost was approximately three times lower compared to using conventional technologies to achieve the same process achievements, with a short payback period of less than 1 year. Another successful implementation of OSN is an API recovery process that concentrates a waste stream containing 1 wt% API to 10 wt%, which in turn gets re-directed to existing downstream units for further purification (Fig. 25).115,116 Similar to the implemented API recovery unit, many fruitful applications exist that can be tapped with OSN; however, extensive process development stage often required for OSN remains to be a hindering factor.
000 barrels per day).114 The installation cost was approximately three times lower compared to using conventional technologies to achieve the same process achievements, with a short payback period of less than 1 year. Another successful implementation of OSN is an API recovery process that concentrates a waste stream containing 1 wt% API to 10 wt%, which in turn gets re-directed to existing downstream units for further purification (Fig. 25).115,116 Similar to the implemented API recovery unit, many fruitful applications exist that can be tapped with OSN; however, extensive process development stage often required for OSN remains to be a hindering factor.
 | ||
| Fig. 25 Photo of API Concentration OSN Unit in GSK; first pharma application of DuraMem™ – 4′′ spiral modules. | ||
The success and economic feasibility of membrane processes in large scale mainly depend on the combination of three factors (Fig. 26): (i) the performance and stability of membrane material; (ii) the technology to fabricate high packing density modules; (iii) innovative process design to overcome bottleneck factors and to reduce operating costs. All of these factors must be fulfilled simultaneously for a membrane process to be feasible in large scale.
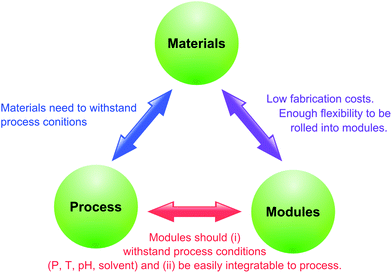 | ||
| Fig. 26 Three interdependent factors that must be satisfied for a membrane process to be successful in large scale. | ||
Among the three, the membrane material often needs to be tailored for specific applications with appropriate stability and performance to fit the overall process. At the same time, the material should allow easy module fabrication at low-cost. On the other hand, the membrane module also needs to exhibit necessary stability (pressure, chemical stability, etc.) in the process conditions. Often, the optimization goes both ways where the process itself needs to be modified to integrate membrane material and the respective membrane module.
Among the three factors, surprisingly, the process design and module fabrication have often hindered membrane processes to become commercially viable, as summarized in Table 7. For instance, pervaporation technology was developed from 1950s but it was only commercialized in 1980s when the technology advanced sufficiently to fabricate high-performance modules.117 Even now, the main bottleneck for pervaporation process is not the membrane selectivity, but the difficulty in making reliable membrane modules that can withstand the necessary harsh conditions.118 In the field of electrodialysis, the deviation of energy consumption from the theoretical limit comes from concentration polarization effect which limits the process.119 On the other hand, in gas separation applications, practical pressure ratios (feed-to-permeate pressure) are in the range of 5–20 considering the compression requirements and equipment costs.120 At these pressure ratios, improving the selectivity of membranes presents less benefit than one might expect.120 For example, air dehydration membranes can typically achieve selectivity more than 200 (water/air) but the processes are completely pressure ratio limited.118 These pressure-ratio limitations should be overcome through process innovations such as the implementation of two-step membrane design to reduce the cost of recompression duty for hydrogen recovery.121 In the reverse osmosis industry, the current plants operate quite close to the thermodynamic minimum (energy of de-mixing) that no significant breakthrough in membrane material is expected in near future.122 Instead, the common bottleneck factors are concentration polarization, chlorine resistance, fouling, and brine management. It is worth mentioning that brine no longer is a burden but rather another fruitful energy source123 with its high osmotic potential.
| Process | Bottleneck | Reference |
|---|---|---|
| Dehydration of air and natural gas | Completely pressure ratio limited (water/air selectivity over 200), concentration polarization | Baker,118 Auvil et al.124 |
| Hydrogen separation (from CO, CH4, N2) | Pressure ratio limited (selectivity over 100) | Ockwig et al.,121 Hayes,125 Zolandz126 |
| Pervaporation | Effective module fabrication technology, Cost | Baker,118 Smith et al.127 |
| Reverse osmosis | Mass transfer resistance, fouling, low chlorine resistance | Elimelech et al.,122 Glater et al.128 |
| Ultrafiltration | Membrane fouling, concentration polarization | Goosen et al.129 |
| OSN | Cost, chemical stability, and poor separation | Vandezande et al.,130 Szekely et al.,52 Kim et al.60 |
As listed in Table 7, it is not uncommon to find that membrane performances are usually more than necessary to achieve a desired outcome yet limited by other factors. Hence, it is vital to critically assess the overall process configuration, factoring in the performance of membranes along with the capital costs as well as the final process aim. As it was critically pointed out in AIChE Separation Roadmap 2020, membrane research must place a high priority on process systems development through economic evaluations over material science research.101
The OSN field is still relatively new to pinpoint what the bottleneck factors are, but they are likely to be the inadequate membrane stability and poor separation performance leading to long development periods. Unfortunately, these limitations often eliminate OSN from being considered as a suitable option from the process development stage.9 Nevertheless, OSN membrane materials are continuously being improved in terms of stability, flux, and separation performance.130 Also, new types of available OSN membranes are increasing in numbers which can withstand wider range of solvents and process conditions. With such solvent resistant NF membranes, innovative process designs previously not possible were made possible such as continuous catalyst recovery131 and membrane reactor.132 A recent work by Schmidt et al. proposed a process development workflow for OSN applications.133
Although the membrane material itself needs to be tailored to specific applications, the module fabrication technology can be shared among different disciplines of the membrane industry. This fabrication technology has improved greatly in the past 30 years and now it is possible to fabricate reliable modules with high packing density. The hurdles that other membrane disciplines have faced do not have to be overcome again. There are mainly four types of membrane modules: spiral wound membrane (SWM), hollow fiber membrane (HFM), plate and frame membrane (PFM), and tubular membrane (TM) module.9 Among the four types, SWM module is the dominant type in the field of OSN but HFM (hollow fibre membrane) modules have also been reported.134 SWM module consists of several membrane leaves (flat sheet membranes), separated by a spacer, wound around a central collection pipe. Logically, the design and geometry of the spacer is crucial to control the axial (and radial) pressure drop and mass transfer. The packing density of SWM modules ranges from 300–1000 m2 m−3. It is important to stress that for OSN applications, all the wetted module components such as spacer, adhesive, and O-rings, need to be resistant to the operating solvent media. Currently, OSN membranes are now commercially available as SWM up to 8′′ module,109,110 but 16′′ module has recently been standardized in RO industry.135
6. Quality by design (QbD) approach for OSN membrane & process development
Nowadays OSN is used for several applications in industries. On one hand, in view of the expected growth of the OSN market, a need still exists to develop more and better membranes to address separation problems in industrial processes as well as to open new applications areas.136 On the other hand, predicting the performance of NF membrane separations is necessary for the design and optimization of processes. Development of predictive/simulative models is therefore an important area. Models often do not include sufficient detail on the physical phenomena taking place and quantification of the effects of basic parameters is not often completely understood. The most common influencing parameters are schematically shown in Fig. 27. | ||
| Fig. 27 Classification of most significant operating parameters for membrane fabrication and performance in OSN. | ||
Typical membrane processes are characterised by complicated matrices of input and output parameters. Membrane fabrication is influenced by polymer concentration, additive concentration, additive type, type of solvent and post-treatment procedures. Membrane performance (i.e. flux through the membrane and retention of target compounds) strongly depend on the nature of solute, solvent and mixture composition,137 process operating parameters and fluid dynamics in the module.138,139 Due to the high number of parameters to take into account, industrial R&D of nanofiltration processes can be expensive, time- and resource-consuming. Following the example of the pharmaceutical industry,140 where the recent regulations from federal agencies have compelled researchers to move from the traditional Quality by Testing (QbT) approach to the more efficient Quality by Design (QbD) approach, chemical and manufacturing industries have also started to look for a more efficient R&D strategy, to reduce costs, energy, solvent consumption, and achieve better quality information. The QbD concept is defined as “a systematic approach to development that begins with predefined objectives and emphasises product and process understanding and process control, based on sound science and quality risk management”.140 This has advantages from economic, technological and environmental points of view, as it can promote better processes, less solvent consumption, less waste production and lower energy requirements.74 Designs of Experiments (DoE) methods141 and machine learning (ML) techniques, such as artificial neural network (ANN) and genetic algorithm (GA) optimization, are extensively applied in process design to help engineers understand the effects of possible combinations and interactions of various parameters on the final product quality.141,142 The purpose of statistically designing an experiment is to collect the maximum amount of relevant information with the minimum expenditure of time and resources. DoE and ML methods are much more efficient than the classical changing one single factor at a time (COST) approach (Table 8). For further reading on the general aspects of DoE the authors recommend the book Design of Experiments – Principles and Applications edited by Eriksson.143
6.1 Experimental design and combinatorial strategies applied to membrane fabrication
Because of the many parameters involved in a typical membrane synthesis, the optimization and testing of membranes can be very time consuming. The challenge in developing and optimizing membranes is to find more efficient search strategies to direct membrane composition toward a product with a better separation of the targeted compounds (i.e., higher selectivity combined with significant fluxes).144 Bulut et al.144 presented the first application of DoE (in the form of evolutionary strategy), to accelerate the development and optimization of membranes. They used genetic algorithms to investigate the preparation of polyimide-based solvent resistant membranes via phase inversion. Thanks to an intelligent search strategy, they could study parameter space of 8 variables with a limited number of experimental data (192 polymeric solutions, instead of 9 × 1021 required by the classical COST approach). Similarly, Vandezande et al.136 adopted a combinatorial strategy, based on a genetic algorithm and a self-adaptive evolutionary strategy to optimize the OSN performance of PI-based membranes, prepared by solidification of emulsified polymer solutions via phase inversion (SEPPI). This approach allowed the screening of a 9-dimensional parameter space, comprising two extra solvents, the two corresponding nanosized zeolite suspensions, as well as another cosolvent. Coupling with high-throughput techniques allowed the preparation of three generations of casting solutions, 176 compositions in total, resulting in 125 testable membranes. Madaeni et al.145 successfully modelled the process of membrane preparation (PES and PSf by immersion participation using different types of solvents and additives) by ANN. The mechanical, chemical and thermal properties of polymers, solvent, non-solvent and additives, as well as the effects of polymer and additive concentrations on membrane performance were elucidated for introducing the ANN. Fabrication of PSf membranes was studied also by applying a full factorial design by Amirabedi et al.146 They successfully modelled the effects of coagulation bath composition, polymer concentration and PEG concentration on tensile strength and porosity, carrying out not more than 18 experiments.6.2 Experimental design and combinatorial strategies applied to the investigation of membrane performance
Many studies in OSN deal with the effects of solute and solvent properties,147–152 membrane properties153 and process parameters, such as solute concentration,100,154 operating pressure,154–158 temperature159,160 and fluid dynamics conditions138,158,161 on the overall process performance. In the majority of these studies, the effect of operating parameters is investigated by varying one factor at a time, and no effects of interactions among operating parameters are identified. Attempts to apply fundamental and semi-empirical models to describe the experimental data have been described,100 however they do not propose an efficient strategy to study multiple effects and their interaction on the overall process performance. Little evidence of statistical significance can be provided by the COST approach.Understanding the effects of the operating parameters on the overall process performance is important for process development and optimisation. Examples of efficient investigation techniques, based on DoE and ANN approaches, have been proposed in recent years to study nanofiltration and ultrafiltration for aqueous applications, while few studies have been done for OSN. In the following, examples from the literature for aqueous applications are shown, together with the examples concerning OSN, as they suggest the significant methodology to apply DoE and ANN to nanofiltration processes. All these studies are summarised in Table 9.
| Application | Methodology | Scale | Reference |
|---|---|---|---|
| Water | COST | Membrane | Darvishmanesh et al.,147,148,151 Machado et al.,149 Geens et al.,150 Marchetti et al.,152 Vankelecom et al.,153 Silva et al.,100,154 Bowen et al.,155 Wijmans et al.,156 Yaroshchuk et al.,157 Whu et al.,158 Tsuru et al.,159,160 Peeva et al.,138 Schock et al.,161 |
| DoE/ANN | Membrane | Ahmad et al.,162 Bahçeci,163 Gozálvez-Zafrilla et al.,164 Guerra et al.,165 Połom et al.,166 Rahmanian et al.,167 Román et al.168 | |
| Organic solvents | DoE | Membrane/Process | Marchetti et al.74,169 |
| Membrane modelling maps | Membrane | Schmidt et al.170 | |
| COST | Process | Sereewatthanawut et al.49 | |
| COST | Process | Caus et al.171 | |
| COST | Process | Lin et al.172 | |
| OSN designer | Process | Peshev et al.173 |
Ahmad et al.162 investigated the separation of dye/salt/water solutions by porous silica/γ-alumina ceramic membranes by using a DoE approach. Temperature, feed concentration, pressure and pH, examined by Response Surface Method with central composite design (CCD), were found to statistically affect the quality of the separation and simple linear and quadratic models were obtained to represent the significant effect of the operational conditions on the responses of interest: the percentage retention of dye, the percentage retention of salt, and the permeate flux. Besides obtaining the models, they determined the optimum conditions for achieving a high rejection of dye, maximum desalting and large permeate flux. Similarly, Bahçeci163 studied the effect of temperature, trans-membrane pressure and pretreatment with filtering aids (antifoulants) on permeate flux and fouling resistance during ultrafiltration of apple juice by a DoE approach based on a central composite design with a quadratic model. They could conclude that pretreatment of apple juice with filtering aids and an increase in the temperature and trans-membrane pressure significantly improved the permeate flux. Gozálvez-Zafrilla et al.164 applied a crossed mixture-process design, based on D-optimal criterion, to predict the rejection behaviour of commercial nanofiltration membranes (NF270, Dow-Filmtec) when treating non-dilute ionic solutions (containing chloride, nitrate, sulfate, sodium and calcium ions). Mixture designs were used as they include factors related with composition. Guerra et al.165 studied the effects of tubular ceramic membrane hydrodynamic conditions (cross flow velocity and transmembrane pressure) on permeate flux using one type of ceramic membrane with two different channel configurations. Factorial experimental design was used to construct a controlled set of experiments in which the effect of varying the operating parameters was measured. Połom and Szaniawska166 studied the effect of operating parameters such as cross-flow velocity, pH in the feed solution, pressure difference across the membrane and lactic acid concentration in the feed solution on lactic acid rejection using an experimental design technique. With increasing lactic acid concentration in the feed solution, low rejection can be maintained by decreasing the cross-flow velocity and pressure difference across the membrane at fixed pH. Comparison of DoE and ANN approaches was performed by Rahmanian et al.167 for micellar-enhanced ultrafiltration to remove zinc ions from wastewater by comparing DoE and ANN approaches. Their results show that, due to the complexity in generalisation of the micellar-enhanced ultrafiltration process by any mathematical model, the neural network proves to be a very promising method in comparison with fractional factorial design by DoE for the purpose of process simulation. Finally, Román et al.168 showed how information from statistical design can be integrated with process modelling. They used concentration-dependent solute rejection obtained by experimental design to describe the dynamics of different membrane filtration processes (constant volume vs. variable volume diafiltration) and select the most appropriate filtration technique for the demineralization of acid whey.
Studies on OSN by DoE are, on the other hand, few and, to the best of our knowledge, no ANN has been applied up to now. Marchetti et al.74,169 investigated the NF performance of model peptides in MeCN–water mixtures through commercial ceramic membranes (HITK), with the dual purpose of characterisation of membrane transport mechanism for the specific model peptides and process selection for the filtration step. Statistical models for solvent flux, peptide and trifluoroacetic acid rejections as a function of five operating parameters (peptide concentration, pH, pressure, cross-flow velocity and ACN concentration) were obtained by performing a DoE fractional factorial design and by analysing the data by statistical Analysis of Variance (ANOVA). The best operating conditions for concentration were found by numerical optimisation of the statistical models with the desirability function method and the most statistically significant interactions are shown in Fig. 28.
 | ||
| Fig. 28 Effect of interaction between MeCN and TFA concentration on peptide rejection by Design Expert® software, at constant Cpeptide, pressure and pump frequency. | ||
Graphical methods have been also proposed to efficiently perform process selection. Schmidt et al.170 characterised flux and rejection performance of two commercial polyimide-based membranes, Starmem 122 and Puramem 280, in multi-component solvent mixtures. The experimental investigation included solvent flux and rejection measurements of five dissolved compounds in binary and ternary mixtures of toluene, n-hexane and 2-propanol. As the solvent choice has a significant impact on membrane performance, membrane rejection maps (MRM) and membrane selectivity maps (MSM) were developed. MRM and MSM are graphical tools that plot in a ternary diagram the rejection of solutes or the selectivity of the membrane for the ternary solvent mixtures. They were used as decision tools in the early stages of conceptual process design by selecting either pure solvents or solvent mixtures that enhance the downstream processing in OSN and thus can replace costly and time-consuming membrane screening or OSN membrane modification. To address the relevance for other solvents, the transferability of these results to classes of similar solvents was also demonstrated.
6.3 Experimental design and combinatorial strategies applied to process modelling and optimization
Investigation of performances at the process level has an important role for a successful application of OSN. Process design and modelling have been approached to describe the dynamics of membrane concentration and diafiltration, spiral wounds modules and membrane cascades.Sereewatthanawut et al.49 demonstrated the applicability of OSN spiral-wound membrane modules to common separation challenges in the synthesis of Active Pharmaceutical Ingredients (APIs) and production of high-value natural compounds, such as the separation of a main product from its impurity. They investigated the separation of Solvent Yellow 7 (model product) from Brilliant Blue (model impurity) in DMF and the separation of a real case-study API from its oligomeric impurities in THF. Solute concentration effects, including concentration polarisation and osmotic pressure were neglected and no selectivity for the solvent was assumed (i.e. the solvent rejection was equal to zero).
Caus et al.171 studied the potential of integrated countercurrent nanofiltration cascades for advanced separation of individual organic components in aqueous solution by means of single-stage filtration experiments using xylose and maltose and cascade simulations. The influence of module recovery, membrane characteristics, recycled fraction and the number of modules was evaluated. Rejection of each module was assumed constant, i.e. independent of pressure and feed concentration.
Lin and Livingston172 developed a model to simulate performance of the cascade with different numbers of stages for the solvent swap from TOABr–toluene to TOABr–methanol, to predict the performance of the solvent exchange and exclude redundant experiments. Effects of system parameters, such as the number of stages in the cascade and the ratio of initial and replacing solvent flows, on the performance of solvent exchange were investigated. As in the previous studies, the rejections and fluxes at each stage were all constants, and consequently flow rates of the permeate and product streams were determined by mass balances.
Finally, Vanneste et al.51 investigated the application of membrane cascades to solvent-based pharmaceutical separations in MIK, THF and methanol through Duramem 150 and Duramem 200. Again, the cascade modelling was based on constant rejection for each stage.
As is clear from the literature shown so far, the attempts to implement the fundamental transport models on a process level are few and the reports that exist use exclusively simple, non-predictive membrane transport models.173 The set of equations for the process modelling has been often solved by considering rejection and solvent flux as being constant, i.e. by neglecting the dependence of flux and rejection on concentration or process operating parameters. For a more efficient and realistic process modelling, however, the effect of the operating parameters on the membrane transport performance should be considered (the importance of considering the effect of operating parameters on flux and rejection was shown in the studies on transport through membranes reported in the previous paragraph). Only few studies show how information from investigation of membrane transport can be used to support process modelling for process selection. These studies report the combination of both statistical and fundamental transport modelling with process modelling for improving process selection and development.
Marchetti et al.169 investigated the diafiltration of peptide solutions in ACN–water mixtures through commercial ceramic membranes (HITK). Empirical models for flux and rejection, obtained by Design of Experiments, were included in the mathematical framework of the diafiltration process, to calculate the evolution of peptide, counter-ion and solvent concentrations over time, compare constant volume vs. variable volume diafiltration modes, and select the best process in terms of operating time and solvent consumption. This work demonstrates that empirical DoE models can simultaneously provide phenomenological understanding of the transport mechanism through nanofiltration membranes for a specific solute of interest and successfully support the process modelling for concentration and diafiltration, providing a methodology to select the most appropriate filtration technique for a given separation problem.
Peshev and Livingston173 proposed to integrated fundamental transport models with process modelling by making OSN unit operations available in process modelling environments, such as Aspen Plus, HYSYS, ProSim Plus. Matlab routines for the membrane transport models were interfaced to Aspen Plus by means of CAPE OPEN. Custom OSN unit operations for common membrane separation processes such as batch concentration, constant volume semi batch and steady state filtration were used to describe the process. By using this approach, solute and solvent properties of databank compounds were also modelled, by accessing thermodynamic and physical property databases and engines. In order to have the possibility to use any molecular structures in the simulations, COSMO SAC method was used to fulfil the minimum input required for COSMO SAC property method in Aspen Plus and model non-databank compounds. They demonstrated the suitability of this technique by comparing the model simulation to the experimental data for ideal systems (rosmarinic acid in ethanol) and non-ideal solutions (tetraoctylammonium bromide in toluene).
Sereewatthanawut et al.174 developed a mathematical model to assess and optimize the separation performance of an enantioselective inclusion complexation – organic solvent nanofiltration (EIC-OSN) process. Enantiomer solubilities, feed concentrations, solvent compositions, permeate solvent volumes, and numbers of nanofiltrations were identified as key factors for process efficiency. The model was first tested by comparing calculated and experimental results for a non-optimized process, and then, calculations were carried out to select the best operating conditions. It was found that the optimal configuration varied with the objective function selected, e.g. resolvability versus yield, with a boundary on product optical purity. The model also suggested that the process efficiency could benefit from (i) diafiltration of the distomer and (ii) higher feed concentrations. Since the latter strategy would result in higher eutomer loss, a multistage process was evaluated using the verified process model.
Abejón et al.89 discusses the design of continuous OSN systems, particularly the configuration of dual membrane cascades through sensitivity analysis of the operation variables, and economic optimization. Analysed configurations include multistage cascades up to three stages, and dual membrane cascades up to three stages (Fig. 29). The total costs were chosen as the formulated objective function to minimize the economic optimization strategy. It was found that the treatment of the residual stream leaving the system is the major contributor of the overall cost of the process being more than 85% for dual cascades, but the solvent recovery units can significantly reduce the costs up to 77%, depending on the required solvent quality.
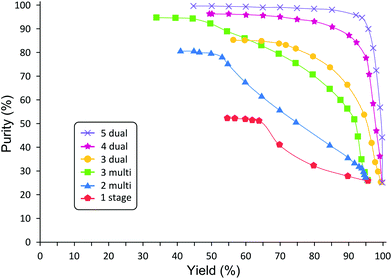 | ||
| Fig. 29 Pareto set of solutions including the product purity, and the process yield for several process configurations: single-stage process; 2- and 3-stage configuration; dual cascades comprising 3, 4 and 5 stages (adapted from Abejón et al.89). | ||
7. Conclusions & future directions
Membrane separations are recognized amongst the best available technologies (BATs) in waste water and gas treatment systems in the chemical sector.175 Based on the present sustainability assessment, it can be concluded that OSN has a huge potential in becoming the BAT among the separation techniques in organic media due to its advantages as listed below:- Low energy requirements;
- Low solid waste generation;
- Low labour intensity;
- Straightforward scale-up;
- Stability in harsh environments allowing wide flexibility (pH, T, solvents);
- Mild operating conditions (T, p);
- Easy solvent swap from high to low boiling point solvents;
- Simultaneous removal of solutes from different chemical classes.
Since the field of OSN has experienced significant growth and advancement in recent years, the green aspects of OSN have now been explored in this review. The main drawbacks identified and its possible solutions are summarized in Table 10. As marked in the table, there are several drawbacks which have not yet been addressed and future research should primarily focus on these topics. When optimizing a green membrane fabrication process at bench scale, one should assess if it would still be green at large scale, taking into account the amount of chemical waste generated as well as the feasibility to scale up that fabrication process.
| Drawbacks | Possible solutions | ||
|---|---|---|---|
| a These topics have not yet been fully addressed → further research work is needed. | |||
| 1 | Membrane fabrication | Large waste water generation containing toxic solvents such as DMFa | Replace solvents used in casting solution with greener solvents to avoid waste water treatment; or use resins to trap the DMF from the water. |
| 2 | Use of toxic solvents and chemicals | Replace toxic solvents by greener solvents; avoid or reduce the amount of toxic chemicals | |
| 3 | Usage of hydrocarbon based polymers | Replace hydrocarbon polymers with renewable biodegradable polymers such as cellulose | |
| 4 | Large chemical waste generation during crosslinkinga | Reduce volume of toxic chemicals during crosslinking; perform dry crosslinking; avoid crosslinking by using solvent stable polymers such as PEEK or cellulose. | |
| 5 | Process development | Time consuming screeninga | Application of QbD/DoE |
| 6 | Low rejection & yield | Tighter membranes are needed; membrane cascades; enlargement of solutes | |
| 7 | Small MW gap between solutes | ||
| 8 | Large solvent consumption | Solvent recovery by adsorption, membranes or distillation. | |
| 9 | Insufficient purity | Hybrid process: coupling OSN with another downstream processing unit. | |
| 10 | Scale-up | Mass transfer and pressure drop | Dedicate more research to understand the process aspects of OSN |
| 11 | Limited number of commercial membranesa | Compile available membrane data effectively | |
| 12 | High cost of modulesa | Expected to drop with rising demand | |
| 13 | Scarce literaturea | Application-driven research up to commercial scale | |
The latest generation of OSN membranes are stable in a wide range of solvents and can withstand harsh operating conditions, i.e. high temperatures and extreme pH. In diafiltration processes, the rejection limitations set by the membranes have been overcome by process chemistry (enlarging catalyst or using anchors) or process engineering (membrane cascades). Nevertheless, for OSN membrane processes to be sustainable, it requires the difference in solute sizes to be around 3–5×. In diafiltration processes, although the energy consumption of OSN is 4 orders of magnitude less than distillation – depending on the solvent – in most of the cases the impurities are too small to be fully retained by OSN membranes. This means that one of the major environmental burdens of OSN is high solvent consumption due to the high number of diavolumes necessary to reach sufficient product purity. Tighter membranes and evaluation of solvent recovery by OSN for the removal of small solutes is necessary to address the needs of fine chemical processing. Hence, OSN can only be sustainable and competitive for purification purposes when a solvent recovery unit is implemented. Interestingly, besides distillation and adsorption, OSN itself was also proposed for solvent recovery. It has been shown that solvent recovery by OSN is progressively advantageous over distillation as the boiling point increases. On the other hand, in case of a solvent exchange the size of the solute is much larger and can easily be retained by the current OSN membranes allowing uniquely sustainable solvent swap from high boiling point to low boiling point solvents. It has been shown that OSN can quickly become CO2 negative technology in comparison to distillation.
It has been shown that membrane processes offer much higher productivity-to-volume ratio with larger scales. In addition, operation time required for a batch of diafiltration is greatly reduced. Several bottleneck factors that often hinder membrane applications to be commercialized are identified and discussed. As for OSN, the main hindrance factors are likely to be material stability and poor performance that often lead to long process development time, a risk that industry is hesitating to take. It has been shown that a simultaneous optimization of membrane materials, process design, and module fabrication must be carried out to develop a successful OSN application. It should be pointed out that industrially implemented OSN processes as well as the literature on OSN scale-up are scarce. For OSN to become a mature technology, the research attention should be focused on scale-up and communicating the possibilities of OSN to industry in order to facilitate its uptake.
The optimization of OSN membranes and processes are mostly carried out by the conventional COST approach leaving the coupled effects of key parameters unnoticed. The main advantages of the statistical design employed to obtain the picture of a complete area of investigation (by DoE or ANN approaches), in comparison with the conventional ones include (i) reduced number of experiments increasing time- and cost-efficiency; (ii) effective estimation of the main effects and their interactions; (iii) reduced environmental, health, and safety concerns.
References
- M. G. Buonomenna, RSC Adv., 2013, 3, 5694–5740 RSC.
- E. Drioli, A. Brunetti, G. Di Profio and G. Barbieri, Green Chem., 2012, 14, 1561–1572 RSC.
- G. Cederlof and E. R. Geus, US Patent7,351,873, 2008 Search PubMed.
- A. P. B. Ribeiro, J. M. L. N. de Mouraa, L. A. G. Goncalves, J. C. C. Petrus and L. A. Viotto, J. Membr. Sci., 2006, 282, 328–336 CrossRef CAS PubMed.
- D. Ormerod, B. Sledsens, G. Vercammen, D. Van Gool, T. Linsen, A. Buekenhoudt and B. Bongers, Sep. Purif. Technol., 2013, 115, 158–162 CrossRef CAS PubMed.
- M. Rabiller-Baudry, G. Nasser, T. Renouard, D. Delaunay and M. Camus, Sep. Purif. Technol., 2013, 116, 46–60 CrossRef CAS PubMed.
- C. J. Pink, H. T. Wong, F. C. Ferreira and A. G. Livingston, Org. Process Res. Dev., 2008, 12, 589–595 CrossRef CAS.
- G. Szekely, J. Bandarra, W. Heggie, B. Sellergren and F. C. Ferreira, Sep. Purif. Technol., 2012, 86, 79–87 CrossRef CAS PubMed.
- J. Micovic, K. Werth and P. Lutze, Chem. Eng. Res. Des., 2014 DOI:10.1016/j.cherd.2014.02.012.
- E. Rundquist, C. Pink, E. Vilminot and A. G. Livingston, J. Chromatogr., A, 2012, 1229, 156–163 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- I. Soroko, Y. Bhole and A. G. Livingston, Green Chem., 2011, 13, 162–168 RSC.
- S. Loeb and S. Sourirajan, Sea Water Demineralization by Means of an Osmotic Membrane, in saline Water Conversion-II, Advances in Chemistry Series Number 28, American Chemical Society, Washington, DC, 1963, pp. 117–132 Search PubMed.
- I. F. J. Vankelecom, K. De Smet, L. E. M. Gevers and P. A. Jacobs, in Nanofiltration, Principles and Applications, ed. A. I. Schaefer, A. G. Fane and T. D. White, Elsevier, 2005 Search PubMed.
- K. Vanherck, G. Koeckelberghs and I. F. J. Vankelecom, Prog. Polym. Sci., 2013, 38, 874–896 CrossRef CAS PubMed.
- J. E. Cadotte and R. J. Petersen, Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Wien, 2te Abteilung a, 63; Desalination, ACS Symp. Ser. No. 153, American Chemical Society, Washington, DC, 1981, p. 305 Search PubMed.
- N. Benmouhoub, N. Simmoonet, N. Agoudjil and T. Coradin, Green Chem., 2008, 10, 957–964 RSC.
- R. H. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Blinks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- K. Alfonsi, J. Colber, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- N. Tanihara, K. Tanaka, H. Kita and K. Okamoto, J. Membr. Sci., 1994, 95, 161–169 CrossRef CAS.
- J. S. Kang, J. Won, H. C. Park, U. Y. Kim, Y. S. Kang and Y. M. Lee, J. Membr. Sci., 2000, 169, 229–235 CrossRef CAS.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 CrossRef CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- R. Renner, Environ. Sci. Technol., 2001, 35, 410A–413A CrossRef CAS.
- D. Y. Xing, N. Peng and T. S. Chung, Ind. Eng. Chem. Res., 2010, 49, 8761–8769 CrossRef CAS.
- D. Y. Xing, S. Y. Chan and T. S. Chung, Green Chem., 2012, 14, 1405–1412 RSC.
- T. S. Chung, J. Macromol. Sci., Polym. Rev., 1997, C37, 277–301 CAS.
- A. B. Conciatori, E. C. Chenevey, T. C. Bohrer and A. E. Prince Jr., J. Polym. Sci., Part C: Polym. Symp., 1967, 19, 49–64 CrossRef.
- D. Y. Xing, S. Y. Chan and T. S. Chung, Chem. Eng. Sci., 2013, 87, 194–203 CrossRef CAS PubMed.
- E. M. V. Hoek and A. K. Ghosh, US patent2010/0224555 A1, 2010 Search PubMed.
- H. Gao, T. Jiang, B. Han, Y. Wang, J. Du, Z. Liu and J. Zhang, Polymer, 2004, 45, 3017–3019 CrossRef CAS PubMed.
- L. Zhu, C. Y. Huang, Y. H. Patel, J. Wu and S. V. Malhotra, Macromol. Rapid Commun., 2006, 27, 1306–1311 CrossRef CAS.
- S. Takezo, S. Takatoshi and S. Masao, US Patent3992495/1976, 1976 Search PubMed.
- S. Li, F. Qin, P. Qin, M. N. Karim and T. Tan, Green Chem., 2013, 15, 2180–2190 RSC.
- H. J. Li, T. M. Cao, J. J. Qin, X. M. Jie, T. H. Wang, J. H. Liu and Q. Yuan, J. Membr. Sci., 2006, 279, 328–335 CrossRef CAS PubMed.
- Y. Zhang, H. Shao, Ch. Wu and X. Hu, Macromol. Biosci., 2001, 1, 141–148 CrossRef CAS.
- Z. Mao, Y. Cao, X. Jie, G. Kang, M. Zhou and Q. Yuan, Sep. Purif. Technol., 2010, 72, 28–33 CrossRef CAS PubMed.
- X. L. Li, L. P. Zhu, B. K. Zhu and Y. Y. Xu, Sep. Purif. Technol., 2011, 83, 66–73 CrossRef PubMed.
- H. Z. Chen, N. Wang and L. Y. Liu, J. Chem. Technol. Biotechnol., 2012, 87, 1634–1640 CrossRef CAS.
- K. Cziner, T. Virkki-Hatakka, M. Hurme and I. Turunen, Chem. Eng. Technol., 2005, 28, 1490–1499 CrossRef CAS.
- M. Degerman, N. Jakobsson and B. Nilsson, Chem. Eng. Technol., 2008, 31, 875–882 CrossRef CAS.
- T. Winkelnkemper and G. Schembecker, Sep. Purif. Technol., 2010, 71, 356–366 CrossRef CAS PubMed.
- C. Jimenez-Gonzalez, D. J. C. Constable and C. S. Ponder, Chem. Soc. Rev., 2012, 41, 1485–1498 RSC.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- C. Jimenez-Gonzalez, P. Poechlauer, Q. B. Broxterman, B. S. Yang, D. am Ende, J. Baird, C. Bertsch, R. E. Hannah, P. Dell'Orco, H. Noorman, S. Yee, R. Reintjens, A. Wells, V. Massonneau and J. Manley, Org. Process Res. Dev., 2011, 15, 900–911 CrossRef CAS.
- W. M. Nelson, Green Solvents for Chemistry – Perspectives and Practice, Oxford University Press, Inc., 2003, ISBN 0-19-515736-2 Search PubMed.
- I. Sereewatthanawut, F. W. Lim, Y. S. Bhole, D. Ormerod, A. Horvath, A. T. Boam and A. G. Livingston, Org. Process Res. Dev., 2010, 14, 600–611 CrossRef CAS.
- G. Szekely, J. Bandarra, W. Heggie, B. Sellergren and F. C. Ferreira, J. Membr. Sci., 2011, 381, 21–33 CrossRef CAS PubMed.
- J. Vanneste, D. Ormerod, G. Theys, D. Van Gool, B. Van Camp, S. Darvishmanesh and B. Van der Bruggen, J. Chem. Technol. Biotechnol., 2013, 88, 98–108 CrossRef CAS.
- G. Szekely, M. Gil, B. Sellergren, W. Heggie and F. C. Ferreira, Green Chem., 2013, 15, 210–225 RSC.
- J. F. Kim, G. Szekely, I. B. Valtcheva and A. G. Livingston, Green Chem., 2014, 16, 133–145 RSC.
- C. Lee, R. Helmy, C. Strulson, J. Plewa, E. Kolodziej, V. Antonucci, B. Mao, C. J. Welch, Z. Ge and M. A. Al-Sayah, Org. Process Res. Dev., 2010, 14, 1021–1026 CrossRef CAS.
- A. Keraani, T. Renouard, C. Fischmeister, C. Bruneau and M. Rabiller-Baudry, ChemSusChem, 2008, 1, 927–933 CrossRef CAS PubMed.
- W. E. Siew, C. Ates, A. Merschaert and A. G. Livingston, Green Chem., 2013, 15, 663–674 RSC.
- S. So, L. G. Peeva, E. W. Tate, R. J. Leatherbarrow and A. G. Livingston, Chem. Commun., 2010, 46, 2808–2810 RSC.
- S. Drioli, I. Adamo, M. Ballico, F. Morvan and G. M. Bonora, Eur. J. Org. Chem., 2002, 3473–3480 CrossRef CAS.
- D. J. Gravert and K. D. Janda, Chem. Rev., 1997, 97, 489–509 CrossRef CAS PubMed.
- J. F. Kim, A. M. Freitas da Silva, I. B. Valtcheva and A. G. Livingston, Sep. Purif. Technol., 2013, 116, 277–286 CrossRef CAS PubMed.
- D. Nair, H. T. Wong, S. Han, I. F. J. Vankelecom, L. S. White, A. G. Livingston and A. T. Boam, Org. Process Res. Dev., 2009, 13, 863–869 CrossRef CAS.
- G. J. Harmsen, Chem. Eng. Process., 2007, 46, 774–780 CrossRef CAS PubMed.
- P. Lutze and A. Gorak, Chem. Eng. Res. Des., 2013, 91, 1978–1997 CrossRef CAS PubMed.
- M. Priske, K. D. Wiese, A. Drews, M. Kraume and G. Baumgarten, J. Membr. Sci., 2010, 360, 77–83 CrossRef CAS PubMed.
- G. Szekely, I. B. Valtcheva, J. F. Kim and A. G. Livingston, React. Funct. Polym, 2014 DOI:10.1016/j.reactfunctpolym.2014.03.008.
- C. S. Slater, M. J. Savelski, W. A. Carole and D. J. C. Constable, in Green Chemistry in the Pharmaceutical Industry, ed. P. J. Dunn, A. S. Wells and M. T. Williams, Wiley-VCH Verlag GmBH & Co., Weinheim, 2010, ch. 3, pp. 49–82 Search PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- E. M. Rundquist, C. J. Pink and A. G. Livingston, Green Chem., 2012, 14, 2197–2205 RSC.
- M. J. Raymond and C. S. Slater, Green Chem., 2010, 12, 1826–1834 RSC.
- American Chemical Society. ACS GCI Pharmaceutical Roundtable, 2011, [Online]. Available from:http://chemistry.org/greenchemistryinstitute/pharma_roundtable.html [Accessed 21 August 2011].
- C. S. Slater, M. J. Savelski, W. A. Carole and D. J. C. Constable, in Green Chemistry in the Pharmaceutical Industry, ed. P. J. Dunn, A. S. Wells and M. T. Williams, Wiley-VCH Verlag GmBH & Co., Weinheim, 2010, ch. 3, p. 77 Search PubMed.
- C. Stewart Slater, Mariano J. Savelski and Marie Nydia Ruiz-Felix, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2013, 48(13), 1602–1608 CrossRef PubMed.
- J. Geens, B. De Witte and B. Van der Bruggen, Sep. Sci. Technol., 2007, 42, 2435–2449 CrossRef CAS.
- P. Marchetti, A. Butté and A. G. Livingston, Reactive Peptide Nanofiltration, in Sustainable Nanotechnology and the Environment: Advances and Achievements, ed. N. Shamim and S. Virender, ACS Symp. Ser., 2013 Search PubMed.
- L. S. White and A. R. Nitsch, J. Membr. Sci., 2000, 179, 267–274 CrossRef CAS.
- R. M. Gould, L. S. White and C. R. Wildemuth, Environ. Prog., 2001, 20, 12–16 CrossRef CAS.
- L. P. Raman, M. Cheryan and N. Rajagopalan, J. Am. Oil Chem. Soc., 1996, 73, 219 CrossRef CAS.
- N. Stafie, D. F. Stamatialis and M. Wessling, J. Membr. Sci., 2004, 228, 103–116 CrossRef CAS PubMed.
- S. S. Koseoglu, J. T. Lawhon and E. W. Lusas, J. Am. Oil Chem. Soc., 1990, 67, 315–322 CrossRef CAS.
- J. C. Wu and E. Lee, J. Membr. Sci., 1999, 154, 251–259 CrossRef.
- J. R. Kwiatkowski and M. Cheryan, J. Am. Oil Chem. Soc., 2005, 82, 221–227 CrossRef CAS PubMed.
- S. Darvishmanesh, T. Robberecht, P. Luis, J. Degréve and B. Van der Bruggen, J. Am. Oil Chem. Soc., 2011, 88, 1255–1261 CrossRef CAS.
- S. Manjula, H. Nabetani and R. Subramanian, J. Membr. Sci., 2011, 366, 43–47 CrossRef CAS PubMed.
- C. Weibin, S. Yanzhi, P. Xianglan, L. Jiding and Z. Shenlin, Chin. J. Chem. Eng., 2011, 19, 575–580 CrossRef.
- L. R. Firman, N. A. Ochoa, J. Marchese and C. L. Pagliero, J. Membr. Sci., 2013, 431, 187–196 CrossRef CAS PubMed.
- L. P. Raman, M. Cheryan and N. Rajagopalan, Lipids, 1996, 98, 10–14 CrossRef CAS.
- W. E. Siew, A. G. Livingston, C. Ates and A. Merschaert, Chem. Eng. Sci., 2013, 90, 299–310 CrossRef CAS PubMed.
- S. Darvishmanesh, L. Firoozpour, J. Vanneste, P. Luis, J. Degrève and B. Van der Bruggen, Green Chem., 2011, 13, 3476–3483 RSC.
- R. Abejón, A. Garea and A. Irabien, AIChE J., 2014, 60, 931–948 CrossRef.
- Y. Kong, D. Shi, H. Yu, Y. Wang, J. Yang and Y. Zhang, Desalination, 2006, 191, 254 CrossRef CAS PubMed.
- B. M. Bhosle, R. Subramanian and K. Ebert, Eur. J. Lipid Sci. Technol., 2005, 107, 746–753 CrossRef CAS.
- T. Fane, Developments in membrane technology are providing significant opportunities and challenges in desalination, Chem. Eng., 2013, 7, 26–29 Search PubMed.
- A. Rathore and A. Velayudhan, Scale-up and Optimization in Preparative Chromatography, Marcel Dekker, Inc., 2003 Search PubMed.
- M. Mulder, Basic Principles of Membrane Technology, Kluwer Academic Publishers, Dordrecht, 1996 Search PubMed.
- A. Criscuoli and E. Drioli, Ind. Eng. Chem. Res., 2007, 46, 2268–2271 CrossRef CAS.
- M. R. Wiesner and S. Chellan, Environ. Sci. Technol., 1999, 33(17), 360A–366A CrossRef CAS PubMed.
- R. C. Gumerman, B. E. Burris and S. P. Hansen, Estimation of Small System Water Treatment Costs; U.S. EPA Contract No. 68-03-3093, U.S., Environmental Protection Agency, U.S. Government Printing Office, Washington, DC, 1984 Search PubMed.
- R. C. Gumerman, R. L. Culp and S. R. Hasen, Estimating Water Treatment Costst EPA-600/2–79–162a-d, U.S. Environmental Protection Agency, U.S. Government Printing Office, Washington, DC, 1979 Search PubMed.
- R. H. Perry and C. H. Chilton, Chemical Engineers’ Handbook, McGraw-Hill, New York, 1999, vol. 19 Search PubMed.
- P. Silva, L. G. Peeva and A. G. Livingston, J. Membr. Sci., 2010, 349, 167–174 CrossRef CAS PubMed.
- AICHE 2020 Roadmap.
- R. W. Baker, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef CAS.
- 2020 Analysis and Forecasts of Bioethanol Market.
- R. L. Goldsmith, R. P. de Filippi, S. Hossain and R. S. Timmins, in Membrane Processes in Industry and Biomedicine, ed. M. Bier, Plenum Press, New York, 1971, pp. 267–300 Search PubMed.
- F. V. Kosikowski, in Membrane Separations in Biotechnology ed. W. C. McGregor, Marcel Dekker, New York, 1986, pp. 201–254 Search PubMed.
- S. Judd, The BMR Book: Principles and Applications of membrane bioreactors in water and wastewater treatment, Elsevier Ltd, Oxford, 2006 Search PubMed.
- A. G. Fane, Submerged membranes, in Advanced Membrane Technology and Applications ed. N. N. Li, A. g. Fane, W. S. W. Ho and T. Matsuura, John Wiley & Sons, Inc., Hoboken, NJ, 2008, pp. 239–270 Search PubMed.
- S. Kennedy and S. J. Churchouse, Progress in membrane bioreactors: new advances. Proceedings of the Water and wastewater Europe Conference, Milan, 2005.
- http://duramem.evonik.com/product/duramem-puramem/en/Pages/default.aspx .
- http://www.solsep.com/ .
- http://www.amsmembrane.com/ .
- http://www.gmtmem.com/en .
- N. A. Bhore, R. M. Gould, S. M. Jacob, P. O. Staffeld, D. McNally, P. H. Smiley and C. R. Wildemuth, Oil Gas J., 1999, 97, 67–74 CAS.
- L. White, J. Membr. Sci., 2006, 286, 26–35 CrossRef CAS PubMed.
- G. Baumgarten, 3rd International Conference on Organic Solvent Nanofiltration, London, September 2010.
- A. Buekenhoudt, H. Beckers, D. Ormerod, M. Bulut, P. Vandezande and R. Vleeschouwers, Chem. Ing. Tech., 2013, 85, 1243–1247 CrossRef CAS.
- H. E. A. Brüschke, State-of-the-art of pervaporation processes in the chemical industry, Membrane Technology in the Chemical Industry, 2001, pp. 127–172 Search PubMed.
- R. W. Baker, Membrane Technology and Applications, Wiley, 2012 Search PubMed.
- L. H. Shaffer and M. S. Mintz, Electrodialysis, in Principles of Desalination, Academic Press, New York, 1966, pp. 200–289 Search PubMed.
- R. W. Baker and J. G. Wiljmans, Polymeric Gas Separation Membranes, CRC Press, Boca Raton, FL, 1994, pp. 353–398 Search PubMed.
- N. W. Ockwig and T. M. Nenoff, Chem. Rev., 2007, 107, 4078 CrossRef CAS PubMed.
- M. Elimelech and W. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- B. J. Feinberg, G. Z. Ramon and E. M. V. Hoek, Environ. Sci. Technol., 2013, 47, 2982–2989 CrossRef CAS PubMed.
- S. R. Auvil, J. S. Choe and L. J. Kellogg, U.S. Patent5,259,869, 1992 Search PubMed.
- R. A. Hayes, U.S. Patent5,141,642, 1992 Search PubMed.
- R. R. Zolandz and G. K. Fleming, Gas Permeation Applications, Chapman & Hall, New York, 1992 Search PubMed.
- B. Smith, D. Suhanya, S. Sridhar and M. Ramakrishna, J. Membr. Sci., 2004, 241, 1–21 CrossRef PubMed.
- J. Glater, S. K. Hong and M. Elimelech, Desalination, 1994, 95, 325–345 CrossRef CAS.
- M. F. A. Goosen, S. S. Sablani, H. Al-Hinai, S. Al-Obeidani, R. Al-Belushi and D. Jackson, Sep. Sci. Technol., 2005, 39, 2261–2297 CrossRef PubMed.
- P. Vandezande, L. E. M. Gevers and I. F. J. Vankelecom, Chem. Soc. Rev., 2008, 37, 365–405 RSC.
- A. Tsoukala, L. Peeva, A. G. Livingston and H.-R. Bjørsvik, ChemSusChem, 2012, 5, 188–193 CrossRef CAS PubMed.
- A. Stankiewicz, Chem. Eng. Process.: Process Intensif., 2003, 42, 137–144 CrossRef CAS.
- P. Schmidt, E. L. Bednarz, P. Lutze and A. Gorak, Chem. Eng. Sci., 2014, 115, 115–126 CrossRef CAS PubMed.
- S. M. Dutczak, C. R. Tanardi, K. K. Kopeć, M. Wessling and D. Stamatialis, Sep. Purif. Technol., 2012, 86, 183–189 CrossRef CAS PubMed.
- P. Sehn, E. Zhao, J. Johnson, M. Hallan and M. Peery, Water Pract. Technol., 2009, 4 Search PubMed.
- P. Vandezande, L. E. M. Gevers, N. Weyens and I. F. J. Vankelecom, J. Comb. Chem., 2009, 11, 243–251 CrossRef CAS PubMed.
- P. Marchetti, A. Butté and A. G. Livingston, J. Membr. Sci., 2013, 444, 101–115 CrossRef CAS PubMed.
- L. G. Peeva, E. Gibbins, S. S. Luthra, L. S. White, R. P. Stateva and A. G. Livingston, J. Membr. Sci., 2004, 236, 121–136 CrossRef CAS PubMed.
- E. A. Mason and H. K. Lonsdale, J. Membr. Sci., 1990, 51, 1–81 CrossRef CAS.
- International Conference On Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html, August 2009.
- Z. R. Lazic, Design of Experiments in Chemical Engineering, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004 Search PubMed.
- R. Soleimani, N. Alavi Shoushtari, B. Mirza and A. Salahi, Chem. Eng. Res. Des., 2013, 91, 883–903 CrossRef CAS PubMed.
- L. Eriksson, E. Johansson, N. Kettaneh-Wold, C. Wikström and S. Wold, Design of Experiments – Principles and Applications, Umetrics Academy Publisher, Malmö, Sweden, 2008 Search PubMed.
- M. Bulut, L. E. M. Gevers, J. S. Paul, I. F. J. Vankelecom and P. A. Jacobs, J. Comb. Chem., 2006, 8, 168–173 CrossRef CAS PubMed.
- S. S. Madaeni, N. T. Hasankiadeh, A. R. Kurdian and A. Rahimpour, Sep. Purif. Technol., 2010, 76, 33–43 CrossRef CAS PubMed.
- P. Amirabedi, R. Yegani and M. K. R. Aghjeh, J. Textiles Polym., 2013, 1 Search PubMed.
- S. Darvishmanesh, J. Degréve and B. Van der Bruggen, ChemPhysChem, 2010, 11, 404–411 CrossRef CAS PubMed.
- S. Darvishmanesh, J. Degréve and B. Van der Bruggen, Phys. Chem. Chem. Phys., 2010, 12, 13333–13342 RSC.
- D. Machado, D. Hasson and R. Semiar, J. Membr. Sci., 2000, 166, 63–69 CrossRef CAS.
- J. Geens, B. Van der Bruggen and C. Vandecastleele, Sep. Purif. Technol., 2006, 48, 255–263 CrossRef CAS PubMed.
- S. Darvishmanesh, A. Buekenhoudt, J. Degréve and B. Van der Bruggen, Sep. Purif. Technol., 2009, 70, 46–52 CrossRef CAS PubMed.
- P. Marchetti, A. Butté and A. G. Livingston, J. Membr. Sci., 2012, 444, 415–416 Search PubMed.
- I. F. J. Vankelecom, K. De Smeta, L. E. M. Gevers, A. G. Livingston, D. Nair, S. Aerts, S. Kuypers and P. A. Jacobs, J. Membr. Sci., 2004, 231, 99–108 CrossRef CAS PubMed.
- P. Silva and A. G. Livingston, J. Membr. Sci., 2006, 280, 889–898 CrossRef CAS PubMed.
- W. R. Bowen and J. S. Welfoot, Chem. Eng. Sci., 2002, 57, 1121–1137 CrossRef CAS.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- E. Yaroshchuk, J. Membr. Sci., 1995, 10, 83–87 CrossRef.
- J. A. Whu, B. C. Balzis and K. K. Sirkar, J. Membr. Sci., 2000, 170, 159–172 CrossRef CAS.
- T. Tsuru, S. Izumi, T. Yoshioka and M. Asaeda, AIChE J., 2000, 46, 565–574 CrossRef CAS.
- T. Tsuru, M. Narita, R. Shinagawa and T. Yoshioka, Desalination, 2008, 233, 1–9 CrossRef CAS PubMed.
- G. Schock and A. Miquel, Desalination, 1987, 64, 339–352 CrossRef CAS.
- A. L. Ahmad, C. P. Leo and S. R. Abd. Shukor, Desalin. Water Treat., 2009, 5, 80–90 CrossRef CAS.
- K. S. Bahçeci, Int. J. Food Sci. Technol., 2012, 47, 315–324 CrossRef PubMed.
- J. M. Gozálvez-Zafrilla, A. Santafé-Moros, J. Carlos García-Díaz, J. M. Gozálvez-Zafrilla, A. Santafé-Moros and J. Carlos García-Díaz, Desalination, 2013, 315, 61–69 CrossRef PubMed.
- K. Guerra, J. Pellegrino and J. E. Drewes, Sep. Purif. Technol., 2012, 87, 47–53 CrossRef CAS PubMed.
- E. Połom and D. Szaniawska, Desalination, 2006, 198, 208–214 CrossRef PubMed.
- B. Rahmaniana, M. Pakizeh, S. A. A. Mansoori and R. Abedini, J. Hazard. Mater., 2011, 187, 67–74 CrossRef PubMed.
- A. Román, G. Vatai, A. Ittzés, Z. Kovács and P. Czermak, J. Food Eng., 2012, 35, 708–714 CrossRef PubMed.
- P. Marchetti, A. Butté and A. G. Livingston, Chem. Eng. Sci., 2013, 101, 200–212 CrossRef CAS PubMed.
- P. Schmidt, T. Köse and P. Lutze, J. Membr. Sci., 2013, 429, 103–120 CrossRef CAS PubMed.
- A. Caus, L. Braeken, K. Boussu and B. Van der Bruggen, J. Chem. Technol. Biotechnol., 2009, 84, 391–398 CrossRef CAS.
- J. C. Lin and A. G. Livingston, Chem. Eng. Sci., 2007, 62, 2728–2736 CrossRef CAS PubMed.
- D. Peshev and A. G. Livingston, Chem. Eng. Sci., 2013, 104, 975–987 CrossRef CAS PubMed.
- I. Sereewatthanawut, F. C. Ferreira, N. F. Ghazali and A. G. Livingston, AIChE J., 2010, 56, 893–904 CAS.
- http://www.epa.ie/whatwedo/advice/bat/ .
Footnotes |
| † The main techniques for the fabrication of the top layer of TFC membranes are: (a) dip coating a solution of a polymer onto a support; (b) dip coating a solution of a reactive monomer onto a support, followed by post-curing with heat or irradiation; (c) interfacial polymerization; (d) casting an ultrathin film separately, then laminating it to a support; (e) depositing a barrier film directly from a gaseous phase monomer plasma. |
| ‡ The mass intensity (MI) and solvent intensity (SI) are defined as the ratio between the total mass of the material or solvent used to generate a quantity of product per unit of target compound produced, respectively. |
| § Membranes discriminate between dissolved molecules of different sizes and are usually characterized by their molecular weight cut-off (MWCO), which is used to classify membranes in terms of selectivity. It is defined as the molecular weight of the molecule which is 90% rejected by the membrane. The value is interpolated from a curve of MW vs. rejection. |
| ¶ The E factor is defined as the mass ratio of total waste to products produced. |
| This journal is © The Royal Society of Chemistry 2014 |