Highly stereoselective anti-Markovnikov hydrothiolation of alkynes and electron-deficient alkenes by a supported Cu-NHC complex†
Abstract
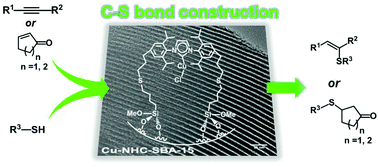
A practical, efficient, and low-cost heterogeneous catalyst consisting of a Cu-NHC (N-heterocyclic carbene) complex grafted to SBA-15 silica for the catalytic hydrothiolation of alkynes and electron-deficient alkenes under mild reaction conditions has been developed. The heterogeneous catalyst displays higher activity and stereoselectivity to Z-anti-Markovnikov isomers compared with the homogeneous analog under otherwise identical reaction conditions. The catalytic system is applicable to a broad range of alkynes and thiols and is recyclable without significant loss in catalytic performance. High activity and perfect selectivity to alkyl sulfides formed by the addition of electron-deficient alkenes to various thiols catalyzed by the supported Cu-NHC complex were also realized.

 Please wait while we load your content...
Please wait while we load your content...