Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient visible light-mediated cross-dehydrogenative coupling reactions of tertiary amines catalyzed by a polymer-immobilized iridium-based photocatalyst†
Woo-Jin
Yoo
and
Shū
Kobayashi
*
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: shu_kobayashi@chem.s.u-tokyo.ac.jp; Fax: +81-3-5634-0033; Tel: +81-3-5841-4794
First published on 5th February 2014
Abstract
The immobilization of an iridium-based heterogeneous photocatalyst via a radical polymerization process is described, and its catalytic activity was evaluated for the aerobic phosphonylation reaction of N-aryl tetrahydroisoquinolines under visible light irradiation.
The direct use of carbon–hydrogen (C–H) bonds in cross-coupling reactions is an exciting and challenging area of research that has the potential to streamline synthetic methods by reducing the amount of materials and energy required to make complex organic molecules.1 Despite the challenges associated with the low reactivity and selectivity in C–H bond functionalization, various successful carbon–carbon (C–C) and carbon–heteroatom (C–X) bond formation reactions have been realized with C–H bonds as reagents. The cross-dehydrogenative coupling (CDC) reaction of the α-C–H bond of nitrogen atoms represents one of the most successful examples of mild and selective C–C and C–X bond formations derived from C–H bonds.2 Although initial protocols relied heavily upon the use of stoichiometric amounts of strong oxidants,3 the rapid progress in this field has led to CDC reactions that can be performed under mild aerobic conditions.4
The use of sunlight, as a renewable and clean source of energy to facilitate organic transformations, represents a new frontier for environmentally sustainable organic chemistry. Recently, ruthenium- and iridium-based polypyridyl complexes, well-known organometallic compounds that absorb strongly in the visible light spectrum to produce long-lived photoexcited states, have emerged as efficient catalysts for organic transformations that are mediated by single-electron transfer (SET) processes.5 For instance, the strong oxidative potential of these photoexcited metal chromophores has been exploited to facilitate the CDC reaction of tertiary amines under ambient conditions in air.6 However, despite their emergence as efficient catalysts for a wide range of bond formation processes, the relative cost associated with ruthenium- and iridium-based photocatalysts limits their practical use. In this context, the development of easily recoverable and reusable heterogeneous visible light photocatalysts would be desirable. However, only limited examples of immobilized ruthenium- and iridium-based photocatalysts have been reported thus far.7
In this communication, we wish to report a suspension polymerization protocol to access an immobilized iridium-based polypyridyl complex, and its evaluation, as a heterogeneous visible light photocatalyst, by examining the aerobic phosphonylation reaction of N-aryl tetrahydroisoquinoline derivatives.
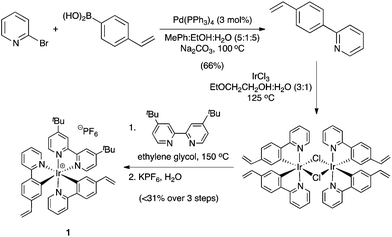
Based on our interest in the CDC reaction of tertiary amines,6c,8 and Stephenson's initial report on the use of an iridium-based photocatalyst for the oxidative aza-Henry reactions of N-aryl tetrahydroisoquinolines,6l we targeted Ir(ppy)2(dtbbpy)PF6 (ppy: 2-phenylpyridyl and dtbbpy: 4,4′-di-tert-butyl-2,2′-dipyridyl) for immobilization. Our strategy involved the synthesis of Ir(vppy)2(dtbbpy)PF6 (vppy: 2-(4-vinylphenyl)pyridyl) (1), with the assumption that the introduction of the aliphatic substituent would not adversely affect the photocatalytic activity of the immobilized iridium complex (Scheme 1).
The synthesis of the photoredox active monomer 1 began with the preparation of 2-(4-vinylphenyl)pyridine via a Suzuki–Miyaura cross-coupling reaction.9 Following the literature procedure for the synthesis of Ir(ppy)2(dtbbpy)PF6,10 the desired polypyridyl iridium complex 1 was obtained, albeit with some impurities that could not be separated. Despite this setback, the crude 1 was subjected to the heterogeneous radical polymerization process, with the understanding that the impurities would not be immobilized, with various well-established monomeric feedstocks (Scheme 2).
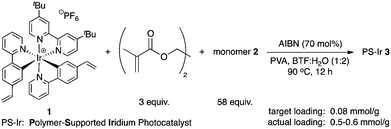
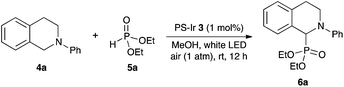
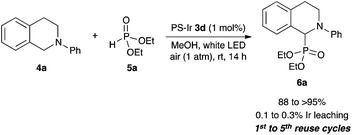
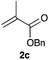
Our initial suspension polymerization protocol was based on our previously reported conditions,11 with a small modification with respect to the solvent choice. In general, these cationic iridium-based photocatalysts are soluble in solvents that are often miscible with water, thus unsuitable for suspension polymerization. Chlorinated solvents were found to dissolve 1 well, but inhibited the radical polymerization reaction. Fortuitously, benzotrifluoride (BTF), known as an excellent solvent for radical reactions,12 was found to be the most suitable solvent for our immobilization efforts. With a polymerization protocol in hand, various monomers were utilized to immobilize 1, and these heterogeneous cross-linked co-polymers 3 were evaluated as catalysts for the aerobic CDC reaction of N-phenyl tetrahydroisoquinoline (4a) with diethyl phosphite (5a) (Table 1).13
| Entry | PS-Ir 3 | Monomer 2 | Ir loadingb (mmol g−1) | Yield of 6ac (%) | Ir leachingd (%) |
|---|---|---|---|---|---|
| a Reaction conditions: amine 4a (0.25 mmol), phosphite 5a (0.25 mmol), and PS-Ir 3 (0.0025 mmol, 1 mol%) in MeOH (0.8 mL) at room temperature for 12 h under a balloon of dry air and 7.1 W white LED illumination. b Ir levels were determined by inductive coupled plasma (ICP) analysis of the acid-digested PS-Ir 3. c Yield based on 4a and determined by 1H NMR analysis using 1,1,2,2-tetrachloroethane as an internal standard. d Ir levels were determined by ICP analysis of the crude reaction filtrate. e PS-Ir 3c was subjected to the polymerization protocol with 2c. f The reaction time was extended to 14 h. | |||||
| 1 | 3a |
|
0.0590 | 74 | 1.0 |
| 2 | 3b |
|
0.0586 | 83 | 2.1 |
| 3 | 3c |
|
0.0523 | 90 | 0.9 |
| 4e,f | 3d |
|
0.0255 | 92 | 0.3 |
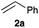
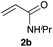
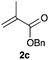
We found that the performance of the immobilized photocatalysts was affected by the monomer choice for the visible light-mediated CDC reaction, with the acrylate-based cross-linked copolymer 3c providing the best results (entries 1–3). However, when we evaluated the effectiveness of our immobilization strategy, we found that small amounts of iridium leaching occurred. We hypothesized that an additional layer of polymer might help minimize metal leaching, and when we subjected 3c to the radical polymerization process with 2c, we found that the resulting polymer-supported iridium photocatalyst 3d was an effective photocatalyst for the aerobic phosphonylation reaction with lower levels of iridium leaching (entry 4).
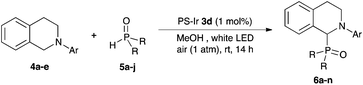
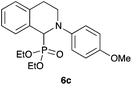
Next, we examined the substrate scope of the visible light-mediated aerobic CDC reaction of N-aryl tetrahydroisoquinolines 4a–e with various phosphites 5a–d and secondary phosphine oxides 5e–j (Table 2). We initially examined various N-aryl tetrahydroisoquinolines (4a–e) and found that the substituents on the aromatic ring influenced the CDC reaction (entries 1–5). In particular, we found that the strong electron-donating methoxy group caused the oxidative coupling reaction to become sluggish, and a longer reaction time and a high catalyst loading were required for complete conversion of 4c (entry 3). On the other hand, when halogen-substituted tertiary amines 4d–e were utilized as substrates, side-product formation caused a decrease in the overall yields of the desired CDC adducts (entries 4 and 5). We then examined various aliphatic phosphites 5a–d as nucleophiles, and found that with the exception of the more bulky diisopropyl phosphite (5d), the yields of the desired oxidative coupled products were excellent (entries 6–8). We also utilized our immobilized Ir photocatalyst 3d for the CDC-type reaction using secondary phosphine oxides 5e–j as P-based nucleophiles (entries 9–14). In general, the phosphine oxides were found to be excellent partners for the aerobic coupling reactions, and good to excellent yields of the expected CDC products were obtained.
| Entry | Ar | R | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: amine 4 (0.50 mmol), phosphite/phosphine oxide 5 (0.50 mmol), and PS-Ir 3d (0.005 mmol, 1 mol%) in MeOH (1.6 mL) at room temperature for 14 h under a balloon of dry air and 7.1 W white LED illumination. b Yield of isolated product 6 was based on 4. c Reaction conditions: amine 4c (0.50 mmol), phosphite 5a (0.50 mmol), and PS-Ir 3d (0.010 mmol, 2 mol%) in MeOH (1.6 mL) at room temperature for 24 h under a balloon of dry air and 7.1 W white LED illumination. | ||||
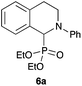
| 1 | Ph | OEt |
|
87 |
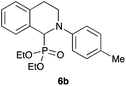
| 2 | 4-Me–C6H4 | OEt |
|
97 |
| 3c | 4-MeO–C6H4 | OEt |
|
87 |
| 4 | 4-Br–C6H4 | OEt |
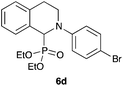
|
39 |
| 5 | 4-Cl–C6H4 | OEt |
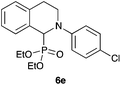
|
47 |
| 6 | Ph | OMe |
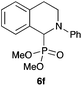
|
95 |
| 7 | Ph | OnBu |
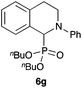
|
88 |
| 8 | Ph | OiPr |

|
67 |
| 9 | Ph | Ph |

|
92 |
| 10 | Ph | 4-Me–C6H4 |
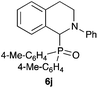
|
85 |
| 11 | Ph | 4-MeO–C6H4 |

|
89 |
| 12 | Ph | 4-Cl–C6H4 |
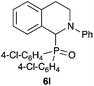
|
87 |
| 13 | Ph | 4-CF3–C6H4 |

|
61 |
| 14 | Ph | c Hexyl |
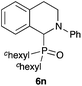
|
77 |
Finally, we examined the viability of recovering and reusing the cross-linked co-polymer 3d for the aerobic phosphonylation reaction. It was found that the catalyst could be reused at least four times without a noticeable loss of catalytic activity with minimal levels of iridium leaching (Scheme 3).
In conclusion, we successfully immobilized an iridium-based polypyridyl complex, through the use of the well-established suspension polymerization method, and demonstrated its effectiveness as a visible light photocatalyst for the aerobic CDC reaction between N-aryl tetrahydroisoquinolines and various P–H nucleophiles under visible light irradiation. The synthetic utility of this heterogeneous photocatalyst was established through the recovery and reuse studies, which showed that the catalyst could be reused up to four times without loss of reactivity. We anticipate that the immobilization strategy described in this report could be easily adopted to access multitudes of heterogeneous visible light photocatalysts derived from metal polypyridyl complexes.
This work was partially supported by a Grant-in-Aid for Science Research from the Japan Society for the Promotion of Science (JSPS), the Global COE Program (Chemistry Innovation through Cooperation of Science and Engineering), The University of Tokyo, the Japan Science and Technology Agency (JST), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Notes and references
- For representative reviews on C–H bond functionalization, please see:
(a) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed
; (b) D. Y.-K. Chen and S. W. Youn, Chem.–Eur. J., 2012, 18, 9452 CrossRef CAS PubMed
; (c) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC
; (d) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed
; (e) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed
.
- For representative reviews on the CDC reactions with tertiary amines, please see:
(a) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed
; (b) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC
; (c) C. J. Scheuermann, Chem.–Asian J., 2010, 5, 436 CrossRef CAS PubMed
; (d) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed
; (e) S.-I. Murahashi and D. Zhang, Chem. Soc. Rev., 2008, 37, 1490 RSC
.
-
(a) T. Nobuta, N. Tada, A. Fujiya, A. Kariya, T. Miura and A. Itoh, Org. Lett., 2013, 15, 574 CrossRef CAS PubMed
; (b) L. Huang, X. Zhang and Y. Zhang, Org. Lett., 2009, 11, 3730 CrossRef CAS PubMed
; (c) L. Chu, X. Zhang and F.-L. Qing, Org. Lett., 2009, 11, 2197 CrossRef CAS PubMed
; (d) C. M. R. Volla and P. Vogel, Org. Lett., 2009, 11, 1701 CrossRef CAS PubMed
; (e) X. Xu and X. Li, Org. Lett., 2009, 11, 1027 CrossRef CAS PubMed
; (f) O. Baslé and C.-J. Li, Org. Lett., 2008, 10, 3661 CrossRef PubMed
; (g) M. Niu, Z. Yin, H. Fu, Y. Jiang and Y. Zhao, J. Org. Chem., 2008, 73, 3961 CrossRef CAS PubMed
; (h) Z. Li, D. S. Bohle and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS PubMed
; (i) S.-I. Murahashi, N. Komiya and H. Terai, Angew. Chem., Int. Ed., 2005, 44, 6931 CrossRef CAS PubMed
; (j) Z. Li and C.-J. Li, Eur. J. Org. Chem., 2005, 3173 CrossRef CAS
; (k) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed
; (l) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef CAS PubMed
; (m) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2004, 126, 11810 CrossRef CAS PubMed
.
-
(a) M. O. Ratnikov, X. Xu and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 9475 CrossRef CAS PubMed
; (b) J. Dhineshkumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 15, 1092 CrossRef CAS PubMed
; (c) Q.-Y. Meng, Q. Liu, J.-J. Zhong, H.-H. Zhang, Z.-J. Li, B. Chen, C.-H. Tung and L.-Z. Wu, Org. Lett., 2012, 14, 5992 CrossRef CAS PubMed
; (d) G. Zhang, Y. Ma, S. Wang, Y. Zhang and R. Wang, J. Am. Chem. Soc., 2012, 134, 12334 CrossRef CAS PubMed
; (e) E. Boess, C. Schmitz and M. Klussmann, J. Am. Chem. Soc., 2012, 134, 5317 CrossRef CAS PubMed
; (f) E. Boess, D. Sureshkumar, A. Sud, C. Wirtz, C. Farès and M. Klussmann, J. Am. Chem. Soc., 2011, 133, 8106 CrossRef CAS PubMed
; (g) L. Huang, T. Niu, J. Wu and Y. Zhang, J. Org. Chem., 2011, 76, 1759 CrossRef CAS PubMed
; (h) O. Baslé, N. Borduas, P. Dubois, J. M. Chapuzet, T.-H. Chan, J. Lessard and C.-J. Li, Chem.–Eur. J., 2010, 16, 8162 CrossRef PubMed
; (i) O. Baslé and C.-J. Li, Chem. Commun., 2009, 4124 RSC
; (j) S.-I. Murahashi, T. Nakae, H. Terai and N. Komiya, J. Am. Chem. Soc., 2008, 130, 11005 CrossRef CAS PubMed
; (k) O. Baslé and C.-J. Li, Green Chem., 2007, 9, 1047 RSC
; (l) S.-I. Murahashi, N. Komiya, H. Terai and T. Nakae, J. Am. Chem. Soc., 2003, 125, 15312 CrossRef CAS PubMed
.
- For reviews on visible light photocatalysis, please see:
(a) T. P. Yoon, ACS Catal., 2013, 3, 895 CrossRef CAS PubMed
; (b) Y. Xi, H. Yi and A. Lei, Org. Biomol. Chem., 2013, 11, 2387 RSC
; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed
; (d) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed
; (e) M. A. Ischay and T. P. Yoon, Eur. J. Org. Chem., 2012, 3359 CrossRef CAS
; (f) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC
; (g) N. Hoffmann, ChemSusChem, 2012, 5, 352 CrossRef CAS PubMed
; (h) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed
; (i) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC
; (j) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed
.
-
(a) J. Xie, Q. Xue, H. Jin, H. Li, Y. Cheng and C. Zhu, Chem. Sci., 2013, 4, 1281 RSC
; (b) X. Ju, D. Li, W. Li, W. Yu and F. Bian, Adv. Synth. Catal., 2012, 354, 3561 CrossRef CAS
; (c) W.-J. Yoo, A. Tanoue and S. Kobayashi, Chem.–Asian J., 2012, 7, 2764 CrossRef CAS PubMed
; (d) S. Cai, X. Zhao, X. Wang, Q. Liu, Z. Li and D. Z. Wang, Angew. Chem., Int. Ed., 2012, 51, 8050 CrossRef CAS PubMed
; (e) D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094 CrossRef CAS PubMed
; (f) J. W. Tucker, Y. Zhang, T. F. Jamison and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2012, 51, 4144 CrossRef CAS PubMed
; (g) M. Rueping, R. M. Koenigs, K. Poscharny, D. C. Fabry, D. Leonori and C. Vila, Chem.–Eur. J., 2012, 18, 5170 CrossRef CAS PubMed
; (h) D. B. Freeman, L. Furst, A. G. Condie and C. R. J. Stephenson, Org. Lett., 2012, 14, 94 CrossRef CAS PubMed
; (i) M. Rueping, S. Zhu and R. M. Koenigs, Chem. Commun., 2011, 47, 12709 RSC
; (j) M. Rueping, D. Leonori and T. Poisson, Chem. Commun., 2011, 47, 9615 RSC
; (k) M. Rueping, S. Zhu and R. M. Koenigs, Chem. Commun., 2011, 47, 8679 RSC
; (l) A. G. Condie, J. C. González-Gómez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464 CrossRef CAS PubMed
.
-
(a) N. Priyadarshani, Y. Liang, J. Suriboot, H. S. Bazzi and D. E. Bergbreiter, ACS Macro. Lett., 2013, 2, 571 CrossRef CAS
; (b) C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2012, 134, 7211 CrossRef CAS PubMed
; (c) C. Wang, Z. Xie, K. E. deKrafft and W. Lin, ACS Appl. Mater. Interfaces, 2012, 4, 2288 CrossRef CAS PubMed
; (d) J.-L. Wang, C. Wang, K. E. deKrafft and W. Lin, ACS Catal., 2012, 2, 417 CrossRef CAS
; (e) C. Wang, Z. Xie, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445 CrossRef CAS PubMed
; (f) H. Shimakoshi, M. Nishi, A. Tanaka, K. Chikama and Y. Hisaeda, Chem. Commun., 2011, 47, 6548 RSC
; (g) Z. Xie, C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 2056 CrossRef CAS PubMed
.
- A. Tanoue, W.-J. Yoo and S. Kobayashi, Adv. Synth. Catal., 2013, 355, 269 CAS
.
- H. Mizuno, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2011, 133, 1251 CrossRef CAS PubMed
.
- Y. Miyake, K. Nakajima and Y. Nishibayashi, J. Am. Chem. Soc., 2012, 134, 3338 CrossRef CAS PubMed
.
- H. Miyamura, G. C. Y. Choo, T. Yasukawa, W.-J. Yoo and S. Kobayashi, Chem. Commun., 2013, 49, 9917 RSC
.
-
(a) J. J. Maul, P. J. Ostrowski, G. A. Ublacker, B. Linclau and D. P. Curran, Top. Curr. Chem., 1999, 206, 79 CrossRef
; (b) A. Ogawa and D. P. Curran, J. Org. Chem., 1997, 62, 450 CrossRef CAS
.
- Upon a reviewer's suggestion, we also examined the aerobic phosphonylation reaction between N-phenyl tetrahydroisoquinoline (4a) and diethyl phosphite (5a) using Ir(ppy)2(dtbbpy)PF6 as a catalyst under our optimized reaction conditions. We found that the reaction proceeds, albeit with undesired side reactions, to provide the desired CDC adduct in 55% yield.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures for the suspension polymerization and the aerobic phosphonylation reaction of N-aryl tetrahydroisoquinoline derivatives, characterization data (1H NMR, 13C NMR, 31P NMR, 19F NMR, IR, high-resolution MS) for all new compounds. See DOI: 10.1039/c4gc00058g |
| This journal is © The Royal Society of Chemistry 2014 |