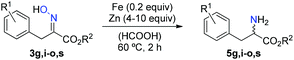Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fixation and recycling of nitrogen monoxide through carbonitrosation reactions†
Cristina
de Salas
and
Markus R.
Heinrich
*
Department für Chemie und Pharmazie, Pharmazeutische Chemie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Schuhstraße 19, 91052 Erlangen, Germany. E-mail: markus.heinrich@fau.de
First published on 11th March 2014
Abstract
The removal of nitrogen monoxide from gas streams through complexation to iron(II) ions in aqueous dimethylsulfoxide can be combined with a new variant of the Meerwein arylation, which incorporates the previously complexed NO into organic compounds to give oximes as final products. The first step of this two-step process has been evaluated regarding the effectiveness of the NO absorption and the sensitivity of the aqueous iron(II)–DMSO solution towards oxygen from air, in both cases in comparison with the known BioDeNOx process. The subsequent Meerwein arylation, which was designed with the intention to make use of nitrogen monoxide as the simplest nitrogen-centered radical scavenger, is shown to tolerate an exceptionally broad spectrum of substituents on the aromatic core of the diazonium salts including electron-donating as well as electron-withdrawing substituents. Under simple conditions the resulting oximes can be converted to racemic amino acid esters.
Introduction
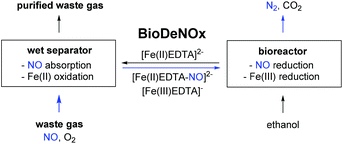
In the last couple of years much attention has been paid to the development of sustainable chemistry processes that are able to convert environmentally problematic components of exhaust gases into valuable products.1 Interestingly, this general objective has so far only very scarcely been extended to nitrogen monoxide or nitrogen dioxide. The removal of these toxic compounds, which is known as dentrification, is today commonly achieved through selective catalytic reduction (SCR)2 or selective non-catalytic reduction (SNCR).3 In both cases, only simple dinitrogen is produced as the final product from NO or NO2 by employing ammonia or urea as reductants. High temperatures, NH3 slippage and the difficult adjustment to unsteady nitrogen monoxide concentrations remain challenges.4 Alternatively, the concentrations of NO and NO2 can be decreased by gas absorption systems using acidic, alkaline or oxidizing solutions.5 In this way, inorganic nitrates and nitric acid can be obtained as commercially exploitable products, from which nitric acid is commonly further applied in nitration reactions.6A unique process among the denitrification strategies based on absorption exists that is known as BioDeNOx (Scheme 1).7 The removal of nitrogen monoxide from the exhaust gas is hereby achieved at relatively low temperatures by using aqueous iron(II)–EDTA complexes as scavengers (left part).8 The thus formed iron(II)–EDTA–NO complexes are then transferred from the scrubber unit to a bioreactor (right part). In the second step of the process, the iron(II)–EDTA complexes are regenerated from the iron-nitrosyl complexes by enzymatic reduction to produce nitrogen and carbon dioxide from ethanol added as a reductant.9,10
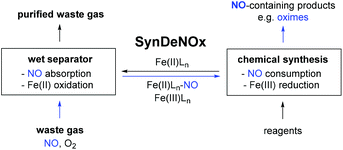
As an extension of the BioDeNOx process, we recently started to investigate whether iron-nitrosyl complexes formed by the absorption of nitrogen monoxide from an NO-containing gas stream could also be used for synthetic purposes (Scheme 2). In this way, valuable chemical products, such as oximes, would be produced from the denitrification instead of simple nitrogen, and the overall strategy could be described as SynDeNOx.
Regarding the amounts and concentrations of nitrogen monoxide that will most probably be required for a successful recycling process of this type, an attractive field of application for SynDeNOx appears to be the multiple industrial processes of metal dissolution, metal processing and metal finishing. With dilute nitric acid as the most common reagent for the treatment of many metals and alloys, gas streams with NO contents of up to 25 vol% can be obtained without difficulty.11–13 Advantageously, exhaust gas streams from such processes usually do not contain large amounts of SO2 or fly ash, which if present could complicate the recycling of NO. From a chemical point of view, and most probably due to the rapid oxidation of nitrogen monoxide to nitrogen dioxide in the presence of oxygen or air,14 only a few radical reactions had been reported for the incorporation of nitrogen monoxide from oxygen-containing gas mixtures into organic substrates at the beginning of our studies.15–17 We were therefore surprised to find that Meerwein-type arylations can be a useful tool for the synthetic reuse of nitrogen monoxide under the desired conditions.18–20 In this communication, we provide detailed insights into the effectiveness of the NO trapping by iron(II) salts in aqueous dimethylsulfoxide, into the scope of the Meerwein arylation and into the transformation of the primarily obtained oximes into amino acids.
Results and discussion
Concerning the potential applicability of iron(II)–EDTA–NO complexes, which are available from the first step of the BioDeNOx process (Scheme 1), as NO donors in radical reactions, preliminary studies in our group had shown that these complexes are not well suited for reactions involving aryl radicals. This may firstly be due to possible hydrogen abstraction from the EDTA ligand by the highly reactive aryl radicals.21 Moreover, aryl radical reactions are triggered by the generation of the aryl radical and since there is no resting state in the radical reaction mechanism of the Meerwein arylation the nitrogen monoxide needs to be released quickly enough from the iron–EDTA complex to ensure an efficient trapping and to avoid oligomerisation.22 This quick release appears quite unlikely due to the relatively high stability of the iron(II)–EDTA–NO complexes.8The conclusion from these initial studies was that a ligand for the iron(II) ions would be required that is largely stable towards hydrogen abstraction by aryl radicals and that does at the same time sufficiently increase the binding affinity of the iron(II) ions towards nitrogen monoxide. Ideally, this complex should not be as sensitive to oxygen as aqueous iron(II)–EDTA. Since aqueous dimethylsulfoxide as a solvent had shown a comparatively high stability towards hydrogen abstraction in earlier studies,23 and had also turned out to be well suited for reactions proceeding via aryl radicals generated from arenediazonium salts by reduction with iron(II)-sulfate, we examined the NO-binding properties of iron(II)-sulfate in mixtures of DMSO and water.
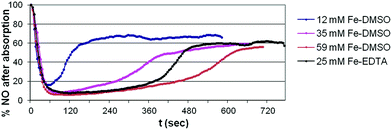
For this purpose, a stream of air containing 0.4 vol% (4 mL/1000 mL) NO was passed through solutions of iron(II)-sulfate in aqueous DMSO. The initial reference value for 100% was obtained by measuring the NO content of the gas stream with a bypass for the absorption vessel. After redirecting the gas stream through the iron(II)–DMSO–water mixture, the NO concentration in the exiting gas stream was determined in close intervals over 10–12 minutes (Fig. 1). Not unexpectedly, the NO binding ability of the iron(II)-containing solution gradually increases with the concentration of iron(II). The comparison with the BioDeNOx setup, in which typically 25 mM solutions of iron(II)–EDTA are used for the NO removal, shows that a 35 mM iron(II)–DMSO solution does not yet reach the iron(II)–EDTA effectiveness of NO removal, but a 59 mM solution shows slightly better properties. Comparing the curve integrals (areas above graphs in Fig. 1) of the three experiments with the iron(II)–DMSO system, the total NO uptake appears to be proportional to the amount of iron(II) ions present in the solution. With iron(II)–EDTA, a comparable total NO uptake can be achieved at a lower concentration of iron(II), since iron(II)–EDTA does bind NO more strongly.8
The curve progressions further demonstrate that all absorption systems, after reaching saturation, do still possess a certain ability to remove NO and to decrease its content to ca. 60% of its original amount. We currently assume that this is due to a partial conversion of NO to NO2, which is readily absorbed into aqueous solutions.
To evaluate and compare the influence of oxygen on the BioDeNOx (Fe–EDTA) and the SynDeNOx (Fe–DMSO) absorption system, we pretreated a 25 mM solution of iron(II)–EDTA and a 59 mM solution of iron(II)–DMSO with air over a defined period of time.24,25 The resulting solutions were then used for the usual absorption experiments. Not surprisingly, the absorption capacity of both systems decreases when the pretreatment with air is prolonged from 1 to 3 and then to 5 minutes (Fig. 2). A comparison of relative absorption values for 3 and 5 minutes of pretreatment indicates that the Fe–DMSO system is slightly less affected through oxidation by air than the Fe–EDTA system. The Fe–DMSO system however contains a more than twofold higher concentration of iron(II) ions.
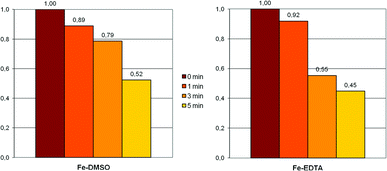 | ||
| Fig. 2 Relative NO absorption by the Fe–DMSO (59 mM) and the Fe–EDTA (25 mM) system after pretreatment with air. | ||
Up to this point, the Fe–DMSO system has shown a lower NO absorption capacity than the Fe–EDTA system and a comparable stability towards oxidation by air, albeit at a twofold higher iron(II) concentration. But most importantly among the prerequisites, the Fe–DMSO system is suitable for a combination with reactions proceeding via highly reactive aryl radicals. Given these first promising results, we then turned towards a closer investigation of the scope and the limitations of the Meerwein arylation (carbonitrosation) which was developed for the incorporation of NO in organic compounds. For the experiments summarized in Table 1, nitrogen monoxide was used as a pure gas under anaerobic conditions.
| Entry | Arenediazonium salt 1: R1= | Alkene 2: R2= | Oximeb3 (%) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z) Z) |
|---|---|---|---|
| a Reaction conditions: see the Experimental section in the ESI for the general procedure. b Yields after purification by column chromatography. | |||
| 1 | 1a: p-NO2 | 2a: Ph |
3a: 60 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 2 | 1b: H | 2a: Ph | 3b: 50 (E) |
| 3 | 1a: p-NO2 | 2b: CN |
3c: 78 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 4 | 1c: p-Cl | 2b: CN |
3d: 71 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5 | 1a: p-NO2 | 2c: CO2Me | 3e: 84 (E) |
| 6 | 1d: p-CN | 2c: CO2Me | 3f: 82 (E) |
| 7 | 1c: p-Cl | 2c: CO2Me | 3g: 70 (E) |
| 8 | 1e: p-OMe | 2c: CO2Me | 3h: 55 (E) |
| 9 | 1f: p-F | 2c: CO2Me | 3i: 62 (E) |
| 10 | 1g: p-Br | 2c: CO2Me | 3j: 69 (E) |
| 11 | 1h: o-Cl | 2c: CO2Me | 3k: 59 (E) |
| 12 | 1i: o-Br | 2c: CO2Me | 3l: 70 (E) |
| 13 | 1j: o-F | 2c: CO2Me | 3m: 59 (E) |
| 14 | 1k: 3,4-(OMe)2 | 2c: CO2Me | 3n: 63 (E) |
| 15 | 1l: o-OMe | 2c: CO2Me | 3o: 79 (E) |
| 16 | 1m: p-CO2Me | 2c: CO2Me | 3p: 55 (E) |
| 17 | 1n: o-CO2Me | 2c: CO2Me | 3q: 68 (E) |
| 18 | 1o: m-OMe | 2c: CO2Me | 3r: 48 (E) |
| 19 | 1c: p-Cl | 2d: CO2Et | 3s: 61 (E) |
| 20 | 1c: p-Cl | 2e: CO2tBu | 3t: 60 (E) |
| 21 | 1c: p-Cl | 2f: CO2H | Traces |
| 22 | 1c: p-Cl | 2g: CONMe2 | 3u: 44 (E) |
The good yields obtained for almost all combinations of diazonium salts 1 and alkenes 2 show that the carbonitrosation reaction is a broadly applicable method, especially with regard to the substituents on the aromatic core of the diazonium salt. Due to the importance of the rate of reduction of the diazonium ions to generate aryl radicals, variants of the Meerwein arylation do not necessarily tolerate acceptor- as well as donor-substituted diazonium salts.26 Our earlier synthetic study had shown that the reaction principle can also be expanded to non-activated alkenes such as allyl acetate.23 With this type of alkenes the corresponding oximes are furnished in slightly lower yields ranging from 40 to 55%. The only, but complete failure in the present series of experiments occurred when acrylic acid (2f) was used as an activated alkene (entry 21).27 Oximes derived from amides, such as 3u from N,N-dimethyl acrylamide (2g) (entry 22), are interesting compounds due to the existence of a number of bioactive natural products with closely related structures.28
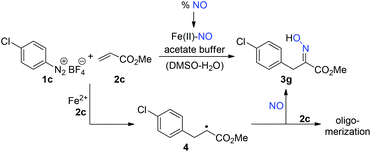
With regard to the application of carbonitrosation reactions for the purpose of recycling, it is necessary to determine the amounts or concentrations of nitrogen monoxide that are required to obtain the desired oximes in satisfactory yields. A closer inspection of the mechanistic background (Scheme 3) reveals that the concentration of nitrogen monoxide cannot be deliberately decreased. In case of lower concentrations of nitrogen monoxide in the exhaust gas stream and thus lower amounts of free or iron(II)-bound NO available in the reaction mixture, it is more likely that the radical adduct 4 adds to another molecule of methyl acrylate (2c) than that it is trapped by nitrogen monoxide.19,29
The results of the related experiments, which are summarized in Table 2, suggest that a NO content of about 10% is necessary to achieve reasonable yields of oxime 3g (entry 3).
Control experiments with NO concentrations of 1% and 0.4% did not lead to a measureable product formation. Repetition of two experiments in the presence of sulfur dioxide showed a certain decrease in yield (entries 2 and 4), but demonstrated that SO2 is generally tolerated.30
As a consequence, and as supposed in the Introduction, industrial processes from the field of metal manufacturing with dilute nitric acid are of particular interest for an application of this methodology. Probably due to the fact that gas mixtures with an NO content of 10–25% are frequently produced in such processes, metal processing plants are grouped among the “top 10 pollution problems”.31 Concerning the overall process, we found that the aqueous DMSO used in the reaction can be easily covered by extraction of the oximes with unipolar organic solvents.
After the investigation of the basic characteristics of carbonitrosation reactions, possible further transformations of the oximes were evaluated. An important field of application for the oximes 3e–t prepared from the acrylic acid esters 2c, 2d and 2e is the conversion to diversely ring-substituted derivatives of the aromatic amino acid phenylalanine.32 The required reduction of the oxime to an amine functionality was hereby conveniently achieved through treatment with zinc and substoichiometric amounts of iron powder in formic acid at slightly elevated temperatures (Table 3).33,34 Otherwise, such ring-substituted phenylalanines have to be prepared by more tedious alkylation of protected glycine derivatives35 or acetamidomalonates36 with much less well accessible benzyl halides. Alternatively, Heck-type reactions of aryl halides with acetamidoacrylates may be employed,37 which are however sensitive to further chloro- or bromo-substituents on the aromatic core. All these synthetic procedures undoubtedly show that high value fine chemicals are available through the newly developed NO recycling strategy, which by far exceed the prices of simple nitroarenes, nitric acid or nitrates being accessible through known NO recycling methodologies.6,38 The high commercial value of the oximes would also justify slightly increased costs for their purification.
| Entry | Oxime | Amineb5 (%) | |
|---|---|---|---|
| 3: R1= | R2= | ||
| a Reaction conditions: see the Experimental section in the ESI for the general procedure. b Yields after purification by column chromatography. | |||
| 1 | 3g: p-Cl | Me | 5g: 96 |
| 2 | 3i: p-F | Me | 5i: 75 |
| 3 | 3j: p-Br | Me | 5j: 92 |
| 4 | 3k: o-Cl | Me | 5k: 68 |
| 5 | 3l: o-Br | Me | 5l: 95 |
| 6 | 3m: o-F | Me | 5m: 93 |
| 7 | 3n: 3,4-(OMe)2 | Me | 5n: 67 |
| 8 | 3o: o-OMe | Me | 5o: 68 |
| 9 | 3s: p-Cl | Et | 5s: 89 |
The most attractive way to further convert racemic amino acid esters, such as the ethyl ester 5s, is to apply an enzymatic dynamic kinetic resolution (DKR).39 Recent progress in this field has been reported by Beller.40 In an alcalase-catalyzed hydrolysis employing 3,5-dinitrosalicylaldehyde for the continuous racemization of the starting materials, the racemic ethyl esters of phenylalanine and tyrosine could be converted to the corresponding L-amino acids in high yields and with excellent enantioselectivities.41
In addition to their conversion into amino acids, ketoximes – as they are now readily available through carbonitrosation reactions – have been valuable starting materials in enantioselective reductions with spiroboranes,42 in syntheses of heterocycles such as pyrroles and indoles,43 and in reactions proceeding via iminyl radicals.44
Summary
Based on our preliminary results, this study shows that the denitrification of nitrogen monoxide-containing gas streams can successfully be combined with the synthesis of oximes through a Meerwein-type carbonitrosation reaction. The aqueous iron(II)–DMSO absorption system used herein was found to be less efficient than the known iron(II)–EDTA system concerning the removal of NO, but to possess a comparable stability towards oxidation by air. Most importantly with regard to future developments aimed at recycling, the iron(II)–DMSO system allows the later incorporation of the iron-complexed NO into organic substrates through simple radical reactions with readily available precursors. The primarily obtained oximes have been shown to be versatile synthetic intermediates enabling novel, quick and broad access to highly valuable diversely ring-substituted phenylalanine derivatives.To our knowledge, the Meerwein-type carbonitrosation is the first reaction type that is suitable for the recycling of nitrogen monoxide from oxygen-containing gas streams through the synthesis of more valuable products than nitroarenes, nitric acid or nitrate salts. Carbonitrosations therefore represent first examples of how to implement the appealing concept of SynDeNOx, which aims at the combination of organic fine chemical synthesis with denitrification. In particular, the basic finding of this work, that iron(II)–DMSO systems are able to capture NO from oxygen-containing gas streams and to insert it into organic substrates via radical reactions, opens up many directions for future research aiming at the chemical problem of NO recycling.
Acknowledgements
We would like to thank the project students Ms Christina Heckel and Ms Stephanie Kindt for experimental assistance. We are further grateful for the generous financial support of this project by the Deutsche Bundesstiftung Umwelt (DBU).Notes and references
- (a) G. W. van Loon and S. J. Duffy, Environmental Chemistry: A global perspective, Oxford University Press, 3rd edn, 2010 Search PubMed; (b) Sustainable chemistry hierarchy published by the U.S. Environmental Protection Agency (EPA): http://www.epa.gov/greenchemistry/pubs/about_gc.html.
- Y. Hu, K. Griffiths and P. R. Norton, Surf. Sci., 2009, 603, 1740–1750 CrossRef CAS.
- M. T. Javed, N. Irfan and B. M. Gibbs, J. Environ. Manage., 2007, 83, 251–289 CrossRef CAS PubMed.
- (a) Y. Li and J. N. Armor, Appl. Catal., B, 1997, 13, 131–139 CrossRef CAS; (b) N. N. Sazonova, A. V. Simakov and H. Veringa, React. Kinet. Catal. Lett., 1996, 57, 71–79 CrossRef CAS.
- (a) B. Somnath, Chem. Eng. Commun., 2007, 194, 1374–1395 CrossRef; (b) J. B. Joshi, Chem. Eng. Commun., 1985, 33, 1–92 CrossRef CAS; (c) J. A. Patwardhan and J. B. Joshi, AIChE J., 2003, 49, 2728–2748 CrossRef CAS.
- (a) T. Matsumura, K. Kaji, T. Furuya and N. Nishiguchi, Japanese Patent JP51129762; Chem. Abstr. 86:138740, 1977 Search PubMed; (b) M. Porebski, B. Brzozowska, W. Kruzewski and P. Skubala, Polish Patent PL152551, Chem. Abstr. 115:91260, 1991 Search PubMed; (c) C. M. H. Brereton and A. A. Guenkel, US Patent US5963878, Chem. Abstr. 131:244827, 1999 Search PubMed; (d) M. R. Naimi-Jamal and G. Kaupp, Adv. Chem. Res., 2012, 11, 75–120 CAS.
- For recent reports on the BioDeNOx process, see: (a) N. Li, Y. Zhang, Y. Li, M. Chen, X. Dong and J. Zhou, J. Chem. Technol. Biotechnol., 2013, 88, 311–316 CrossRef CAS; (b) P. van der Maas, I. Manconi, B. Klapwijk and P. Lens, Biotechnol. Bioeng., 2008, 100, 1099–1107 CrossRef CAS PubMed; (c) P. van der Maas, P. van den Brink, S. Utomo, B. Klapwijk and P. Lens, Biotechnol. Bioeng., 2006, 94, 575–584 CrossRef CAS PubMed; (d) P. van der Maas, L. Harmsen, S. Weelink, B. Klapwijk and P. Lens, J. Chem. Technol. Biotechnol., 2004, 79, 835–840 CrossRef CAS.
- For the stability of iron-nitrosyl complexes, see: (a) M. Wolak and R. van Eldik, Coord. Chem. Rev., 2002, 230, 263–282 CrossRef CAS; (b) T. Schneppensieper, A. Wanat, G. Stochel, S. Goldstein, D. Meyerstein and R. van Eldik, Eur. J. Inorg. Chem., 2001, 2317–2325 CrossRef CAS; (c) T. Schneppensieper, S. Finkler, A. Czap, R. Van Eldik, M. Heus, P. Nieuwenhuizen, C. Wreesmann and W. Abma, Eur. J. Inorg. Chem., 2001, 491–501 CrossRef CAS.
- For a Pseudomonas sp. strain DN-2 with the ability to reduce not only Fe(II)–EDTA–NO but also Fe(III)–EDTA, see: S. Zhang, W. Li, C. Wu, H. Chen and Y. Shi, Appl. Microbiol. Biotechnol., 2007, 76, 1181–1187 CrossRef CAS PubMed.
- Drawbacks are the sensitivity of the iron(II)–EDTA complexes towards oxygen, long reaction times for the enzymatic regeneration of the complexes, cost-intensive enzymes as well as the relatively short lifetimes of the enzymes: P. van der Maas, P. van den Brink, B. Klapwijk and P. Lens, Chemosphere, 2009, 75, 243–249 CrossRef CAS PubMed.
- Highly concentrated NO is for example obtained from dilute nitric acid and copper: (a) Holleman-Wiberg's Inorganic Chemistry, ed. N. Wiberg and A. F. Holleman, Academic Press Inc., 2001 Search PubMed; (b) D. R. Kamperman, U.S., Patent No3945865; Chem. Abstr. 86:21269, 1977 Search PubMed.
- Personal information by Umicore, Precious Metals Competence Center.
- For a recent report on the purification of highly concentrated NOx gas (206 000 ppm) produced in a metal dissolution process, see: M. Yasuda, N. Tsugita, K. Ito, S. Yamauchi, W. R. Glomm, I. Tsuji and H. Asano, Environ. Sci. Technol., 2011, 45, 1840–1846 CrossRef CAS PubMed.
- S. Goldstein and G. Czapski, J. Am. Chem. Soc., 1995, 117, 12078–12084 CrossRef CAS.
- Radical reactions with nitrogen monoxide in the presence of oxygen usually proceed via the intermediate formation (more or less small amounts) of nitrogen dioxide. For a recent example, see: I. Jovel, S. Prateeptongkum, R. Jackstell, N. Vogl, C. Weckbecker and M. Beller, Adv. Synth. Catal., 2008, 350, 2493–2497 CrossRef CAS.
- For a review article presenting an overview of reactions that are suitable for the incorporation of (non-oxygen containing) nitrogen monoxide into organic molecules, see: J. Hartung, Chem. Rev., 2009, 109, 4500–4517 CrossRef CAS PubMed.
- Organocobalt compounds have been found to be especially suitable radical precursors with regard to incorporation of (non-oxygen containing) nitrogen monoxide: (a) T. Okamoto and S. Oka, J. Chem. Soc., Chem. Commun., 1984, 289–290 RSC; (b) V. F. Patel and G. Pattenden, Tetrahedron Lett., 1987, 28, 1451–1454 CrossRef CAS; (c) A. Ghosez, T. Göbel and B. Giese, Chem. Ber., 1988, 121, 1807–1811 CrossRef CAS; (d) A. Veit and B. Giese, Synlett, 1990, 166 CrossRef CAS; (e) M. Kijima, H. Yamashita and T. Sato, J. Organomet. Chem., 1992, 426, 399–404 CrossRef CAS; (f) T. Okamoto, K. Kobayashi, S. Oka and S. Tanimoto, J. Org. Chem., 1987, 52, 5089–5092 CrossRef CAS; (g) V. F. Patel and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1990, 2703–2708 RSC.
- For a first report, see: H. Meerwein, E. Büchner and K. v. Emster, J. Prakt. Chem., 1939, 152, 237–266 CrossRef CAS.
- For review articles on the Meerwein arylation, see: (a) M. R. Heinrich, Chem.–Eur. J., 2009, 15, 820–833 CrossRef CAS PubMed; (b) C. S. Rondestvedt, Org. React., 1976, 24, 225–259 CAS.
- For a first example of SynDeNOx, see: C. de Salas, O. Blank and M. R. Heinrich, Chem. – Eur. J., 2011, 17, 9306–9310 CrossRef CAS PubMed.
- C. Galli, Chem. Rev., 1988, 88, 765–792 CrossRef CAS.
- When organocobalt complexes are used as radical precursors in reactions with NO, a resting state is given, since the carbon-centered radicals can (in a reverse reaction) add to the cobalt complexes to regenerate the starting material.
- (a) M. R. Heinrich, O. Blank and A. Wetzel, J. Org. Chem., 2007, 72, 476–484 CrossRef CAS PubMed; (b) M. R. Heinrich, A. Wetzel and M. Kirschstein, Org. Lett., 2007, 9, 3833–3835 CrossRef CAS PubMed; (c) M. R. Heinrich, O. Blank, D. Ullrich and M. Kirschstein, J. Org. Chem., 2007, 72, 9609–9616 CrossRef CAS PubMed; (d) O. Blank, A. Wetzel, D. Ullrich and M. R. Heinrich, Eur. J. Org. Chem., 2008, 3179–3189 CrossRef CAS; (e) O. Blank, N. Raschke and M. R. Heinrich, Tetrahedron Lett., 2010, 51, 1758–1760 CrossRef CAS.
- A close investigation of the BioDeNOx process revealed that approximately 75% of the amount of iron(II)–EDTA is oxidized while only 25% serves for NO capture. This ratio was calculated from the amounts of ethanol required for the reduction of the iron(III)-complexes and NO in the bioreactor. For related studies, see: R. Kumaraswany, U. van Dongen, J. G. Kuenen, W. Abma, M. C. M. van Loosdrecht and G. Muyzer, Appl. Environ. Microbiol., 2005, 71, 6345–6352 CrossRef PubMed.
- For attempts to raise the stability of the iron(II)–EDTA complexes towards oxidation by the addition of fluoride ions, see: J. Maigut, R. Meier and R. van Eldik, Inorg. Chem., 2008, 47, 6314–6321 CrossRef CAS PubMed.
- P. Mastrorilli, C. F. Nobile and N. Taccardi, Tetrahedron Lett., 2006, 47, 4759–4762 CrossRef CAS.
- We currently assign the failure of the experiments with acrylic acid to the increased polarity of this particular alkene, which might complicate trapping of the alkyl radical intermediate with NO as well as extraction of the products from the aqueous phase.
- (a) L. Calcul, W. D. Inman, A. A. Morris, K. Tenney, J. Ratnam, J. H. McKerrow, F. A. Valeriote and P. Crews, J. Nat. Prod., 2010, 73, 365–372 CrossRef CAS PubMed; (b) P. Proksch, A. Putz, S. Ortlepp, J. Kjer and M. Bayer, Phytochem. Rev., 2010, 9, 475–489 CrossRef CAS; (c) F. Hentschel and T. Lindel, Synthesis, 2010, 181–204 CAS.
- For the rate of combination of carbon-centered radicals with NO, see: (a) T. J. Wallington, H. Egsgaard, O. J. Nielsen, J. Platz, J. Sehested and T. Stein, Chem. Phys. Lett., 1998, 290, 363–370 CrossRef CAS; (b) See also ref. 16.
- W. Shan, F. Liu, H. He, X. Shi and C. Zhang, Appl. Catal., B, 2012, 115–116, 100–106 CrossRef CAS.
- See http://www.worstpolluted.org.
- For recent review articles on the synthesis of amino acids, see: (a) A. Perdih and M. S. Dolenc, Curr. Org. Chem., 2011, 15, 3750–3799 CrossRef CAS; (b) J. Martens, ChemCatChem, 2010, 2, 379–381 CrossRef CAS; (c) H. Groeger and F. R. Dietz, in Encyclopedia of Chemical Biology, ed. T. P. Begley, Wiley, 2009, vol. 1, pp. 191–204 Search PubMed; (d) J. Deska in Amino Acids, Peptides and Proteins in Organic Chemistry, ed. A. B. Hughes, Wiley, 2011, vol. 3, pp. 115–141 Search PubMed.
- T. Kitagawa, D. Khandmaa, A. Fukumoto and M. Asada, Chem. Pharm. Bull., 2004, 52, 1137–1139 CrossRef CAS PubMed.
- For a recent report on the reduction of oximes to amines, see: S. Liu, Y. Yang, X. Zhen, J. Li, H. He, J. Feng and A. Whiting, Org. Biomol. Chem., 2012, 10, 663–670 CAS.
- Y.-N. Zhang, W. Zhang, D. Hong, L. Shi, Q. Shen, J.-Y. Li, J. Li and L.-H. Hu, Bioorg. Med. Chem., 2008, 16, 8697–8705 CrossRef CAS PubMed.
- E. Specker, J. Boettcher, A. Heine, C. A. Sotriffer, H. Lilie, A. Schoop, G. Müller, N. Griebenow and G. Klebe, J. Med. Chem., 2005, 48, 6607–6619 CrossRef CAS PubMed.
- B. G. Szczepankiewicz, G. Liu, P. J. Hajduk, C. Abad-Zapatero, Z. Pei, Z. Xin, T. H. Lubben, J. M. Trevillyan, M. A. Stashko, S. J. Ballaron, H. Liang, F. Huang, C. W. Hutchins, S. W. Fesik and M. R. Jirousek, J. Am. Chem. Soc., 2003, 125, 4087–4096 CrossRef CAS PubMed.
- DL-4-Chlorophenylalanine methyl ester: 5480 € per kg; DL-4-chlorophenylalanine methyl ester hydrochloride: 1055€ per 25 g; sodium nitrate: 18 € per kg from VWR chemicals catalogue.
- K. Drauz, H. Gröger and O. May, Enzyme Catalysis on Organic Synthesis, Wiley-VCH, 2012 Search PubMed.
- (a) D. A. Schichl, S. Enthaler, W. Holla, T. Riermeier, U. Kragl and M. Beller, Eur. J. Org. Chem., 2008, 3506–3512 CrossRef CAS; (b) M. J. Hateley, D. A. Schichl, C. Fischer and M. Beller, Synlett, 2001, 25–28 CAS.
- According to ref. 40a, L-phenylalanine was obtained from racemic phenylalanine methyl ester in 95% yield (99% ee) and L-tyrosine was obtained from racemic tyrosine methyl ester in 94% yield (95% ee).
- X. Huang, M. Ortiz-Marciales, K. Huang, V. Stepanenko, F. G. Merced, A. M. Ayala, W. Correa and M. De Jesús, Org. Lett., 2007, 9, 1793–1795 CrossRef CAS PubMed.
- (a) S. Ngwerume and J. E. Camp, J. Org. Chem., 2010, 75, 6271–6274 CrossRef CAS PubMed; (b) H.-Y. Wang, D. S. Müller, R. M. Sachwani, H. N. Londino and L. L. Anderson, Org. Lett., 2010, 12, 2290–2293 CrossRef CAS PubMed; (c) S. Chiba, L. Zhang, S. Sanjaya and G. Y. Ang, Tetrahedron, 2010, 66, 5692–5700 CrossRef CAS.
- A. Caballero, P. J. Campos, M. A. Rodríguez and R. Alonso, Tetrahedron, 2010, 66, 8828–8831 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analytical data. See DOI: 10.1039/c3gc42432d |
| This journal is © The Royal Society of Chemistry 2014 |