Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems†
Ana Filipa M.
Cláudio
,
Carlos F. C.
Marques
,
Isabel
Boal-Palheiros
,
Mara G.
Freire
* and
João A. P.
Coutinho
*
Departamento de Química, CICECO, Universidade de Aveiro, 3810-193 Aveiro, Portugal. E-mail: maragfreire@ua.pt; jcoutinho@ua.pt; Fax: +351 234370084; Tel: +351 234370200
First published on 17th October 2013
Abstract
In the last decade, aqueous two-phase systems (ATPS) composed of ionic liquids (ILs) and inorganic salts have been largely explored as novel extractive platforms. The use of ILs as phase-forming components in ATPS has led to outstanding extraction performances compared to more traditional approaches. Nevertheless, despite those exceptional achievements, IL regeneration, recycling and reuse lagged behind and still remain a challenging task towards the development of greener cost-effective processes. Aiming at overcoming these shortcomings, the phase diagrams of novel ATPS composed of imidazolium-based ILs and Na2CO3 or Na2SO4 were determined and their extraction efficiencies for a model antioxidant – gallic acid – were evaluated. The most promising IL-based ATPS were then used in sequential two-step cycles (product extraction/IL recovery) so as to evaluate the efficacy on the IL recyclability and reusability. Extraction efficiency values ranging between 73% and 99% were obtained in four sequential partitioning experiments involving gallic acid while allowing the regeneration of 94–95% of the IL and further reutilization. Moreover, to support the vast applicability of the back-extraction routes and the recyclability concept proposed here, the most prominent systems were further tested with two additional antioxidants, namely syringic and vanillic acids. In both examples, the extraction efficiencies were higher than 97%. The remarkable results obtained in this work support the establishment of IL-based ATPS as a sound basis of greener cost-effective strategies with a substantial reduction in the environmental footprint and economical issues.
Introduction
Aqueous two-phase systems (ATPS) are recognized as a promising technique to separate and purify biomolecules because of their selectivity, easy scale-up and continuous operation mode. Those systems are formed when two distinct substances, both miscible with water, are employed; above a critical concentration of the phase-forming components spontaneous phase separation takes place and an aqueous liquid–liquid system is formed. Besides the typical polymer-based ATPS largely explored since the 50s, ionic liquids (ILs) emerged as a valuable option to polymers1 because they usually provide a faster phase separation, a significant reduction in viscosity2 and a tailoring of the coexisting phases’ polarities in the sense that complete extraction efficiencies can always be foreseen.3 Ionic liquids are salts with melting temperatures below 100 °C – a result of their ions’ delocalized charge and frequent asymmetry that prevent crystallization. Moreover, due to their ionic nature, most ILs are also characterized by a negligible volatility at ambient conditions, high thermal and chemical stabilities, and a large solubilisation ability for a wide variety of compounds.4,5 These characteristics, together with the vast possibility of their ions rearrangement, bestow ILs with an outstanding tailoring ability for extraction and purification routes.To effectively apply IL-based ATPS as extractive platforms, their phase diagrams must be established experimentally, and there is today a large amount of literature data reporting ternary phase diagrams of systems constituted by ILs, salts and water.3 In addition to the phase diagrams, several studies have been demonstrating the outstanding performance of IL-based ATPS for extraction purposes.4 Improved extraction efficiencies, in some examples up to complete extraction, have been obtained for proteins, enzymes, alkaloids, antioxidants, antibiotics, endocrine disruptors, among others.4
ILs have been publicized as “green solvents” due to their negligible vapour pressures preventing thus further emissions to the atmosphere. Nevertheless, the design of entirely green ILs is still very limited and in most cases their toxicity, biocompatibility and biodegradability issues are only now being addressed.6 In industrial applications, ILs are inevitably mixed with other products/solvents, and the development of efficient separation and recycling routes is a crucial attempt to decrease their environmental footprint.7,8 Most ILs are also still expensive in comparison with more conventional molecular solvents9 reinforcing therefore the need for their recycling and reuse.10 Envisaging this crucial objective, in the past few years, some researchers have been addressing the development of novel methodologies for the recovery and further reuse of ILs.7,11,12 The main operations to recover ILs from aqueous solutions are based on the addition of salting-out species12,13 and barrier (membrane) separations;8 yet, the former were shown to be more adequate for industrial implementation.12 For instance, hydrophobic (water immiscible) ILs were successfully employed in the extraction of amino acids, phenols and amines11 with the IL-rich phase being regenerated by back-extraction of the product, enabling thus the phase-forming compounds to be recycled. Hydrophilic (water-miscible) ILs used to extract α-tocopherol from a model mixture with methyl linoleate and caffeine from guaraná seeds were also recovered making use of organic solvents and reused several times.11 The regeneration of ILs by liquid–liquid techniques may be economically viable; however, the use of volatile organic solvents in the overall process is a significant drawback. Hydrophilic ILs may be also recovered using scCO2, but the process is expensive and consequently not easily amenable to the industrial scale.12 The use of the unique solvent properties of ILs through the development of efficient separation/purification methods has been thoroughly investigated;4 even though IL regeneration, recycling and reuse remain a demanding challenge that needs to be urgently faced.
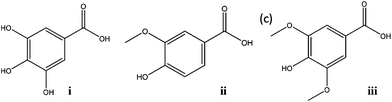
In this work we propose a new strategy for the recycling and reuse of hydrophilic ILs commonly used as phase-forming components of ATPS and usually employed as extractive systems. For such a purpose, a combination of two salt-type ATPS was employed for the extraction and back-extraction of gallic acid (pKa = 4.4; 9.4; 11.0),14 and further confirmed with two other species, namely vanillic acid (pKa = 4.2; 10.2)14 and syringic acid (pKa = 4.0; 9.6).14 These 3 compounds are representative molecules of phenolic acids and/or antioxidants (Fig. 1). Phenolic compounds possess beneficial roles in the reduction of cancer appearance, neurodegenerative disorders, hypertension and cardiovascular diseases.15 Besides, some phenolic compounds are also phytotoxic and bactericidal, and can be used in biological wastewater treatments.16
In previous studies2,17 we thoroughly studied the partition behaviour of phenolic compounds using IL-based ATPS and determined the optimal conditions for gallic acid extraction, such as the IL nature and media composition.17 In this context, improving the sustainability of the process was the step to follow. Hence, in this work, we aimed at recycling and reusing the ILs after the extraction stage to drastically minimize the environmental concerns and economical costs. After the efficient extraction of gallic acid for the IL-rich phase with Na2SO4-based ATPS we therein investigated the combined use of IL-Na2CO3-based ATPS to perform the antioxidant back-extraction for the salt-rich phase aiming at purifying the IL-rich phase. Na2CO3 was selected because it is highly soluble in water, environmentally safe, inexpensive and has a strong salting-out effect (forming ATPS with a wide variety of ILs);3 under these circumstances, the ILs may be further chosen according to their ability to extract the molecule of interest. To additionally validate the back-extraction and recyclability/reusability of the IL, the enhanced ATPS were further used in the extraction–back-extraction cycle of two additional molecules: vanillic and syringic acids.
Results and discussion
Planning of the back-extraction and recyclability strategy
In a previous study we have shown the improved ability of IL-Na2SO4-based ATPS for the extraction of gallic acid (extraction efficiencies up to 99%).17 In this step the gallic acid migrates towards the IL-rich phase, requiring a further process for the recovery of both the product and the IL. Furthermore, we have found that the partition of phenolic acids is strongly pH dependent.17 Charged species tend to partition into the salt-rich phase whereas neutral molecules preferentially partition into the IL-rich phase.17 This pH-dependent behaviour was initially demonstrated by Visser et al. using a simple indicator dye, thymol blue, although using ILs non-miscible with water (at room temperature).18 In this context, it is conceivable to use this reversible partitioning ability controlled by the pH of the medium to develop the back-extraction process. Amongst the possible salts capable of providing an alkaline medium, Na2CO3 was chosen because of its advantageous properties specified before. As a result, we designed a two-step ATPS scheme to perform a greener IL-recyclable extraction procedure: (i) the biomolecule is extracted/separated into the IL-rich phase using Na2SO4-based ATPS; (ii) the IL-rich aqueous phase is separated and reused to form a new ATPS with Na2CO3 to carry out the back-extraction of the product of interest while regenerating the IL aqueous solution for subsequent reutilization.In the first stage, and to manipulate the extraction of gallic acid for the IL-rich phase, Na2SO4 was used as the two-phase promoter alongside several imidazolium-based ILs. This salt was chosen since it has a neutral character regarding the pH of its aqueous medium and is suitable to form ATPS with distinct ILs. The phase diagrams of IL-Na2SO4-based ATPS and their ability to perform the extraction of gallic acid were previously reported by us.17,19 Nonetheless, it should be remarked that an optimization was carried out here to improve the overall extraction efficiencies. In the second step, novel ATPS composed of several imidazolium-based ILs and Na2CO3, which produces alkaline media, were determined with the goal of defining their immiscibility regions and to ascertain on the phases’ composition.
Both ATPS were individually evaluated in what concerns their extraction efficiencies either for the IL- or salt-rich phases. The most promising ILs were then employed in sequential two-step cycles (product extraction/IL recovery) so as to demonstrate the efficacy of the IL reuse and inherent recyclability. Finally, to guarantee the broad applicability of the product extraction/IL recovery cycle, two other species (vanillic and syringic acids) were evaluated regarding their extraction efficiencies with the optimized ATPS.
ATPS formation ability
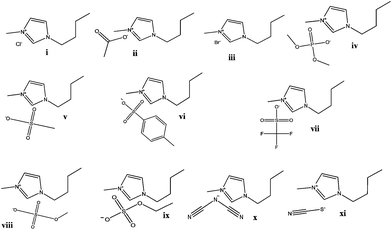
The ability of eleven imidazolium-based ILs to form ATPS with Na2CO3 was first evaluated. The addition of inorganic salts to aqueous solutions of ILs leads to liquid–liquid demixing due to a preferential hydration of the high charge-density salt ions over the IL. In fact, the salting-out effect is the key element behind the formation of ATPS comprising ILs and conventional salts.20 Hence, salts composed of highly charged ions, such as PO43−, CO32− and SO42−, display a high capability to form ATPS with the low charge density ILs. Compared to the common salting-out inducing salts, ILs are generally weakly hydrated since they are made up of low-symmetry and charge-delocalized ions only capable of weak directional intermolecular interactions.4 Consequently, the competition of the ions for water molecules will lead to the dehydration of the IL and to the liquid–liquid demixing.The chemical structures of the ILs evaluated in this work, either in their ability to form ATPS or in their extractive performance, are depicted in Fig. 2. The description of their acronyms is provided as a footnote.‡
With the exception of [C4C1im][CH3CO2], all the ILs displayed in Fig. 2 are able to form ATPS at 25 °C in the presence of appropriate concentrations of Na2CO3. With the acetate-based IL, instead of the coexisting liquid phases, a solid–liquid equilibrium was observed in the whole composition range of the IL. In the ATPS composed of Na2CO3, the IL is almost completely segregated into the upper phase whereas the bottom layer corresponds to the inorganic-salt-rich phase. Only one exception was verified with [C4C1im][CF3SO3] where an inversion on the phases’ densities occurs due to the high density afforded by the anion fluorine atoms.
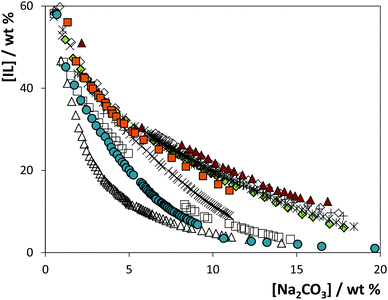
The solubility curves for the systems composed of IL + Na2CO3 + water are depicted in Fig. 3. The detailed weight fraction data and respective correlation, tie-lines and tie-line lengths are provided in the ESI.† As mentioned before, sodium carbonate is a strong salting-out agent and leads to the liquid–liquid demixing of a wide variety of ILs in aqueous medium. This may be imparted to the carbonate's hydration ability. CO32− is a base and acts as a hydrogen acceptor towards water, which certainly has an important contribution in the extensive formation of complexes with water.21
As may be appreciated in Fig. 3, the solubility curves show a strong dependency on the IL; the closer the curve is to the origin, the less IL and/or salt is needed to induce the phase splitting and the wider is the biphasic region. From the depicted data, for 10 wt% of salt, a decreasing order for the ability of the [C4C1im]-based ILs to form ATPS can be established as follows: [C4C1im][CF3SO3] > [C4C1im][SCN] > [C4C1im][N(CN)2] > [C4C1im][TOS] > [C4C1im][C2H5SO4] > [C4C1im]Cl ≈ [C4C1im][CH3SO4] ≈ [C4C1im]Br > [C4C1im][CH3SO3] > [C4C1im][DMP]. Since all ILs share the same cation, this order is a direct result of the IL anion. The anions interact with water molecules by an approximately linear hydrogen bond, suggesting that the dominant interactions are short range forces of a chemical nature.22 As a result, the ability of a given anion to be preferentially hydrated depends on its ability to hydrogen bond with water, which further rules the ATPS formation aptitude. In fact, the IL anion forming ability for ATPS closely follows the trend of their H-bond basicity values,5,23 as previously discussed by us.24
The phase diagrams (solubility curves and tie-lines) at 25 °C for the ATPS composed of several ILs and Na2SO4 were previously determined and are reported elsewhere.19 A similar pattern is observed when comparing the solubility curves here obtained using Na2CO3 with the correspondent ones using Na2SO4, reinforcing the assumption of the major role that the IL anion plays in the ATPS formation process. This allows the IL to be chosen according to the extraction purpose.
Optimization of the gallic acid extraction in individual ATPS
In the ATPS constituted by Na2CO3, both aqueous phases present an alkaline pH imparted by the inorganic salt (the pH of the coexisting phases varies between 10 and 12 as presented in ESI†). In this range of pH values, gallic acid (pKa = 4.41)14 is predominantly in its deprotonated form, which is negative and has reduced affinity for the IL-rich phase bearing the less polar and more diffusedly charged ions. Although differences are observed amongst the different ILs, gallate ions tend to migrate to the salt-rich phase in most of the studied systems. The extraction efficiencies of gallic acid for the Na2CO3-rich phase, defined as the percentage ratio of the amount of gallic acid in the inorganic-salt-rich phase to that in the total mixture, are shown in Fig. 4.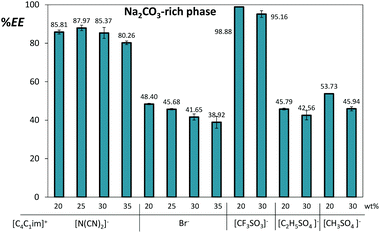 | ||
| Fig. 4 Extraction efficiencies (%EE) of gallic acid for the inorganic-salt-rich phase in ATPS composed of 10 wt% of Na2CO3 and variable concentrations of [C4C1im]-based ILs at 25 °C. | ||
The extraction efficiencies of gallic acid range between 39% and 99% and are mostly dependent on the IL nature. The IL weight fraction was varied between 20% and 35%. On the other hand, the corresponding extraction efficiencies differed only slightly as a result of the IL content. This means that ATPS with the lowest IL weight fraction (20%) still keep the optimal extracting performance – a result that has two major advantages: reduction of the downstream cost and decrease of the environmental impact that these systems may present.
Remarkable extraction efficiencies were observed with the most hydrophobic ILs investigated, namely 88% with [C4C1im][N(CN)2] and 99% with [C4C1im][CF3SO3]. When analysing the distribution behaviour of gallic acid it is reasonable to admit that its migration closely correlates with the aptitude of each IL for ATPS formation (Fig. 3). The hydrophobic character of the triflate anion accounts for the [C4C1im][CF3SO3] strong liquid–liquid demixing ability as well as to its reduced affinity for gallate anions; dicyanamide ion displays a complex solvation shell with a subtle balance between the anion–water and water–water interactions25 that may contribute to the lack of affinity towards gallate ions. The more moderate two phase promoters, [C4C1im][CH3SO4] and [C4C1im][C2H5SO4], exhibit a considerable affinity for the phenolic molecule due to their more hydrophilic nature and higher water content at the IL-rich phase – cf. ESI† with the phases’ compositions.
In a previous study we have shown that gallic acid preferentially migrates towards the IL-rich phase in ATPS composed of imidazolium-based ILs and Na2SO4.17 In this work we expanded that study of the extraction efficiencies of gallic acid using several imidazolium-based ILs and different concentrations aiming at finding the best systems to be coupled with the back-extraction procedure. The extraction efficiencies of gallic acid for the IL-rich phase, now defined as the percentage ratio of the amount of gallic acid in the IL-rich phase to that in the total mixture, are depicted in Fig. 5.
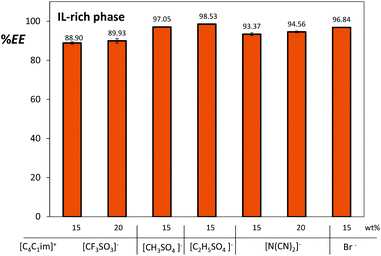 | ||
| Fig. 5 Extraction efficiencies (%EE) of gallic acid for the IL-rich phase in ATPS composed of 25 wt% of [C4C1im]-based ILs and variable concentrations of Na2SO4 at 25 °C. | ||
The preferential migration of gallic acid towards the IL-rich phase was observed for all the studied systems with extraction efficiencies ranging between 89% and 99%. Those differences can be attributed to the intrinsic nature of IL anions and also to the concurrent pH effect which they produce in the aqueous media and in the biomolecule itself. Being a neutral salt, Na2SO4 aqueous solutions have no buffering capacity, and hence, the inherent IL acidic/alkaline characteristics largely control the pH of the corresponding ATPS.17 Except for [C4C1im][N(CN)2], the pH values of both IL and Na2SO4 aqueous phases are slightly acidic as presented in ESI.†
Amongst the studied ATPS constituted by [C4C1im]-based ILs, outstanding results were obtained with [C4C1im][CF3SO3] and [C4C1im][N(CN)2] – more hydrophobic ILs with a lower water content at the IL-rich phase as can be appreciated in their phases’ compositions presented in the ESI.†
Taking into account the overall results obtained it is evident that the best ILs to be considered in the following steps of back-extraction and recyclability are those that allowed the higher extraction efficiencies for both the IL- and salt-rich phase, namely [C4C1im][CF3SO3] and [C4C1im][N(CN)2].
Back-extraction of gallic acid and ILs recyclability and reusability
As mentioned before, the main goal of this work was to develop a procedure that uses the promising IL-based ATPS, not only for the extraction/purification of biomolecules as has been extensively shown in the literature,4 but also for efficiently accomplishing their back-extraction while allowing the IL recyclability and reusability. This combined procedure should allow an enhanced recovery of the added-value biomolecules and the IL cleaning and recycling, reducing therefore both economic and environmental loads.Using the extraction efficiencies obtained in single-step procedures, either into the IL-rich phase using Na2SO4 or into the inorganic-salt-rich phase when Na2CO3 was employed, we proceeded to investigate the back-extraction of gallic acid and the recyclability of the ILs in a combined approach. For this purpose, the extraction of gallic acid was performed in two cycles, each involving a first step with Na2SO4 + IL ATPS, followed by a second step, using Na2CO3 + IL aqueous systems. This second step “cleans” the IL aqueous solution thus preparing it for reutilization in a new cycle using identical procedures. For this study the two most efficient ILs in both extractions, [C4C1im][N(CN)2] and [C4C1im][CF3SO3], were chosen and the most favourable compositions were used. The results obtained for the gallic acid extraction in each of the four sequential single-step procedures, in the two cycles, are summarized in Table 1.
| Step | [C4C1im][N(CN)2] | [C4C1im][CF3SO3] | |||
|---|---|---|---|---|---|
| Non-adjusted pH | Adjusted pH | Non-adjusted pH | Adjusted pH | ||
| 1st Cycle | |||||
| 1 (to IL-rich phase) | % EEIL | 94.6 ± 0.5 | 93.1 ± 0.2 | 89.9 ± 1.1 | 93.3 ± 3.7 |
| pH IL/salt | 7.4/7.7 | 7.5/7.5 | 4.6/4.6 | 4.0/3.3 | |
| 2 (to Na2CO3-rich phase) | % EESalt | 70.6 ± 0.7 | 72.4 ± 1.2 | 98.7 ± 2.5 | 98.3 ± 1.7 |
| pH IL/salt | 11.2/11.3 | 11.7/11.7 | 11.1/11.5 | 11.1/11.1 | |
| 2nd Cycle | |||||
| 3 (to IL-rich phase) | % EEIL | 50.1 ± 3.7 | 96.7 ± 3.3 | 35.6 ± 5.0 | 95.5 ± 2.6 |
| pH IL/salt | 8.7/9.9 | 6.4/6.3 | 8.6/8.5 | 2.9/2.9 | |
| 4 (to Na2CO3-rich phase) | % EESalt | 79.4 ± 4.2 | 78.7 ± 2.0 | 91.1 ± 2.1 | 99.2 ± 0.8 |
| pH IL/salt | 11.3/11.8 | 11.1/11.3 | 11.4/11.5 | 11.4/11.3 | |
As may be appreciated, although differences between the two ILs were observed for each of the different steps, the second cycle always produces poorer results at non-adjusted pH conditions. A closer observation reveals that there is an increase in the medium pH after the first back-extraction with sodium carbonate; this salt being a base of considerable strength, its concentrated solution imparts to both aqueous phases an alkaline pH (9 < pH < 12). This effect is even more remarkable for the least acidic IL, [C4C1im][N(CN)2]. The pH effect is thus responsible for the reduced migration of gallic acid towards the IL-rich phase in the recycling step. At alkaline pH values the biomolecule is mostly in its anionic form preferring, therefore, the inorganic salt enriched phase as discussed before.
If the media pH are neutralized or adjusted to slight acidic values better results can be obtained. The whole extraction procedure was then repeated for both ILs. At this stage we conducted a pH adjustment between the cycles with the addition of H2SO4 4 M to the systems composed of [C4C1im][CF3SO3] and [C4C1im][N(CN)2], guaranteeing that SO42− is the main anion in the ATPS, and outstanding results were achieved as expected. This addition was carried out under a continuous control of the medium pH and until attaining the desired value. The small amount of acid solution required to change the pH has an insignificant impact on the overall system composition (lower than 0.2 wt%). Fig. 6 depicts the extraction efficiencies of gallic acid in the four sequential steps.
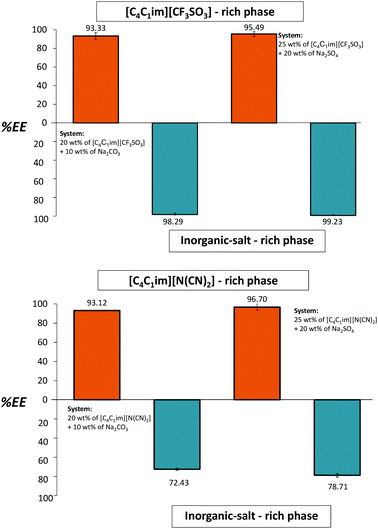 | ||
| Fig. 6 Extraction efficiencies (%EE) of gallic acid in sequential ATPS composed of 25 wt% of IL + 20 wt% Na2SO4 (orange bars) and 20 wt% of IL + 10 wt% Na2CO3 (blue bars) at 25 °C. | ||
For the ATPS composed of [C4C1im][CF3SO3], the extraction efficiency values are always higher than 93%. This excellent performance is similar in the two cycles, further confirming the IL aqueous phase regeneration and reusability. For the other IL studied, [C4C1im][N(CN)2], the extraction efficiency values are higher than 93% for the IL-rich phase and 72% for the salt-rich layer. In summary, the initial extraction efficiencies are always reached after the IL recyclability without a decrease in their extractive performance. In addition, due to the high ability of Na2CO3 as a two-phase promoter, a relatively small amount of salt is enough to induce the liquid–liquid demixing (10 wt% of Na2CO3 is sufficient to produce ATPS with a wide range of ILs).
After the last stage, the biomolecule is mainly concentrated at the carbonate-rich aqueous solution (with a concentration of IL lower than 2 wt% – cf. ESI† with the phases’ compositions). The biomolecule isolation may finally be achieved through the addition of HCl, leaving NaCl in solution – a biocompatible salt – although it can be removed by ion exchange if high purity standard levels of the antioxidant are foreseen.
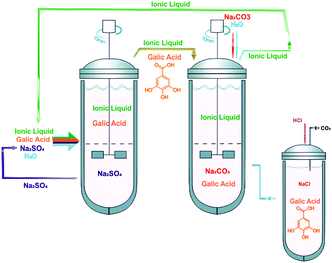
Fig. 7 depicts the optimized strategy for the extraction and back-extraction of gallic acid while allowing the reutilization of the IL aqueous phase. This optimized procedure highlights the “greener” nature of IL-based ATPS if a proper selection of the inorganic salts and ILs is carried out based on the extraction efficiencies that they afford. In this context, the use of combined ATPS for the extraction and recovery of other value-added compounds is straightforwardly envisaged.
The combination of ATPS constituted by inorganic salts and ILs that is here reported yielded an almost complete recovery of gallic acid and a subsequent IL phase depletion, thus regenerating the IL aqueous solution for subsequent reutilizations with the same efficacy shown in the first cycle. Taking into account the flow chart sketched in Fig. 7, the IL is only lost in the Na2CO3-rich solution during the back-extraction step. The IL present in the Na2SO4-rich phase is completely recovered and reused. Based on the TL compositions presented in ESI,† and on the mixture compositions and total weight of each phase, there is a loss of 6% of [C4C1im][N(CN)2] and 5% of [C4C1im][CF3SO3] at the end of each cycle of two steps. Further process improvements aiming at the complete recovery of ILs are thus required. The addition of stronger salting-out salts to generate a second IL-rich phase or their adsorption onto activated carbon, as previously demonstrated by us,26 are two of the possibilities to be evaluated.
Since the proposed process deals in aqueous solution with two charged species (ionic liquid + inorganic salt), one of the major concerns regarding the development of the back-extraction procedure relies on the possibility of ion exchange between the salts and the ionic liquid. Therefore, aiming at evaluating if any ion exchange takes place during the formation of the ATPS and further phase separation, several analytical and spectroscopic techniques were additionally applied to the characterization and quantification of the coexisting phases of the systems composed of [C4C1im][CF3SO3] and [C4C1im][N(CN)2], combined with both salts, at the mixture compositions presented in Fig. 6. The detection of the sulphate and carbonate anions was carried out with Fourier Transform Infrared spectroscopy. The sodium cation, at both aqueous phases, was quantified by Inductively Coupled Plasma Optical Emission Spectrometry. The imidazolium cation content was determined by UV-Vis spectroscopy. From the results obtained it is possible to establish that there is no significant ion exchange in the studied systems, and that the amount of each ion at each phase is in good agreement with those estimated from the tie-lines. The experimental results are shown in ESI.† These results are in close agreement with previous studies involving ILs and other inorganic salts.27 The inorganic anions of the salts commonly used to form ABS with ILs are of high charge density and with a great propensity to form hydration complexes.28 Furthermore, these anions usually exhibit a stronger cation–anion interaction strength with less organic (more complex) and high charge density cations.29 All of these factors contribute to a negligible ion exchange in these type of systems (composed of inorganic salts with high charge density ions versus low charge density IL ions).
Application of the back-extraction concept to other species
To further support the applicability of the back-extraction process, we further used the ATPS composed of [C4C1im][CF3SO3] + Na2SO4/Na2CO3 to study the extraction efficiencies and back-extraction possibility of two additional antioxidants: vanillic acid and syringic acid. Fig. 8 depicts the results obtained. In both examples the extraction efficiencies are above 97%. The detailed results and phases’ compositions are provided in ESI.†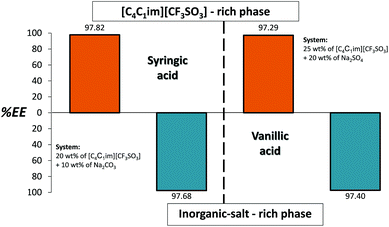 | ||
| Fig. 8 Extraction efficiencies (%EE) of syringic and vanillic acid in sequential ATPS composed of 25 wt% of IL + 20 wt% Na2SO4 (orange bars) and 20 wt% of IL + 10 wt% Na2CO3 (blue bars) at 25 °C. | ||
In summary, the results reported here demonstrate that a judicious choice of the ATPS forming components, media composition and pH ensures outstanding results in the separation/extraction of added-value products and in the following back-extraction procedures. The industrial implementation of the proposed recovery procedure does not only minimize the environmental load of the industrial wastes but performs, at the same time, the retrieval of the valuable ILs.
Conclusions
Despite the large interest devoted to IL-based ATPS as extractive systems of outstanding performance, the recovery of the extracted added-value products and the systems recyclability and reusability have been seldom studied. An efficient and environmentally safe extraction/purification combined process for IL-based ATPS was here proposed. As a proof of principle, a model antioxidant was firstly employed – gallic acid. In a first step, Na2SO4 + IL ATPS were used to extract gallic acid from an aqueous solution to the IL-rich phase; then, another ATPS composed of IL and Na2CO3 was used to perform the back-extraction of the biomolecule and to “clean” the IL-rich phase for its subsequent reutilization. The use of Na2SO4 allowed near complete extractions into the IL enriched phase. The back-extraction that followed, using Na2CO3, also led to remarkable results, stripping the biomolecule almost entirely from the IL. In each cycle, the IL recovery efficiency was between 94 and 95%. The recycling of the IL and its further recyclability was established without losses in the extractive performance of the corresponding ATPS. This two-step cycle was repeated twice, proving the IL recyclability and reusability. Furthermore, the optimized systems were also applied to the extraction/back-extraction of syringic and vanillic acids. Extraction efficiencies above 97% were obtained, supporting thus the vast applicability of the recommended strategy.The proposed methodology guarantees that IL-based ATPS are recyclable extractive platforms decreasing therefore all the associated environmental and economic concerns. IL-based ATPS are indeed sustainable and cost-efficient extraction routes if a suitable reutilization of the phase-forming components is achieved and with high potential to be applied at an industrial scale.
Experimental
Materials
The ILs used in this work to form ATPS with Na2CO3 were [C4C1im][CF3SO3], [C4C1im][SCN], [C4C1im][CH3SO3], [C4C1im][C2H5SO4], [C4C1im][CH3SO4], [C4C1im][TOS], [C4C1im]Br, [C4C1im][N(CN)2], [C4C1im][DMP], [C4C1im]Cl, and [C4C1im][CH3CO2]. All ILs were supplied by Iolitec. To reduce the volatile impurities content to negligible values, ILs individual samples were kept at constant agitation under vacuum and at a moderate temperature (50 °C), for a minimum of 24 h. After this purification step, the purity of each IL was further checked by 1H, 13C and 19F NMR (when applicable) spectra and found to be >98 wt% for all samples and according to the purity levels given by the supplier.Na2SO4 was acquired from LabSolve (purity >99.8 wt%) and Na2CO3 was from Prolabo (purity >99.9 wt%). Gallic, vanillic and syringic acids were acquired from Merck (>99.5 wt% pure), Sigma-Aldrich (>97 wt% pure) and Alfa Aesar (>98 wt% pure), respectively. H2SO4, 95% pure, was from Sigma-Aldrich.
The water used was double distilled, passed across a reverse osmosis system and further treated using a Milli-Q plus 185 water purification equipment. The buffers used in the calibration of the pH meter equipment were the citric acid/sodium hydroxide/sodium chloride solution with a pH value of 4.00 (±0.02), and the potassium dihydrogen phosphate/disodium hydrogen phosphate solution with a pH value of 7.00 (±0.02), acquired from Fluka.
Methods
The compositions of the biphasic mixtures used for the gallic acid partitioning were chosen based on the phase diagrams determined in this study and also in a previous one.19 Several compositions were adopted while varying the IL and salt compositions. After the optimization investigations with gallic acid, the partitioning of vanillic and syringic acids was also investigated. The initial mixtures and respective phases’ compositions are presented in the ESI.† In all of these compositions an aqueous solution containing gallic, syringic or vanillic acids at 3 × 10−3 mol dm−3 was used. All mixtures were prepared by weight with an uncertainty of ±10−4 g. The mixtures were prepared and vigorously stirred in small ampoules of approximately 10 cm3 specially built for extraction procedures. After this procedure, the ATPS were left in equilibrium (without stirring) for at least 12 h. The temperature was controlled at 25 ± 1 °C by keeping the glass ampoules in an air bath equipped with a Pt 100 probe and a temperature controller. After the equilibration period at a constant temperature, the phases were carefully separated and the amount of each acid was measured with the help of a UV-Vis spectrophotometer (and using calibration curves beforehand established). A careful verification was previously made to analyse the effects of the media composition, namely IL and salt, and medium pH in the quantification by UV spectroscopy. The imidazolium-based ILs were observed to cause a minimal interference with the analytical method at the dilutions carried out for quantification; nonetheless, to minimize these interferences, ternary mixtures at the same weight fraction composition were prepared for each individual system, using pure water instead of the antioxidants aqueous solution, as blank samples. At least three individual samples were analysed to determine the extraction efficiencies and the respective standard deviations – the obtained numerical values are presented in the ESI.†
The extraction efficiencies (%EE) of each acid within the Na2CO3-based ATPS (and thus into the salt-rich phase) was determined according to
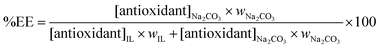 | (1) |
On the other hand, the extraction efficiencies (%EE) of gallic, syringic and vanillic acids into the IL-rich phase in the Na2SO4-based systems were determined according to
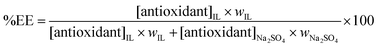 | (2) |
After all the optimization, the same procedure was applied to the extraction/back-extraction of vanillic and syringic acids (one cycle and 2 steps) using [C4C1im][CF3SO3]. The vanillic and syringic acid aqueous solutions were at 3.20 × 10−3 mol dm−3.
Acknowledgements
This work was financed by national funding from FCT- Fundação para a Ciência e a Tecnologia, through projects PTDC/QUI-QUI/121520/2010 and Pest-C/CTM/LA0011/2013. The authors also acknowledge FCT for the PhD grant SFRH/BD/74503/2010 of A.F.M. Cláudio.Notes and references
- (a) P. A. Albertsson, Nature, 1958, 182, 709–711 CrossRef CAS; (b) K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS PubMed.
- (a) A. F. M. Cláudio, M. G. Freire, C. S. R. Freire, A. J. D. Silvestre and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 75, 39–47 CrossRef PubMed; (b) C. L. S. Louros, A. F. M. Cláudio, C. M. S. S. Neves, M. G. Freire, I. M. Marrucho, J. Pauly and J. A. P. Coutinho, Int. J. Mol. Sci., 2010, 11, 1777–1791 CrossRef CAS PubMed.
- (a) M. G. Freire, C. M. S. S. Neves, I. M. Marrucho, J. N. Canongia Lopes, L. P. N. Rebelo and J. A. P. Coutinho, Green Chem., 2010, 12, 1715–1718 RSC; (b) C. F. C. Marques, T. Mourão, C. M. S. S. Neves, A. S. Lima, I. Boal-Palheiros, J. A. P. Coutinho and M. G. Freire, Biotechnol. Prog., 2013, 29, 645–654 CrossRef CAS PubMed; (c) H. Passos, A. C. A. Sousa, M. Ramiro Pastorinho, A. J. A. Nogueira, L. P. N. Rebelo, J. A. P. Coutinho and M. G. Freire, Anal. Methods, 2012, 4, 2664–2667 RSC; (d) S. Shahriari, L. C. Tomé, J. M. M. Araújo, L. P. N. Rebelo, J. A. P. Coutinho, I. M. Marrucho and M. G. Freire, RSC Adv., 2013, 3, 1835–1843 RSC.
- M. G. Freire, A. F. M. Cláudio, J. M. M. Araújo, J. A. P. Coutinho, I. M. Marrucho, J. N. Canongia Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 4966–4995 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- (a) D. Coleman and N. Gathergood, Chem. Soc. Rev., 2010, 39, 600–637 RSC; (b) J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS PubMed; (c) S. Stolte, S. Abdulkarim, J. R. Arning, A. K. Blomeyer-Nienstedt, U. Bottin-Weber, M. Matzke, J. Ranke, B. Jastorffa and J. Thoming, Green Chem., 2007, 10, 214–224 RSC; (d) S. P. M. Ventura, A. M. M. Gonçalves, F. Gonçalves and J. A. P. Coutinho, Aquat. Toxicol., 2010, 96, 290–297 CrossRef CAS PubMed; (e) S. P. M. Ventura, C. S. Marques, A. A. Rosatella, C. A. M. Afonso, F. Gonçalves and J. A. P. Coutinho, Ecotox. Environ. Saf., 2012, 76, 162–168 CrossRef CAS PubMed; (f) S. P. M. Ventura, A. M. M. Gonçalves, T. Sintra, J. L. Pereira, F. Gonçalves and J. A. P. Coutinho, Ecotoxicol., 2013, 22, 1–12 CrossRef CAS PubMed.
- J. F. Fernández, J. Neumann and J. Thöming, Curr. Org. Chem., 2011, 15, 1992–2014 CrossRef.
- K. Haerens, S. V. Deuren, E. Matthijs and B. V. Bruggen, Green Chem., 2010, 12, 2182–2188 RSC.
- (a) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; (b) Y. Zhang, B. R. Bakshi and E. S. Demessie, Environ. Sci. Technol., 2008, 42, 1724–1730 CrossRef CAS.
- S. Abu-Eishah, in Ionic Liquids - Classes and Properties, ed. S. T. Handy, 2011, InTech Search PubMed.
- (a) A. F. M. Cláudio, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Green Chem., 2013, 15, 2002–2010 RSC; (b) V. M. Egorov, S. M. Smirnova and I. V. Pletnev, Sep. Purif. Technol., 2008, 63, 710–715 CrossRef CAS PubMed; (c) L. Huaxi, L. Zhuo, Y. Jingmei, L. Changping, C. Yansheng, L. Qingshan, Z. Xiuling and W. B. Urs, Green Chem., 2012, 14, 1721–1727 RSC; (d) X. Ni, H. Xing, Q. Yang, J. Wang, B. Su, Z. Bao, Y. Yang and Q. Ren, Ind. Eng. Chem. Res., 2012, 51, 6480–6488 CrossRef CAS; (e) E. M. Siedlecka, M. Czerwicka, J. Neumann, P. Stepnowski, J. F. Fernández and J. Thöming, in Ionic Liquids: Theory, Properties, New Approaches, ed. A. Kokorin, 2011 Search PubMed.
- B. Wu, W. Liu, Y. Zhang and H. Wang, Chem.–Eur. J., 2009, 15, 1804–1810 CrossRef CAS PubMed.
- C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, RSC Adv., 2012, 2, 10882–10890 RSC.
- (a) P. Chuysinuan, N. Chimnoi, S. Techasakul and P. Supaphol, Macromol. Chem. Phys., 2009, 210, 814–822 CrossRef CAS; (b) ChemSpider – The free chemical database at http://www.chemspider.com.
- J. Teixeira, T. Silva, S. Benfeito, A. Gaspar, E. M. Garrido, J. Garrido and F. Borges, Eur. J. Med. Chem., 2013, 62, 289–296 CrossRef CAS PubMed.
- A. Noubigh, A. Mgaidi, M. Abderrabba, E. Provost and W. Furst, J. Sci. Food Agric., 2007, 87, 783–788 CrossRef CAS.
- A. F. M. Cláudio, A. M. Ferreira, C. S. R. Freire, A. J. D. Silvestre, M. G. Freire and J. A. P. Coutinho, Sep. Purif. Technol., 2012, 97, 142–149 CrossRef PubMed.
- A. E. Visser, R. P. Swatloski and R. D. Rogers, Green Chem., 2000, 2, 1–4 RSC.
- A. F. M. Cláudio, A. M. Ferreira, S. Shahriari, M. G. Freire and J. A. P. Coutinho, J. Phys. Chem. B, 2011, 115, 11145–11153 CrossRef PubMed.
- (a) M. G. Freire, P. J. Carvalho, A. M. S. Silva, L. M. N. B. F. Santos, L. P. N. Rebelo, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 202–211 CrossRef CAS PubMed; (b) M. G. Freire, C. M. S. S. Neves, A. M. S. Silva, L. M. N. B. F. Santos, I. M. Marrucho, L. P. N. Rebelo, J. K. Shah, E. J. Maginn and J. A. P. Coutinho, J. Phys. Chem. B, 2010, 114, 2004–2014 CrossRef CAS PubMed; (c) T. Mourão, A. F. M. Cláudio, I. Boal-Palheiros, M. G. Freire and J. A. P. Coutinho, J. Chem. Thermodyn., 2012, 54, 398–405 CrossRef PubMed.
- (a) S. Shahriari, C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, J. Phys. Chem. B, 2012, 116, 7252–7258 CrossRef CAS PubMed; (b) Y. Marcus, Chem. Rev., 2009, 109, 1346–1370 CrossRef CAS PubMed.
- K. D. Collins, G. W. Neilson and J. E. Enderby, Biophys. Chem., 2007, 128, 95–104 CrossRef CAS PubMed.
- C. Reichardt, Org. Process Res. Dev., 2006, 11, 105–113 Search PubMed.
- S. P. M. Ventura, C. M. S. S. Neves, M. G. Freire, I. M. Marrucho, J. Oliveira and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 9304–9310 CrossRef CAS PubMed.
- B. Jagoda-Cwiklik, X. B. Wang, H. K. Woo, J. Yang, G. J. Wang, M. Zhou, P. Jungwirth and L. S. Wang, J. Phys. Chem. A, 2007, 111, 7719–7725 CrossRef CAS PubMed.
- (a) C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, RSC Adv., 2012, 2, 10882–10890 RSC; (b) J. Lemus, C. M. S. S. Neves, C. F. C. Marques, M. G. Freire, J. A. P. Coutinho and J. Palomar, Environ. Sci. Proc. Impacts, 2013, 15, 1752–1759 RSC.
- (a) K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS PubMed; (b) C. M. S. S. Neves, M. G. Freire and J. A. P. Coutinho, RSC Adv., 2012, 2, 10882–10890 RSC.
- (a) M. G. Freire, P. J. Carvalho, A. M. S. Silva, L. M. N. B. F. Santos, L. P. N. Rebelo, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 202–211 CrossRef CAS PubMed; (b) M. G. Freire, C. M. S. S. Neves, A. M. S. Silva, L. M. N. B. F. Santos, I. M. Marrucho, L. P. N. Rebelo, J. K. Shah, E. J. Maginn and J. A. P. Coutinho, J. Phys. Chem. B, 2010, 114, 2004–2014 CrossRef CAS PubMed.
- A. M. Fernandes, M. A. A. Rocha, M. G. Freire, I. M. Marrucho, J. A. P. Coutinho and L. M. N. B. F. Santos, J. Phys. Chem. B, 2011, 115, 4033–4041 CrossRef CAS PubMed.
- (a) C. M. S. S. Neves, S. P. M. Ventura, M. G. Freire, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 5194–5199 CrossRef CAS PubMed; (b) J. F. B. Pereira, Á. S. Lima, M. G. Freire and J. A. P. Coutinho, Green Chem., 2010, 12, 1661–1669 RSC; (c) M. G. Freire, C. L. S. Louros, L. P. N. Rebelo and J. A. P. Coutinho, Green Chem., 2011, 13, 1536–1545 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Weight fraction data and respective correlation of the binodal curves, tie-lines and tie-line lengths, weight fraction compositions of the systems used in the extractions, extraction efficiencies of gallic acid and pH of the coexisting phases of the different systems. See DOI: 10.1039/c3gc41999a |
| ‡ Acronyms of ILs: 1-butyl-3-methylimidazolium trifluoromethanesulfonate (triflate), [C4C1im][CF3SO3]; 1-butyl-3-methylimidazolium thiocyanate, [C4C1im][SCN]; 1-butyl-3-methylimidazolium methanesulfonate, [C4C1im][CH3SO3]; 1-butyl-3-methylimidazolium ethylsulfate, [C4C1im][C2H5SO4]; 1-butyl-3-methylimidazolium methylsulfate, [C4C1im][CH3SO4]; 1-butyl-3-methylimidazolium tosylate, [C4C1im][TOS]; 1-butyl-3-methylimidazolium bromide, [C4C1im]Br; 1-butyl-3-methylimidazolium dicyanamide, [C4C1im][N(CN)2]; 1-butyl-3-methylimidazolium dimethylphosphate, [C4C1im][DMP]; 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl; 1-butyl-3-methylimidazolium acetate, [C4C1im][CH3CO2]. |
| This journal is © The Royal Society of Chemistry 2014 |