Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: opportunities and obstacles in the food industry
Rebecca
Walker
a,
Eric A.
Decker
ab and
David Julian
McClements
*ab
aDepartment of Food Science, University of Massachusetts, Amherst, MA 01003, USA. E-mail: mcclements@foodsci.umass.edu; Fax: +1 (413) 545-1262; Tel: +1 (413) 545-1019
bDepartment of Biochemistry, Faculty of Science, King Abdulaziz University, P. O. Box 80203, Jeddah 21589, Saudi Arabia
First published on 11th November 2014
Abstract
Consumption of biologically active amounts of omega-3 fatty acids is linked to improved human health, which has partly been attributed to their important role in brain development and cardiovascular health. Western diets are relatively low in omega-3 fatty acids and many consumers turn to supplements or functional foods to increase their intake of these healthy lipids. Fish oil is one of the most widely used sources of omega-3 fatty acid for supplementation and has greater health benefits than plant sources because of its higher concentration of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The incorporation of omega-3 fatty acids into foods and beverages is often challenging due to their low water-solubility, poor oxidative stability, and variable bioavailability. Nanoemulsions offer a promising way to incorporate omega-3 fatty acids into liquid food systems like beverages, dressing, sauces, and dips. Nanoemulsions are colloidal dispersions that contain small oil droplets (r < 100 nm) that may be able to overcome many of the challenges of fortifying foods and beverages with omega-3 fatty acids. The composition and fabrication of nanoemulsions can be optimized to increase the chemical and physical stability of oil droplets, as well as to increase the bioavailability of omega-3 fatty acids.
1. Introduction
EPA and DHA are the long chain polyunsaturated fatty acids (LC-PUFAs) most commonly found in fish oil and are linked to brain development, cardiovascular health, and inflammation.1–4 Western diets have been reported to be severely lacking in the amount of omega-3 fatty acids (FA) consumed.1–4 Consumption of sufficient levels of omega-3 FAs has been identified as a way to reduce mortality risks, especially for cardiovascular disease.5 It is estimated that the mortality risk of low omega-3 intake was responsible for 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in the US in 2005. Low consumption of EPA and DHA is due to numerous factors such as the high cost of fish, dislike of seafood by many consumers, presence of methyl mercury, and low availability in many geographical locations.6–8 The low consumption of EPA and DHA mean that fortification of foods may be one of the most effective ways to increase omega-3 intake and improve health.
000 deaths in the US in 2005. Low consumption of EPA and DHA is due to numerous factors such as the high cost of fish, dislike of seafood by many consumers, presence of methyl mercury, and low availability in many geographical locations.6–8 The low consumption of EPA and DHA mean that fortification of foods may be one of the most effective ways to increase omega-3 intake and improve health.
Much of the early research on omega-3 FAs focused on enrichment of foods using alpha-linolenic acid (ALA), however more attention is now being paid to eicosapentaenoic acid (EPA) and docosahexaenoic (DHA). This rise in interest may be a result of the specific recommendation for EPA and DHA intake by the National Academies and the Dietary Guidelines for Americans in 2010 or the FDA's approval of a qualified health claim for foods or supplements that contain EPA and DHA in 2004.2,9–12
The food industry is now taking measures to help consumers increase their omega-3 FA consumption by introduction of various kinds of functional foods. Functional foods provide health benefits over and above their basic nutritional aspects.13 Omega-3 enriched foods are quite popular, especially beverages, and there are large areas of growth for omega-3 products in countries with both small and large existing omega-3 markets.14 At present, there are a number of functional foods enriched with omega-3 FAs that are on the market, such as milk, eggs, yogurts, breads, and spreads. Some of these products have been naturally enriched through the diet of the chicken or cow they were obtained from, while others have been enriched through the addition of omega-3 FAs as bulk oils, emulsions, or powders.14–16 Nevertheless, there are considerable challenges to incorporating omega-3 FAs into many types of functional food products due to their low water-solubility, poor chemical stability, and variable bioavailability. Consequently, there has been growing interest in the development of appropriate delivery systems to encapsulate, protect, and release omega-3 fatty acids.
Nanoemulsions have great potential for overcoming the challenges associated with developing omega-3 enriched food and beverage products. They can be used to encapsulate oils and increase their water-dispersibility.17 They can be designed to have good kinetic stability and high optical clarity, which is important for application in many food and beverage products.18 They can also be designed to increase the oral bioavailability of encapsulated lipophilic components.19,20 Despite these advantages, nanoemulsion-based delivery systems must still be carefully designed to ensure good physical and chemical stability, and high bioavailability. The purpose of this article is to highlight the potential of nanoemulsions for the encapsulation, protection and release of omega-3 fatty acids. These delivery systems could be used in the food industry to fortify foods and beverages with these bioactive lipids, or they could be used in the supplement or pharmaceutical industry to increase the bioactivity of therapeutic omega-3 fatty acid formulations.
2. Omega-3 fatty acids
2.1. Chemistry and health benefits
Fat consumption is necessary for human development, health, and longevity.21 There are two fatty acids that have been identified as being essential in the human diet: linoleic acid (LA) (18:2 n-6) and alpha-linolenic acid (ALA) (18:3 n-3), which are also known as omega-6 and omega-3 FAs, respectively. These substances are part of a lipid group collectively known as long chain polyunsaturated fatty acids. These fatty acids are considered essential because they cannot be synthesized by the human body as a result of the lack of enzymes that can form double bonds beyond the Δ9 carbon.21 After consumption, the essential fatty acids can then be converted in the human body by desaturation and elongation into longer chained and more unsaturated fatty acids, which are more bioactive than their precursors.22 The most common derivative of LA is arachidonic acid (20:4 n-6).21,22 ALA is converted to eicosapentaenoic acid (EPA) (20:5 n-3), which is further elongated to docosahexaenoic acid (DHA) (22:6 n-3).22The conversion of ingested ALA to EPA and DHA within the body is not usually considered to be a reliable source of LC-PUFAs in the human diet. The elongation and desaturation conversions are highly inefficient as most of the fatty acid precursors are utilized for energy.22 Furthermore, the conversion yield of ALA to EPA and DHA in men is only 0.3–8% and <4%, respectively.23 In women, the conversion yield of ALA to EPA and DHA is 21% and 9%, respectively. This poor production of LC-PUFA in the body makes it more beneficial to consume omega-3 FAs as preformed EPA and DHA, rather than as ALA.
2.2. Food sources
There are many dietary sources of omega-3 FAs including fish, krill, algae, and land plants.6 The type and amount of omega-3 FAs varies between sources. Fish is the most common source of omega-3 FAs and the amount of EPA and DHA varies between fish species, time of year, the fish's diet, and geography. Cold water, pelagic fish usually have the highest levels of EPA and DHA. Overall, in marine fish the most important factor is their total fat content, with high fat fish having the highest amount of omega-3 s per serving. Sardine, mackerel, herring, and halibut have some of the highest omega-3 PUFA levels but are uncommon in many diets.6 In the United States, salmon, anchovies, herring, sardines, Pacific oysters, trout, and Atlantic and Pacific mackerel are the most commonly consumed low mercury seafood varieties.11The frequent consumption of fish does raise some safety and environmental concerns. Fish is susceptible to bioaccumulation of toxins and pollutants, one of the most common being mercury.6 An advantage of using fish oils (rather than consuming whole fish) is that oil refining removes the majority of these toxins. Another concern is overfishing of the supply that could strain the sustainability of the market.8
Alternative marine sources are available as a source of omega-3 FA, without facing some of the challenges associated with using fish. Krill oil can achieve higher levels of EPA and DHA than fish oil but the product has a higher cost so it is usually used in supplements.6 In addition, there are marine plant sources of omega-3 FA that can be used commercially in food products. Algae are a primary producers of omega-3 FAs, which can be cultivated to produce a continuous supply of omega-3 FAs. While algae produce high amounts of DHA, the EPA levels are often lower than those found in fish oil.22,24 Until recently, relatively high production and purification costs limited the large scale manufacturing of algae oils, however, considerable advances have been made in recent years that have led to their increased commercial use.22,24–26
The Dietary Guidelines and American Dietetic Association encourage nutrient consumption from food rather than supplements however, people may choose to consume supplements or fortified foods for many reasons including cost, their dislike of seafood, allergies, a vegan diet, convenience, and the inability to meet recommended EPA and DHA levels from their normal diet.3,8,11 Consumers seeking alternative sources of omega-3 FAs should be aware if the products contain ALA, EPA or DHA in order to receive maximum health benefits.
Land plant sources of omega-3 FAs include canola, soy, flaxseed, and walnuts mainly in the form of ALA.11,22,27 An increased consumption of omega-3 FAs from these sources may have a limited effect in decreasing cardiovascular disease or a stroke because of the inefficient conversion of ALA to EPA and DHA.22
Supplements may contain EPA and DHA in different forms than the common triglyceride form typically found in fish oil.6 Ethyl esters of omega-3 FAs are commonly used in dietary supplements and pharmaceuticals because of their ability to be distilled into ethyl esters to produce highly concentrated oils.6 The ethyl esters of EPA and DHA have a different absorption route in the human body than triglyceride forms, but plasma lipid levels appear to be equivalent, however the triglyceride form can be better utilized in the body.6,28
2.3. Fish oil
In the remainder of this section, we will primarily focus on fish oil as it is considered to be the most common, least expensive, and best source of both EPA and DHA in the human diet.27 However, other sources of omega-3 fatty acids are becoming more economically viable, such as genetically engineered oil seeds.29,30The EPA and DHA found in fish oil is also associated with the prevention and possible treatment of inflammatory disease like asthma, cystic fibrosis, and rheumatoid arthritis.27,33 The anti-inflammatory properties of omega-3 fatty acids may also help patients recover after surgery. Omega-3 FAs administered through a parenteral route to patients after undergoing a liver transplant had positive effects including decreasing the duration of the post-transplant hospital care, reducing infectious morbidities, and protecting the liver from injury partially as a result of the anti-inflammatory effects of the PUFA.34
DHA has been associated with brain development because of the large amounts of DHA in the human nervous system.33 The cell membranes of the brain and retina of the eye experience a surge of DHA inclusion between the third trimester and the first year after birth.35 Omega-3 FAs are essential for proper brain functioning and development and studies have found connections between maternal consumption of fish and the visual acuity, higher developmental scores at 18 months, and higher IQ of infants.33,35–37 These preliminary studies highlight the potential importance of DHA consumption for pregnant women.
Besides brain development, omega-3 FAs have also been investigated for connections with mental health conditions including attention deficit hyperactivity disorder (ADHD), dyslexia, depression, and adult cognitive decline including dementia and Alzheimer's disease.33 All of these areas require further investigation for various reasons including small sample sizes, inconsistencies in regimes, drug interactions, or conflicting conclusions.
The National Academies (USA) has made its omega-3 FA recommendations using adequate intake values. An adequate intake value is used if a recommended daily allowance cannot be established and is determined based on the intake of healthy people.3 For males and females 14 years old and above, the adequate intake value of ALA, EPA and DHA are 1.6 and 1.1 g per day, respectively with most of the recommendation coming from ALA.38 Pregnant and lactating women have an adequate intake value of 1.4 and 1.3 g omega-3 s per day, respectively.
The American Dietetic Association and Dieticians of Canada recommend 2 servings of fatty fish per week; 8 oz of cooked fish should provide 500 mg of EPA and DHA per day.39 The American Diabetes Association suggest at least 2 servings of fish per week for adequate omega-3 FA consumption.40 Commercially fried fish filets are excluded from this recommendation. The American Heart Association recommends 2 servings of fatty fish per week, a total of 8 oz in order to obtain beneficial amounts of EPA and DHA.41
The European Food Safety Agency proposes the dietary intake of 250–500 mg of EPA and DHA per day for adults.27,42 They also acknowledge that supplementing up to 1 g of DHA per day is safe. The Scientific Advisory Committee on Nutrition of Great Britain recommends at least 2 servings of fish (140 g) per week with at least one of the servings from oily fish.2 In France, the French National Nutrition and Health Program (PNNS) recommends eating fish two times a week.43 The French Food Safety Agency (AFFSA) recommends that individuals over the age of 10, including pregnant and lactating women, should consume 500 mg of EPA and DHA per day and a minimum of 250 mg of DHA per day.
The World Health Organization recommends 2 servings of fish per week in order for the consumer to intake about 200 to 500 mg of EPA and DHA per day.44 The Australian and New Zealand National Health and Medical Research Council recommends 430 and 610 mg per day of DHA/EPA/DPA (docosapentaenoic acid) for women and men between the ages of 19 and 69 years.45 For pregnant and lactating women from 19–50 years old, 115 and 145 mg per day of DHA/EPA/DPA is recommended.
Western diets in general do not provide satisfactory omega-3 FA intakes. American's current consumption of EPA and DHA is lower than the recommended values.1,4 On average, Americans are currently consuming 3.5 ounces (99 g) of seafood per week and much of it is low in omega-3 FAs.3,11 The National Health and Nutrition Examination Survey (NHANES) determined the mean intake of EPA and DHA through food sources by people over 19 years is 23 and 63 mg per day, respectively.1,4 For individuals over the age of 19 consuming EPA and DHA through both food and supplement sources, they are consuming 41 and 72 mg per day, respectively. As of the 2008/2009 and 2010/2011 surveys, the actual consumption of oily fish by the population of Great Britain was not meeting the recommendation.2,46 On average, only 54 g of oily fish were consumed per week across the age range of 19–64 years. Adults over 65 consumed an average of 90 g of oily fish per week. In contrast, Japanese diets easily provide sufficient omega-3 FA. The Japanese population achieves the recommended intake values of DHA and EPA through their diet high in seafood, and their use of dietary fats high in ALA.47 Japanese adults consume about 80 g of fish and shellfish per day, resulting in around 1–2 g of omega-3 FA per day.
Although many of the dietary recommendations for omega-3 encourage consumption of fish, this is not always convenient: some people do not like fish; some people cannot afford fish; fresh fish spoils rapidly; fish may contain undesirable contaminants (such as heavy metals); overfishing may reduce the supply of fish available; the growing global population puts a higher demand on the available fish.6–8 Consequently, there is great interest in the development of alternative means of incorporating omega-3 fatty acids into the human diet.8
3. Nanoemulsions
Emulsion-based delivery systems offer a number of potential benefits for introducing omega-3 oils into foods and beverages.48–50 Nanoemulsions are a class of emulsion-based delivery systems that are becoming increasingly popular because of their ease of preparation, small particle size, relatively high stability, and high bioavailability.3.1. Characteristics of nanoemulsions
Oil-in-water nanoemulsions, which are the most suitable for encapsulating omega-3 oils, consist of emulsifier-coated lipid droplets dispersed within an aqueous continuous phase. Nanoemulsions have been defined as emulsions that have mean particle radii below 100 nm.18,51 Unlike microemulsions, which also contain small lipid droplets dispersed in water, nanoemulsions are thermodynamically unstable systems.52,53 Nanoemulsions have been utilized in the food and pharmaceutical industries as delivery systems to encapsulate, protect, and control the release of a variety of bioactives.48,49,54 The small particle size provides both benefits and challenges for nanoemulsions.The bioavailability of lipophilic bioactive components encapsulated in small particles is usually greater than those in larger particles, which may be due to various mechanisms.19,54 Smaller particles have a larger specific surface area allowing for increased enzyme activity at the oil-water interface and therefore faster lipid digestion.17,54 Smaller particles can also penetrate into the mucus layer coating the epithelium cells of the small intestine, thereby increasing the time for lipid digestion and absorption. In addition, smaller particles may be able to pass through the mucus layer and be absorbed by epithelium cells. Lastly, smaller particle sizes increase the solubility of encapsulated lipophilic components in the aqueous phase close to the particle surfaces due to a curvature effect, thereby increasing the driving force for absorption.17,54
Nanoemulsions are not thermodynamically stable since the separate oil and water phases have a lower free energy than the emulsified system.17,51 Nevertheless, they can be designed to have high kinetic stability.55 For example, nanoemulsions typically are more resistant to gravitational separation, flocculation, and coalescence than conventional emulsions.17 Their high stability to gravitational separation can be attributed to two reasons: (i) the creaming or sedimentation velocity is proportional to the square of the particle size; (ii) Brownian motion dominates gravitational forces for small droplets.51 The high stability of nanoemulsions to droplet aggregation is due to the fact that the attractive forces that normally promote flocculation or coalescence weaken with decreasing droplet size.51 On the other hand, nanoemulsions are often more susceptible to Ostwald ripening than conventional emulsions. Ostwald ripening in O/W nanoemulsions involves the diffusion of the oil phase from small droplets to larger ones resulting in an increase in the mean droplet size.17,51,56 Droplet growth due to this mechanism can be inhibited by careful selection of the oil phase or by addition of ripening inhibitors.49
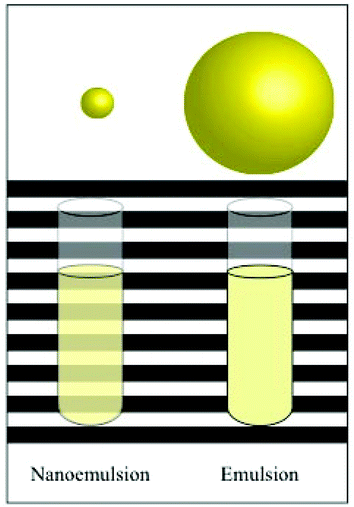
Another potential advantage of nanoemulsions for certain applications is that they can appear transparent or only slightly turbid when they are fabricated to have particle sizes much smaller than the wavelength of light (Fig. 1).18 Typically, the mean droplet radius should be less than about 20–25 nm to ensure high optical clarity of a nanoemulsion, which requires careful control of fabrication conditions and product formulation.
3.2. Fabrication methods
Typically, nanoemulsions require the use of high mechanical energy, high surfactant levels, or both in order to be produced.57 In general, nanoemulsion production can be divided into high-energy and low-energy methods.58 High-energy methods rely on the application of mechanical energy to disrupt the separate oil and water phases, mix the two phases together, and form tiny oil droplets.49,51 High-energy methods based on this principle include high pressure valve homogenizers, microfluidizers and sonicators.17 Droplet size is dependent on many variables including the production method, operation settings, and system components.17 Typically, the droplet size decreases with increasing energy input and duration, provided there is sufficient surfactant present and the oil, water, and surfactant type are carefully selected.In contrast, low-energy methods rely on changes in the environment or solution conditions to promote the spontaneous formation of tiny oil droplets.49 The ability of low-energy methods to produce nanoemulsions are closely related to the physicochemical properties of the surfactant, and depend on the type and amount of surfactant, oil and water present.55 Low-energy methods for nanoemulsion fabrication are becoming more popular because they can better create smaller particles sizes compared to high energy methods, they have lower manufacturing costs, and they have simple production methods.49
A number of low-energy emulsification methods are available, including the spontaneous emulsification (SE), phase inversion temperature (PIT), phase inversion composition (PIC), and emulsion inversion point (EIP) methods.17 SE uses simple mixing as one phase is slowly added to another to spontaneously form an emulsion, e.g., an organic phase containing surfactant and oil is added to an aqueous phase containing water.59 The final emulsion can be manipulated by controlling many variables including which phase is added into the other, the composition of the phases, environmental factors (i.e. temperature and pH), and mixing conditions (i.e. stir speed and rate of addition).49 The PIT method utilizes alterations in temperature to change the solubility or optimum curvature (molecular geometry) of non-ionic surfactants, which results in the conversion of an oil-in-water to a water-in-oil emulsion or vice versa.17,59,60 Typically, a surfactant–oil–water mixture of appropriate composition is heated above the PIT, and then rapidly cooled with continuous stirring to form a nanoemulsion. The PIC method is similar to the PIT method as it relies again on a change in the solubility or optimum curvature of the surfactant, however instead of changing the temperature of the system, the formulation of the system is altered, e.g., salt concentration.59 Both the PIT and PIC methods rely on a transitional-phase inversion which utilizes the change in surfactant's functional characteristics.49 The EIP method however relies on catastrophic-phase inversion instead of transitional-phase inversion methods. Catastrophic-phase inversion changes the ratio between the oil and water phases while maintaining the surfactant's properties.49 This may occur by preparing a water-in-oil emulsion and then adding water while stirring. The water will initially form more droplets in the oil however when excess water is added, the water becomes the continuous phase and the oil becomes droplets leading to the formation of an oil-in-water emulsion.
3.3. Formulating safe nanoemulsions
When formulating nanoemulsions for food systems, food safety is one of the greatest concerns,19 followed by the consumer's desire for clean labels on their foods.61 Reducing the particle size into the nano-range (r < 100 nm) may substantially change the gastrointestinal fate of ingested foods, which has led to some concern about the presence of engineered nanoparticles in foods.19 As mentioned earlier, there may be a considerable increase in the oral bioavailability of encapsulated bioactive agents when they are incorporated into nanoemulsions. In many cases, this increase may be desirable, but in some cases it may be undesirable. For example, a bioactive agent may have an optimum blood level concentration for efficacy, but may become toxic at higher levels. If a nanoemulsion greatly increased the concentration of this type of bioactive agent, it may lead to high blood levels that increase toxicity. However, this should not be a problem with omega-3 oils because they can usually be consumed at high levels without causing health problems. Nevertheless, if the oil is highly oxidized then it may contain toxic reaction products that would cause a problem, although consumers usually reject this type of product due to poor sensory characteristics.The presence of certain components in nanoemulsions may also cause concern, particularly high levels of surfactants or solvents. Surfactants are commonly used to stabilize nanoemulsions by adsorbing to droplet surface and protecting them from aggregation.48 Large amounts of surfactant are typically needed to fabricate nanoemulsions using low-energy methods (such as the spontaneous emulsification or phase inversion temperature methods), but this is less of a problem with the high-energy methods commonly used in the food industry (such as high pressure homogenization or sonication).62,63 In addition, the surfactants used to form nanoemulsions are typically small molecule synthetic surfactants (such as Tweens), although some progress has been achieved forming nanoemulsions using natural surfactants such as phospholipids or saponins.64,65 There are some health concerns associated with using high amounts of certain types of synthetic surfactants in foods, and so their use is limited by government regulations.66 Natural biopolymer-based emulsifiers, such as polysaccharides and proteins, cannot currently be used to form nanoemulsions by low-energy fabrication methods,49 although they can be used to form nanoemulsions by high-energy methods.67 Toxicity may also arise from the utilization of organic solvents in certain solvent displacement or evaporation methods used to prepare nanoemulsions.49 Small traces of these solvents may remain in the emulsion and must be monitored. However, most of the fabrication methods currently used to create food emulsions do not require the utilization of organic solvents. Another important issue affecting the potential toxicity of nanoemulsions is the fact that lipid nanoparticles may behave differently in the human body than the larger particles conventionally used in foods, e.g., the location, rate, and extent of absorption.19
3.4. Formulating label-friendly nanoemulsions
Consumers are increasingly demanding products that are perceived to have “clean labels”.64,68 Changing to natural surfactants may be one way to meet these demands. One natural surfactant that has been investigated is extracted from the bark of the Quillaja saponin Molina tree and is marketed commercially as Q-Naturale® (Ingredion, New Jersey). This surfactant has been compared to Tween 80, a common nonionic surfactant used in the food industry, to form nanoemulsions by a high-energy method (microfluidization) using medium chain triglycerides as the oil phase.64 Q-Naturale exhibited effective surfactant properties, as it was able to form stable nanoemulsions under certain circumstances at relatively low surfactant-to-oil ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). The use of clean label ingredients also extends to any cosolvents or antioxidants that are added to the emulsion formulation to increase physical and chemical stability, which may narrow the formulation possibilities for omega-3 nanoemulsions.
10). The use of clean label ingredients also extends to any cosolvents or antioxidants that are added to the emulsion formulation to increase physical and chemical stability, which may narrow the formulation possibilities for omega-3 nanoemulsions.
4. Applications of nanoemulsions in foods and beverages
The most widely used delivery systems for incorporating omega-3 oils into foods and beverages are bulk oils, emulsions, and powders.69 These powders are typically formed by spray drying emulsions. Microencapsulation has proved to be a popular way of creating powdered omega-3 that can be incorporated into a variety of food products including baked goods, spreads, and fruit beverages.70 However, this technology typically only delivers relatively small levels of bioactive lipids since powders usually only contain around 1 to 30% omega-3 FAs.16 Microencapsulated emulsions for food applications have previously been discussed in detail elsewhere and will therefore not be reviewed further here.16,70Nanoemulsions offer a convenient means of fortifying many aqueous-based food and beverage products with omega-3 oils. Fortified nanoemulsions could be introduced into food systems such as beverages, salad dressings, sauces, dips, and desserts.71,72 Current liquid or semisolid food products that have been enriched with omega-3 FAs using emulsion-based delivery systems include table spreads, yogurts, and milk.73–76 None of these products requires the delivery system to be optically transparent, and therefore emulsions or nanoemulsions could be used, although there may be some advantages in terms of long-term stability and bioavailability from using nanoemulsions.77 The optical transparency that can be achieved with nanoemulsions allows their application within clear food and beverage products, which would expand the functional food market for lipophilic bioactives. Low-energy fabrication methods are also becoming a larger area of interest because of their beneficial characteristics mentioned previously, e.g., simplicity, low cost, and gentle processing conditions.59,72 That being said, nanoemulsions must be carefully formulated to create physically and chemically stable systems suitable for food applications.
5. Obstacles to incorporating Omega-3 nanoemulsions in foods
A number of obstacles must be overcome before omega-3 fortified nanoemulsions can be successfully incorporated into commercial food products,48 such as their susceptibility to lipid oxidation, ensuring the physical stability of the system, delivering a nutritionally beneficial quantity of bioactive in a bioavailable form, and providing a palatable product that is acceptable to consumers. A number of these challenges are discussed in more detail in the remainder of this section.5.1. Oxidation
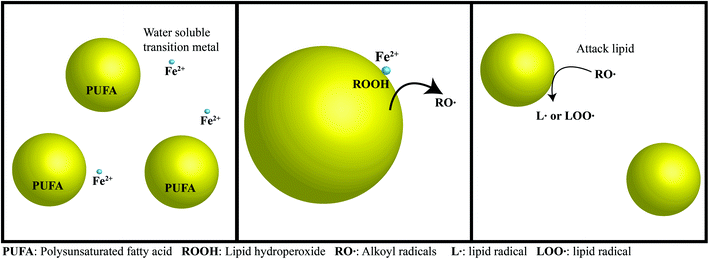
Lipid oxidation in food products causes multiple problems that impact shelf-life, safety, nutritional value, functionality, and flavor.78,79 Oxidation is readily noticed by consumers because the products of the reaction cause undesirable sensory attributes in food products at very low levels.48 Oxidation is the reaction of unsaturated FAs, free radicals and oxygen (Fig. 2) and occurs in three stages: initiation/induction, propagation, and termination.78,80,81 The most common mechanism for oxidation in emulsions is the reaction of free radicals with unsaturated lipids leading to the formation of lipid radicals. These lipid radicals react with oxygen and other lipids, thus beginning the chain reaction (propagation) stage of lipid oxidation.80 Before oxidation occurs, there is a lag phase, which is the phase that food processors attempt to extend through means of storage in cooler temperatures, decreased oxygen exposure, and addition of antioxidants.81 Once the initiation phase has begun, the rate of oxidation increases exponentially and the food is spoiled.Lipid oxidation is promoted by exposure of unsaturated lipids to air, light, heat, and irradiation.78 Many factors contribute to an emulsion-based delivery system's susceptibility to oxidation including the composition, structure and organization of the oil, water and interfacial phases, as well as the type, amount, and location of any antioxidants present.82 Fish oil nanoemulsions are particularly susceptible to lipid oxidation for a number of reasons: high degree of lipid unsaturation; high surface area of exposed lipids; and greater light penetration.82 Indeed, experimental studies have shown that lipid oxidation is faster in protein-stabilized nanoemulsions than in conventional emulsions with similar compositions, which was attributed to the higher lipid surface area.83 Consequently, it may be necessary to take additional steps to stabilize omega-3 oils encapsulated within nanoemulsions when compared to conventional emulsions.
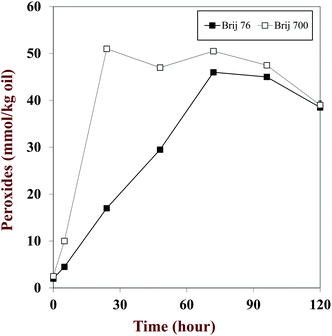
Oxidation in nanoemulsions can be partially managed by controlling their physicochemical characteristics. Surfactants can influence the droplet charge, thickness, and permeability, all of which control the ability of pro-oxidants, free radicals, and oxygen to interact with the lipids in the droplets.82,84 Several studies have shown that anionic surfactants attract cationic transition metals while cationic surfactants repulse them thereby decreasing the rate of oxidation.82,85–88 In addition, the interfacial layer of an emulsion can form a physical (steric) barrier against the aqueous phase of a system that contains pro-oxidants.82,88 Thicker interfacial layers offer more protection, which depends on the dimensions and composition of the surfactant's head and tail group. A surfactant with a larger head group (Brij 700) was found to be better at slowing lipid oxidation in salmon oil-in-water emulsions than one with a smaller head group (Brij 76) (Fig. 3).88 Conversely, surfactant tail length has been shown to have only a minor impact on oxidative stability.89
To prevent oxidation in food systems, radical scavenging and metal chelation are the main antioxidant strategies.78,82,90 Free radical scavengers react with free radicals before they can react with unsaturated FAs, and their effectiveness depends on their ability to donate a hydrogen atom to the free radical.91 Flavonoids tend to be effective free radical scavengers by donating a hydrogen from their hydroxyl groups, however their ability to act as an antioxidant depends on their volatility, pH sensitivity, and polarity. Metal chelation is a mechanism by which an antioxidant reduces the reactivity of the transition metal or physically blocks it from interacting with the lipid.82 Metal chelators in oil-in-water emulsions have been shown to promote the movement of iron out of the lipid phase and to remove it from the surface of oil droplets, thereby inhibiting lipid oxidation.86,92,93 Studies of the chemical degradation of β-carotene in nanoemulsions (another polyunsaturated bioactive lipid) have shown that the rate of oxidation depends on system conditions (such as pH, ionic strength, temperature, droplet size, and emulsifier type) and can be inhibited by adding appropriate antioxidants.94–97 The addition of antioxidants has also been found to improve the stability of citral oil in nanoemulsions.98 Similar factors are likely to affect the rate of omega-3 oxidation in nanoemulsions.
Flavonoids can act as antioxidants through the means of radical and oxygen scavenging and have been found to be successful in inhibiting oxidation in fish oil emulsions.99 Two Flavonoids from apples (phloretin and phloridzin) have been tested for their ability to inhibit oxidation of PUFA methyl esters in oil-in-water emulsions.100 Both of these natural components had a significant effect in preventing lipid oxidation, with phloretin having a higher antioxidant activity than phloridzin, which was attributed to the fact that it was more lipophilic and therefore tended to accumulate within the lipid droplets where oxidation occurs. Certain flavanols (quarcetine glucosides) have also been evaluated for their antioxidant activity in bulk fish oil and in fish oil-in-water emulsions, and compared with butylated hydroxytoluene (BHT) and alpha-tocopherol.101 The emulsions were formed with methyl linolenate or DHA as the lipid phase. In oil-in-water emulsions, the flavanols were less effective than BHT but more effective than alpha-tocopherol in preventing oxidation. In addition, the flavanols were more effective than both BHT and alpha-tocopherol in the bulk oil oxidation prevention.
5.2. Physical stability
The physical stability of nanoemulsions impacts their shelf life, appearance, functionality, and acceptability to consumers. As previously mentioned, nanoemulsions are most susceptible to Ostwald ripening, which is driven by the degree of water-solubility of the oil phase in the aqueous phase.49,102 Oils with a higher water-solubility are more susceptible to Ostwald ripening because it is easier for them to migrate through the aqueous continuous phase. Oils with a lower degree of water-solubility, like long chain triglycerides, rarely experience Ostwald ripening. Fish oils contain long chain triglycerides, which makes them resistant to droplet growth due to Ostwald ripening.103 If nanoemulsion-based delivery system are formulated using more water-soluble oils (such as flavor oils to mask off flavors), then it may be necessary to carefully design them to avoid Ostwald ripening. For example it may be necessary to mix a certain amount of water-insoluble oil (such as fish, flaxseed, or algae oil) with a flavor oil to prevent droplet growth.49 In this case, the water-insoluble oil acts as a ripening inhibitor.The surfactant type and concentration used to create a nanoemulsion or emulsions impacts its susceptibility to flocculation and coalescence.18,49,104 Non-ionic surfactant-coated and polysaccharide-coated droplets tend to be stable across a wide range of salt and pH conditions because they are mainly stabilized by steric repulsion. On the other hand, phospholipid-coated and protein-coated droplets tend to be highly susceptible to changes in pH and ionic strength because they are mainly stabilized by electrostatic interactions. Non-ionic surfactant stabilized nanoemulsions are influenced by other factors, such as surfactant characteristics and temperature. For example, nanoemulsions formed by spontaneous emulsification experienced coalescence during one month storage when using surfactants with intermediate hydrophilic/lipophilic balance (HLB) numbers (5–9). These surfactants tend to be soluble in both oil and water and form lamellar structures instead of micelles due to their optimum curvature, which do not stabilize nanoemulsions very effectively.72 Non-ionic surfactant stabilized nanoemulsions may also coalesce upon heating due to changes in the optimum curvature of the surfactant monolayer at elevated temperatures, i.e., dehydration of the head group.62,105
Protein-coated lipid droplets are highly susceptible to flocculation at high salt levels or at pH values close to their isoelectric point (pI) due to a reduction in electrostatic repulsion between the droplets.106,107 Protein-stabilized nanoemulsions should therefore only be used under conditions that favor a strong electrostatic repulsion between the droplets, i.e., low ionic strength and/or pH far from pI. Alternatively, they should be incorporated into products that are highly viscous or gel-like, since then even if aggregation does occur the nanoparticles will not separate from the product due to gravitational separation.
5.3. Reaching the RDA
For a product to be considered to be a functional food, it must provide health benefits exceeding those of basic nutrition.13 The incorporation of fish oil in foods and beverages meets this definition based on the potential health benefits previously mentioned. However, it is important that the amount of omega-3 FAs present in a functional food is large enough to demonstrate a beneficial health effect.108 Thus products should be fortified with an amount of fish oil that is a substantial amount of the recommended intake value if not the total amount. The total amount of omega-3 FAs in a functional food product (mw-3) depends on the fraction of omega-3 FAs in the oil phase (Φw-3), the fraction of oil phase in a nanoemulsion-based delivery system (ΦnE), the amount of nanoemulsion added to the food product (ΦP), and the serving size of the product (mP):| mw-3 = mP × Φw-3 × ΦnE × ΦP |
For example, for a fish oil containing 50% omega-3 fatty acids (Φw-3 = 0.5), that is converted into a 20 wt% oil-in-water nanoemulsion (ΦnE = 0.2), that is added to a food product that has a serving size of 280 g at a level of 10 wt% (ΦP = 0.1), then the final amount of omega-3 oil present is 2.8 g (2800 mg). As mentioned earlier, the recommended intake values of omega-3 fatty acids are around 250 to 1000 mg per day, and therefore this amount should be achievable. The amount of nanoemulsion added to a food product may be limited by changes in optical properties if the nanoemulsion is not completely transparent. Typically, the smaller the droplet size, the more transparent the nanoemulsion and therefore the more that can be incorporated before the system becomes turbid. It is also important to ensure that the droplets do not grow after the food product has been manufactured, or this could result in an increase in turbidity during storage.
5.4. Bioavailability
With the growing use of emulsion-based delivery systems for human consumption, it is important to evaluate the gastrointestinal fate of the systems to ensure that there are no adverse health effects, and that the bioactive being delivered is indeed being absorbed into the body.19,109In vitro and in vivo digestion models have become instrumental in undertaking this kind of evaluation.110–112 Bioaccessibility is an important marker used in these studies that describes the fraction of an ingested compound (the bioactive) that is transferred into a mixed micelle after lipid digestion.113An ingested nanoemulsion will pass through the mouth and stomach before reaching the small intestine where lipid absorption normally occurs.114,115 The size, composition, and surface characteristics of the lipid droplets within a nanoemulsion may change appreciably when they are exposed to gastrointestinal conditions.19 Upon entering the small intestine, lipase adsorbs to the surfaces of emulsified fats and coverts triacylglycerols into monoacylglycerols and free fatty acids (FFA).21 These FFAs are then incorporated into mixed micelles, travel through the mucus layer, and are absorbed by epithelium cells. The bioavailability of encapsulated fatty acids may be inhibited if the ability of the lipase to adsorb to the surface of lipid droplets and hydrolyze the triglycerides is prevented. The type and amounts of surfactants in a nanoemulsion may therefore impact the rate and extent of lipid digestion and FFA release. For example, corn oil nanoemulsions made using high-energy methods experienced a lag period before FFA release that ranged from 5 to 20 minutes as the mean droplet radius increased.116 This was a result of the lipase not being able to adsorb to the surface of the droplets due to the presence of excess surfactant that competed for the droplet surfaces. In these emulsion, 61–71% of the FFAs were released with higher amounts of FFA being release as the particle radius decreased. The obstruction of lipase as a result of high surfactant concentrations was also seen in medium chain triglyceride nanoemulsions containing vitamin E acetate made from both high and low energy methods.117 In this study, both the high and low energy emulsions had comparable particle sizes and similar behaviors throughout the in vitro digestion and both released similar amounts of FFA.
Surfactants can also impact the rate of lipid digestion based on their molecular and physicochemical characteristics. A study by Speranza et al. evaluated the effect of nonionic and anionic surfactants with a range of HLB numbers on the bioaccessibility of lipids (trioctanoyl glycerol) in emulsions using an in vitro digestion model.118 The results showed that an increasing HLB number increased the lag time in the jejunum and decreased the rate of lipolysis. In contrast, increasing the length of the aliphatic chain decreased the lag time in the jejunum, but increased the rate of lipolysis in the small intestine.
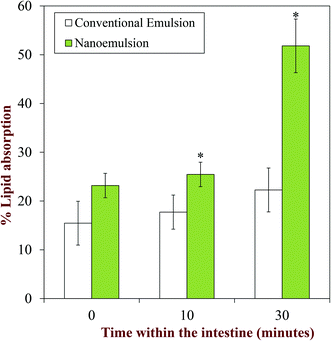
After FFA and bioactives are liberated from the lipid droplets, they form mixed micelles that travel through the mucus layer, and are then absorbed by the intestinal epithelial cells. When conventional fish oil emulsions were compared with fish oil nanoemulsions, the nanoemulsions had a significantly higher percentage of lipid absorbed compared to the conventional emulsions, which was attributed to their smaller particle size (Fig. 4).119 A recent study showed that the bioaccessibility of an oil-soluble bioactive component (vitamin E acetate) was higher in nanoemulsions prepared using a low-energy method (EPI) than in those prepared using a high-energy method (microfluidization).117 It was suggested that the high levels of surfactant used in the low-energy method may have increased the amount of bioactive incorporated into the mixed micelles. The surfactant characteristics can also impact FFA absorption.118 An increasing surfactant HLB has been reported to increase the bioaccessibility of FFAs in the small intestine.
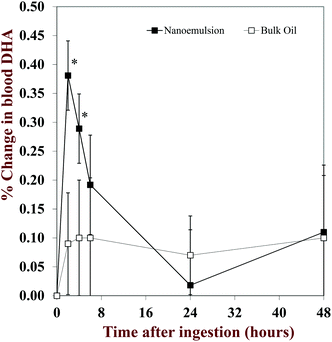
Lastly, the absorption of fish oils from ingested foods is important when developing functional food systems. Researchers investigated the absorption of fish oil in capsules versus microencapsulated fish oil incorporated into a milk shake.120 Both treatments resulted in similar increases of EPA and DHA in blood plasma. Another study looked at yogurt as a carrier product for algal oil nanoemulsions (mean droplet size 258 nm) versus bulk oil.74 In this study, both the nanoemulsion and bulk oil increased DHA levels in blood lipids however; the DHA from the nanoemulsion was more bioavailable than the bulk oil during the first four hours of digestion (Fig. 5). Both of these studies support the use of microencapsulated or emulsified fish oil in food products and provide an alternative way for consumers to supplement their EPA and DHA intake without swallowing a large pill. The properties of a food system that accompanies the fish oil also has importance. When supplements were consumed with a higher fat meal compared to a lower fat meal, more long chain omega-3 PUFA were available, possibly due to the higher fat content stimulating more digestive enzymes and more mixed micelles.28 This again supports the use of functional foods to incorporate omega-3 FA and increase the absorption of the fats as an alternative to supplements.
5.5. Flavor
As previously mentioned, some consumers must find alternative sources of omega-3 FAs because they do not like the flavor or texture of fish or seafood. High quality refined fish oils have little to no flavor. This is unlike oils used in some dietary supplement that are low quality and have strong fishy flavors. Some consumers avoid soft gel capsules of fish oil supplements because of the reflux of fish oil resulting in “fish burps”.121 This is caused by the formation of a layer of the fish oil on top of the stomach contents because the oils have a lower density than the gastric juices. By using a fish oil nanoemulsions incorporated into food products, consumers can receive the benefits of EPA and DHA in a form other than seafood. In addition, nanoemulsions can be designed to be resistant to coalescence and creaming within gastric environments by selecting appropriate emulsifiers so that the oil will not form a layer of oil on the top of the stomach contents and cause reflux issues.122–124 When functional foods are concerned, consumers will not sacrifice the taste of a product, even if the consumer is aware of the potential health benefits of the functional food.1255.6. Consumer acceptance
Studies disagree about which types of food a bioactive component, such as omega-3 oils, should be added for maximum consumer interest. In a study by Ares and Gámbaro consumers were more accepting of a functional food when the carrier food was perceived as being healthy.126 In a separate study by Bech-Larsen and Grunert, it was concluded that functional foods with a healthier base food were perceived as healthier compared to functional foods with an unhealthy base food, however this study also stated that consumers rationalized the enrichment of less healthy foods better than that of already healthy foods.127 Some consumers have concerns about unhealthy foods that have been fortified because they may now be perceived as a health food by others when in fact they are not.128Regardless of the carrier product, it is important to the consumer that the bioactive ingredient and base food are compatible; this is a stronger driving force for the purchasing of functional food products compared to health benefits and attitude towards functional foods.129 For example, products where fish oil appears to be a more natural fit such as fish balls, rye bread, and tuna salad were expected to receive more positive attention by consumers.128 Another characteristic of fish oil enriched foods that should be considered when choosing an appropriate food carrier and in the product formulation is the sweetness profile. Participants in a study evaluating the acceptance of fish oil fortified foods were put off by sweet products such as yogurt drinks and sports bars having the addition of fish oil.128 In a separate study, women between the ages of 40 and 60 years did not accept the addition of sweeteners into a functional food and would rather consume a more natural product.125
It is suggested that the use of health claims on functional food labels will have a positive impact on the consumer's view of the healthfulness of that food.127 The source of omega-3 fatty acids used in the fortification of foods can affect the cost of the products but also their health benefits. ALA omega-3 s may give a cleaner label because they are from plant sources along with a lower price for consumers however, the conversion of ALA to LC-PUFA is quite low, decreasing its actual health benefits.22 The FDA health claim for EPA and DHA containing foods can aid in the marketing and advertising for qualifying products while differentiating them from products that only provide ALA.
Finally, sensory aspects also play a key role in consumer acceptance of foods. Few studies have researched the effect of nanoemulsions on the sensory properties of enriched foods. Dairy products have been the main focus of these studies. One study evaluated the fishy off flavor intensity of strawberry yogurt containing emulsified omega-3 oils after 14 days storage.73 This study found no significant difference between the control and fortified yogurt samples amongst an untrained consumer panel. Another study evaluated a strawberry drinking yogurt fortified with bulk algae oil and algae oil nanoemulsion for smell, appearance, flavor, texture, consistency, aftertaste, and overall acceptability.130 Consumers were able to identify a sensory difference between yogurts fortified with either bulk oil or nanoemulsions in a triangular test. However, no statistically significant differences were found between the nanoemulsion-fortified, bulk oil-fortified, and unfortified yogurts in terms of their consistency and appearance. The sensory properties of cheese fortified with bulk fish oil or fish oil nanoemulsion have also been evaluated.131 Fishy off flavor was dependent on the concentration of fish oil in the sample and was more easily detected in the bulk oil-fortified samples compared to the nanoemulsion-fortified samples. Clearly, more research should be conducted to evaluate the sensory aspects of foods fortified with nanoemulsions to better understand their effect on consumer acceptance.
6. Conclusions
The low consumption of omega-3 FAs in Western diets clearly shows the need for alternative food sources on the market that provide these essential fatty acids. Fish oil is an effective functional food ingredient because it is a good source of both EPA and DHA. Consumers will be more likely to buy functional foods with fish oil if the carrier food is compatible with the fat and if the foods are more savory instead of sweet. Whether the fish oil should be added to healthy or unhealthy foods is debated and should be evaluated on a product-by-product basis. Nanoemulsions are a promising way to deliver fish oils into liquid food systems with the capabilities to protect the oil from oxidation, mask undesirable off-flavors, and increase oral bioavailability. Most importantly, the ability of nanoemulsions to be added to clear products increases the range of products that omega-3 FA enrichment can be applied to. That being said, there is still a need to expand omega-3 nanoemulsion research in order to optimize the fabrication method and formulation as a way to increase palatability, shelf life, and other physical characteristics of the food product.Acknowledgements
This material is based upon work supported by the Cooperative State Research, Extension, Education Service, United State Department of Agriculture, Massachusetts Agricultural Experiment Station and United States Department of Agriculture, NIFA Grant and a USDA/EPA/NSF grant (2011-03539 and 2013-03795).References
- Center for Disease Control and Protections, National Center for Health Statistics, 2003.
- Great Britain Scientific Advisory Committee on Nutrition, The Nutritional Wellbeing of the British population, The Stationary Office, Norwich, 2008 Search PubMed.
- P. M. Kris-Etherton, J. A. Grieger and T. D. Etherton, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2009, 81, 99–104 CrossRef CAS PubMed.
- Y. Papanikolaou, J. Brooks, C. Reider and V. L. Fulgoni, Nutr. J., 2014, 13, 31–36 CrossRef PubMed.
- G. Danaei, E. L. Ding, D. Mozaffarian, B. Taylor, J. Rehm, C. J. L. Murray and M. Ezzati, PLoS Med., 2009, 6, 1–23 Search PubMed.
- R. A. Racine and R. J. Deckelbaum, Curr. Opin. Clin. Nutr. Metab. Care, 2007, 10, 123–128 CrossRef CAS PubMed.
- K. Glanz, M. Basil, E. Maibach, J. Goldberg and D. A. N. Snyder, J. Am. Diet. Assoc., 1998, 98, 1118–1126 CrossRef CAS.
- E. T. Kennedy, H. Luo and L. M. Ausman, J. Nutr., 2012, 142, 605S–609S CrossRef CAS PubMed.
- P. J. Gillies, W. S. Harris and P. M. Kris-Etherton, Curr. Atheroscler. Rep., 2011, 13, 467–473 CrossRef CAS PubMed.
- Center for Food Safety and Applied Nutrition, 2013.
- U. S. Department of Agriculture and U. S. Department of Health and Human Services, Dietary Guidelines for Americans, U.S. Government Printing Office, Washington DC, 7th edn, 2010 Search PubMed.
- U. S. Department of Agriculture and U. S. Department of Health and Human Services, Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, USDA, 2010.
- C. Hasler, A. Bloch and C. Thomson, J. Am. Diet. Assoc., 2004, 104, 814–826 CrossRef PubMed.
- E. Hudson, Nutraceutical Business & Technology, 2010, 6, 30–32 Search PubMed.
- I. Fraeye, C. Bruneel, C. Lemahieu, J. Buyse, K. Muylaert and I. Foubert, Food Res. Int., 2012, 48, 961–969 CrossRef CAS PubMed.
- M. L. Garg, L. G. Wood, H. Singh and P. J. Moughan, J. Food Sci., 2006, 71, R66–R71 CrossRef CAS PubMed.
- D. J. McClements, Soft Matter, 2011, 7, 2297–2316 RSC.
- T. G. Mason, J. N. Wilking, K. Meleson, C. B. Chang and S. M. Graves, J. Phys.: Condens. Matter, 2006, 18, R635–R666 CrossRef CAS.
- D. J. McClements, Prog. Lipid Res., 2013, 52, 409–423 CrossRef CAS PubMed.
- M. F. Yao, H. Xiao and D. J. McClements, Annu. Rev. Food Sci. Technol., 2014, 5, 53–81 CrossRef PubMed.
- S. A. S. Gropper, J. L. Smith and J. L. Groff, Advanced nutrition and human metabolism, Wadsworth Cengage Learning, 2009 Search PubMed.
- R. J. Deckelbaum and C. Torrejon, J. Nutr., 2012, 142, 587S–591S CrossRef CAS PubMed.
- L. M. Arterburn, E. B. Hall and H. Oken, Am. J. Clin. Nutr., 2006, 83, S1467–1476S Search PubMed.
- K. C. Maki, K. Yurko-Mauro, M. R. Dicklin, A. L. Schild and J. G. Geohas, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 91, 141–148 CrossRef CAS PubMed.
- T. C. Adarme-Vega, D. K. Y. Lim, M. Timmins, F. Vernen, Y. Li and P. M. Schenk, Microb. Cell Fact., 2012, 11, 1–10 CrossRef PubMed.
- G. Lenihan-Geels, K. Bishop and L. Ferguson, Nutrients, 2013, 5, 1301–1315 CrossRef CAS PubMed.
- J. A. Tur, M. M. Bibiloni, A. Sureda and A. Pons, Br. J. Nutr., 2012, 107(Suppl. 2), 523–552 Search PubMed.
- J. P. Schuchardt and A. Hahn, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2013, 89, 1–8 CrossRef CAS PubMed.
- S. M. Kitessa, M. Abeywardena, C. Wijesundera and P. D. Nichols, Nutrients, 2014, 6, 2035–2058 CrossRef CAS PubMed.
- L. Pottel, M. Lycke, T. Boterberg, I. Foubert, H. Pottel, F. Duprez, L. Goethals and P. R. Debruyne, Phytochem. Rev., 2014, 13, 223–244 CrossRef CAS PubMed.
- J. N. Din, S. A. Harding, C. J. Valerio, J. Sarma, K. Lyall, R. A. Riemersma, D. E. Newby and A. D. Flapan, Atherosclerosis, 2008, 197, 290–296 CrossRef CAS PubMed.
- R. A. Harrison, M. Sagara, A. Rajpura, L. Armitage, N. Birt, C. A. Birt and Y. Yamori, Nutr. Metab. Cardiovasc. Dis., 2004, 14, 344–350 CrossRef CAS.
- C. H. S. Ruxton, S. C. Reed, M. J. A. Simpson and K. J. Millington, J. Hum. Nutr. Diet., 2004, 17, 449–459 CrossRef CAS PubMed.
- X.-H. Zhu, World J. Gastroenterol., 2012, 18, 6141–6147 CrossRef CAS PubMed.
- M. H. Jorgensen, O. Hernell, E. L. Hughes and K. F. Michaelsen, J. Pediatr. Gastroenterol., 2001, 32, 293–296 CrossRef CAS PubMed.
- J. L. Daniels, M. P. Longnecker, A. S. Rowland and J. Golding, Epidemiology, 2004, 15, 394–402 CrossRef.
- I. B. Helland, L. Smith, K. Saarem, O. D. Saugstad and C. A. Drevon, Pediatrics, 2003, 111, e39–e44 CrossRef.
- National Research Council, Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients), National Academies Press, Washington DC, 2005 Search PubMed.
- P. M. Kris-Etherton and S. Innis, J. Am. Diet. Assoc., 2007, 107, 1599–1161 CrossRef CAS PubMed.
- American Diabetes Association, Diabetes Care, 2008, 31, S61–S78 CrossRef PubMed.
- A. H. Lichtenstein, L. J. Appel, M. Brands, M. Carnethon, S. Daniels, H. A. Franch, B. Franklin, P. Kris-Etherton, W. S. Harris, B. Howard, N. Karanja, M. Lefevre, L. Rudel, F. Sacks, L. V. Horn, M. Winston and J. Wylie-Rosett, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 2186–2191 CrossRef CAS PubMed.
- EFSA Panel on Dietetic Products, Nutrition and Allergies, EFSA Journal, 2012, 10, 2815–2863 Search PubMed.
- French Food Safety Agency (AFFSA), The Director General, 2010.
- World Health Organisation and Food and Agriculture Organization, Diet, Nutrition and the Prevention of Chronic Diseases Report of a Joint WHO/FAO Expert Consultation, World Health Organization (WHO), Geneva, CHE, 2003 Search PubMed.
- Autralia and New Zealand National Health and Medical Research Council, Nutrient Reference Values for Australia and New Zealand, 2006 Search PubMed.
- Public Health England and Food Standards Agency, ed. B. Bates, A. Lennox, A. Prentice, C. Bates, P. Page, S. Nicholson and G. Swan, Public Health England, London, UK, 2014 Search PubMed.
- M. Sugano and F. Hirahara, Am. J. Clin. Nutr., 2000, 71, 189S–196S CAS.
- D. J. McClements, E. A. Decker and J. Weiss, J. Food Sci., 2007, 72, R109–R124 CrossRef CAS PubMed.
- D. J. McClements and J. Rao, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef CAS PubMed.
- X. Li, N. Anton and T. Vandamme, Nano-Emulsions: Overview and Applications, in Nanopharmaceutics, ed. X. J. Liang, World Scientific, London, U.K., 2012, pp. 21–48 Search PubMed.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108–109, 303–318 CrossRef CAS PubMed.
- D. J. McClements, Soft Matter, 2012, 8, 1719–1729 RSC.
- N. Anton and T. F. Vandamme, Pharm. Res., 2011, 28, 978–985 CrossRef CAS PubMed.
- E. Acosta, Curr. Opin. Colloid Interface Sci., 2009, 14, 3–15 CrossRef CAS PubMed.
- N. Anton, J.-P. Benoit and P. Saulnier, J. Controlled Release, 2008, 128, 185–199 CrossRef CAS PubMed.
- A. Kabalnov, J. Dispersion Sci. Technol., 2001, 22, 1–12 CrossRef CAS PubMed.
- S. M. Jafari, Y. He and B. Bhandari, Eur. Food Res. Technol. – Z. Lebensm.-Unters. -Forsch. A, 2007, 225, 733–741 CAS.
- A. Forgiarini, J. Esquena, C. González and C. Solans, Langmuir, 2001, 17, 2076–2083 CrossRef CAS.
- N. Anton and T. F. Vandamme, Int. J. Pharm., 2009, 377, 142–147 CrossRef CAS PubMed.
- J. M. Gutierrez, C. Gonzalez, A. Maestro, I. Sole, C. M. Pey and J. Nolla, Curr. Opin. Colloid. Interface Sci., 2008, 13, 245–251 CrossRef CAS PubMed.
- A. E. Sloan, Food Technol., 2012, 66, 1–5 Search PubMed.
- A. H. Saberi, Y. Fang and D. J. McClements, Food Res. Int., 2013, 54, 812–820 CrossRef CAS PubMed.
- F. Ostertag, J. Weiss and D. J. McClements, J. Colloid Interface Sci., 2012, 388, 95–102 CrossRef CAS PubMed.
- Y. Yang, M. E. Leser, A. A. Sher and D. J. McClements, Food Hydrocolloids, 2013, 30, 589–596 CrossRef CAS PubMed.
- B. Ozturk, S. Argin, M. Ozilgen and D. J. McClements, J. Food Eng., 2014, 142, 57–63 CrossRef CAS PubMed.
- I. Kralova and J. Sjöblom, J. Dispersion Sci. Technol., 2009, 30, 1363–1383 CrossRef CAS.
- C. Qian and D. J. McClements, Food Hydrocolloids, 2011, 25, 1000–1008 CrossRef CAS PubMed.
- Ö. Güçlü-Üstündağ and G. Mazza, Crit. Rev. Food Sci. Nutr., 2007, 47, 231–258 CrossRef PubMed.
- C. Jacobsen, Ann. N. Y. Acad. Sci., 2010, 1190, 141–150 CrossRef CAS PubMed.
- S. Drusch, in Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals, ed. N. Garti and D. J. McClements, 2012, pp. 488–504 Search PubMed.
- K. Velikov and E. Pelan, Soft Matter, 2008, 4, 1964–1980 RSC.
- Y. Yang, C. Marshall-Breton, M. E. Leser, A. A. Sher and D. J. McClements, Food Hydrocolloids, 2012, 29, 398–406 CrossRef CAS PubMed.
- C. P. Chee, J. J. Gallaher, D. Djordjevic, H. Faraji, D. J. McClements, E. A. Decker, R. Hollender, D. G. Peterson, R. F. Roberts and J. N. Coupland, J. Dairy Res., 2005, 72, 311–316 CrossRef CAS PubMed.
- K. E. Lane, W. Li, C. Smith and E. Derbyshire, Int. J. Food Sci. Technol., 2014, 49, 1264–1271 CrossRef CAS PubMed.
- S. P. O’ Dwyer, D. O’ Beirne, D. Ni Eidhin, A. A. Hennessy and B. T. O’ Kennedy, LWT – Food Sci. Technol., 2013, 51, 484–491 CrossRef PubMed.
- R. Sharma, Aust. J. Dairy Technol., 2005, 60, 195–198 Search PubMed.
- D. J. McClements and H. Xiao, Food Funct., 2012, 3, 202–220 CAS.
- E. Arab-Tehrany, M. Jacquot, C. Gaiani, M. Imran, S. Desobry and M. Linder, Trends Food Sci. Technol., 2012, 25, 24–33 CrossRef CAS PubMed.
- C. D. Dacaranhe and J. Terao, J. Food Sci., 2001, 66, 422–427 CrossRef CAS PubMed.
- D. J. McClements and E. A. Decker, J. Food Sci., 2000, 65, 1270–1282 CrossRef CAS PubMed.
- D. J. McClements and E. A. Decker, in Food Chemistry, ed. S. Damodaran, K. L. Parkin and F. O. R., CRC Press, Boca Raton, 4th edn, 2008, pp. 155–216 Search PubMed.
- T. Waraho, D. J. McClements and E. A. Decker, Trends Food Sci. Technol., 2011, 22, 3–13 CrossRef CAS PubMed.
- S. J. Lee, S. J. Choi, Y. Li, E. A. Decker and D. J. McClements, J. Agric. Food Chem., 2011, 59, 415–427 CrossRef CAS PubMed.
- A. Villiere, M. Viau, I. Bronnec, N. Moreau and C. Genot, J. Agric. Food Chem., 2005, 53, 1514–1520 CrossRef CAS PubMed.
- C. S. Boon, Z. Xu, X. Yue, D. J. McClements, J. Weiss and E. A. Decker, J. Agric. Food Chem., 2008, 56, 1408–1414 CrossRef CAS PubMed.
- J. R. Mancuso, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 1999, 47, 4112–4116 CrossRef CAS PubMed.
- J. R. Mancuso, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2000, 48, 213–219 CrossRef CAS PubMed.
- M. P. C. Silvestre, W. Chaiyasit, R. G. Brannan, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2000, 48, 2057–2061 CrossRef CAS PubMed.
- W. Chaiyasit, M. P. C. Silvestre, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2000, 48, 3077–3080 CrossRef CAS PubMed.
- N. Belhaj, E. Arab-Tehrany and M. Linder, Process Biochem., 2010, 45, 187–195 CrossRef CAS PubMed.
- S. Damodaran, K. L. Parkin and O. R. Fennema, Fennema's food chemistry, CRC Press, 4th edn, 2008 Search PubMed.
- Y. J. Cho, J. Alamed, D. J. McClements and E. A. Decker, J. Food Sci., 2003, 68, 1952–1957 CrossRef CAS PubMed.
- L. Mei, D. J. McClements, J. Wu and E. A. Decker, Food Chem., 1998, 61, 307–312 CrossRef CAS.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 135, 1036–1043 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 132, 1221–1229 CrossRef CAS PubMed.
- J. Yi, Y. Li, F. Zhong and W. Yokoyama, Food Hydrocolloids, 2014, 35, 19–27 CrossRef CAS PubMed.
- D. X. Xu, X. Y. Wang, J. P. Jiang, F. Yuan, E. A. Decker and Y. X. Gao, LWT–Food Sci. Technol., 2013, 54, 236–241 CrossRef CAS PubMed.
- Q. Zhao, C. T. Ho and Q. R. Huang, J. Agric. Food Chem., 2013, 61, 7462–7469 CrossRef CAS PubMed.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radic. Biol. Med., 1996, 20, 933–956 CrossRef CAS.
- H. P. Vasantha Rupasinghe and A. Yasmin, Molecules, 2010, 15, 251–257 CrossRef CAS PubMed.
- G. M. Huber, H. P. Vasantha Rupasinghe and F. Shahidi, Food Chem., 2009, 117, 290–295 CrossRef CAS PubMed.
- T. J. Wooster, M. Golding and P. Sanguansri, Langmuir, 2008, 24, 12758–12765 CrossRef CAS PubMed.
- A. Gulotta, A. H. Saberi, M. C. Nicoli and D. J. McClements, J. Agric. Food Chem., 2014, 62, 1720–1725 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 135, 1440–1447 CrossRef CAS PubMed.
- A. H. Saberi, Y. Fang and D. J. McClements, J. Colloid Interface Sci., 2013, 411, 105–113 CrossRef CAS PubMed.
- S. J. Lee and D. J. McClements, Food Hydrocolloids, 2010, 24, 560–569 CrossRef CAS PubMed.
- E. Troncoso, J. M. Aguilera and D. J. McClements, J. Colloid Interface Sci., 2012, 382, 110–116 CrossRef CAS PubMed.
- C. S. Patch, L. C. Tapsell and P. G. Williams, J. Nutr. Educ. Behav., 2005, 37, 235–241 CrossRef.
- H. Bouwmeester, S. Dekkers, M. Y. Noordam, W. I. Hagens, A. S. Bulder, C. de Heer, S. ten Voorde, S. W. P. Wijnhoven, H. J. P. Marvin and A. Sips, Regul. Toxicol. Pharmacol., 2009, 53, 52–62 CrossRef CAS PubMed.
- D. J. McClements and Y. Li, Food Funct., 2010, 1, 32–59 CAS.
- C. J. H. Porter and W. N. Charman, Adv. Drug Delivery Rev., 2001, 50, S127–S147 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, Food Funct., 2014, 5, 1113–1124 CAS.
- C. Sy, B. Gleize, O. Dangles, J.-F. Landrier, C. C. Veyrat and P. Borel, Mol. Nutr. Food Res., 2012, 56, 1385–1397 CAS.
- D. J. McClements, E. A. Decker and Y. Park, Crit. Rev. Food Sci., 2009, 49, 48–67 CrossRef PubMed.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2008, 92–100 Search PubMed.
- E. Troncoso, J. M. Aguilera and D. J. McClements, Food Hydrocolloids, 2012, 27, 355–363 CrossRef CAS PubMed.
- S. Mayer, J. Weiss and D. J. McClements, J. Colloid Interface Sci., 2013, 404, 215–222 CrossRef CAS PubMed.
- A. Speranza, M. G. Corradini, T. G. Hartman, D. Ribnicky, A. Oren and M. A. Rogers, J. Agric. Food Chem., 2013, 61, 6505–6515 CrossRef CAS PubMed.
- T. k. Dey, S. Ghosh, M. Ghosh, H. Koley and P. Dhar, Food Res. Int, 2012, 49, 72–79 CrossRef CAS PubMed.
- P. A. Morrissey, S. Higgins, Y. L. Carroll and N. M. O'Brien, J. Hum. Nutr. Diet., 1999, 12, 265–271 CrossRef.
- I. J. Haug, L. B. Sagmo, D. Zeiss, I. C. Olsen, K. I. Draget and T. Seternes, Eur. J. Lipid Sci. Technol., 2011, 113, 137–145 CrossRef CAS.
- P. J. Wilde and B. S. Chu, Adv. Colloid Interface Sci., 2011, 165, 14–22 CrossRef CAS PubMed.
- L. Marciani, M. Wickham, G. Singh, D. Bush, B. Pick, E. Cox, A. Fillery-Travis, R. Faulks, C. Marsden, P. A. Gowland and R. C. Spiller, Gastroenterology, 2006, 130, A227–A227 Search PubMed.
- L. Marciani, M. Wickham, G. Singh, D. Bush, B. Pick, E. Cox, A. Fillery-Travis, R. Faulks, C. Marsden, P. A. Gowland and R. C. Spiller, Am. J. Physiol.: Gastrointest. Liver Physiol., 2007, 292, G1607–G1613 CrossRef CAS PubMed.
- D. N. Cox, A. Koster and C. G. Russell, Appetite, 2004, 43, 55–64 CrossRef CAS PubMed.
- G. Ares and A. Gámbaro, Appetite, 2007, 49, 148–158 CrossRef PubMed.
- T. Bech-Larsen and K. G. Grunert, Appetite, 2003, 40, 9–14 CrossRef.
- R. Krutulyte, K. G. Grunert, J. Scholderer, K. S. Hagemann, P. Elgaard, B. Nielsen and J. P. Graverholt, Appetite, 2008, 51, 137–147 CrossRef PubMed.
- R. Krutulyte, K. G. Grunert, J. Scholderer, L. Lähteenmäki, K. S. Hagemann, P. Elgaard, B. Nielsen and J. P. Graverholt, Food Quality and Preference, 2011, 22, 11–16 CrossRef PubMed.
- K. E. Lane, E. J. Derbyshire, C. J. Smith, K. Mahadevan and W. Li, Proc. Nutr. Soc., 2013, 72, E99–E99 CrossRef.
- A. Ye, J. Cui, A. Taneja, X. Zhu and H. Singh, Food Res. Int., 2009, 42, 1093–1098 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |