Resveratrol down-regulates a glutamate-induced tissue plasminogen activator via Erk and AMPK/mTOR pathways in rat primary cortical neurons†
Abstract
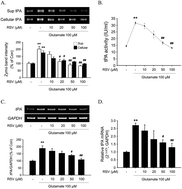
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, RSV) is a polyphenolic compound present in a variety of plant species (including grapes) that produces a myriad of biological activities including anti-inflammatory, antioxidant and neuroprotective effects. In this study, we investigate the effects of resveratrol on the basal and glutamate-stimulated expression and activity of a tissue plasminogen activator (tPA) that plays neuromodulatory or neurotoxic roles in many different neurological situations. Under basal conditions, resveratrol decreased the tPA expression and activity without affecting the tPA mRNA level in rat primary cortical neurons. RSV induced AMPK phosphorylation and inhibited mTOR phosphorylation. Inhibition of AMPK phosphorylation using compound C prevented resveratrol-induced down-regulation of tPA activity. This suggested that AMPK/mTOR-dependent translational inhibition contributes to the down-regulation of the tPA. Under glutamate-stimulated conditions of rat primary cortical neurons, tPA activity and expression were increased along with increased tPA mRNA expression but afterward treatment of RSV inhibited the glutamate-induced increase in tPA activity and expression and tPA mRNA expression. Glutamate stimulation induced activation of Akt and MAPK pathways as well as mTOR which were inhibited by RSV. Interestingly, the Erk pathway inhibitor U0126, but neither PI3K-Akt inhibitor LY294002 nor p38 inhibitor SB203580, mimicked the inhibitory action of RSV on glutamate-induced tPA up-regulation. This suggested the essential role of Erk in the transcriptional up-regulation of tPA expression, which is targeted by RSV. Glutamate stimulation induced neuronal cell death as determined by PI staining and MTT assay. However, RSV protected the cultured rat primary cortical neurons from glutamate-induced cell death as paralleled with the changes in tPA expression. These results suggested that RSV can modulate tPA activity under basal and stimulated conditions by both translational and transcriptional mechanisms. The regulation of the tPA by RSV provides additional therapeutic targets on top of the growing number of molecular substrates of RSV's action in the brain.

 Please wait while we load your content...
Please wait while we load your content...