Environmental release, fate and ecotoxicological effects of manufactured ceria nanomaterials†
Blanche
Collin
*ab,
Mélanie
Auffan
cd,
Andrew C.
Johnson
e,
Inder
Kaur
f,
Arturo A.
Keller
g,
Anastasiya
Lazareva
g,
Jamie R.
Lead
fh,
Xingmao
Ma
i,
Ruth C.
Merrifield
h,
Claus
Svendsen
e,
Jason C.
White
j and
Jason M.
Unrine
*ab
aDepartment of Plant and Soil Sciences, University of Kentucky, Lexington, KY, USA. E-mail: jason.unrine@uky.edu; blanche.collin@uky.edu
bCenter for the Environmental Implications of NanoTechnology (CEINT), Duke University, Durham, NC, USA
cCNRS, Aix-Marseille Université, CEREGE UM34, UMR 7330, 13545 Aix en Provence, France
dInternational Consortium for the Environmental Implications of Nanotechnology iCEINT, Aix en Provence, France
eCenter for Ecology and Hydrology, Wallingford, Oxfordshire, UK
fUniversity of Birmingham, Department of Geography, Earth and Environmental Sciences, Birmingham, UK
gUniversity of California Center for Environmental Implications of Nanotechnology, University of California, Santa Barbara, California, USA
hCenter for Environmental Nanoscience and Risk, University of South Carolina, Columbia, USA
iDepartment of Civil and Environmental Engineering, Southern Illinois University Carbondale, Carbondale, Illinois, USA
jDepartment of Analytical Chemistry, The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, CT 06504, USA
First published on 13th October 2014
Abstract
Recent interest in the environmental fate and effects of manufactured CeO2 nanomaterials (nanoceria) has stemmed from its expanded use for a variety of applications including fuel additives, catalytic converters, chemical and mechanical planarization media and other uses. This has led to a number of publications on the toxicological effects of nanoceria in ecological receptor species, but only limited information is available on possible environmental releases, concentrations in environmental media, or environmental transformations. Increasing material flows of nanoceria in many applications is likely to result in increasing releases to air, water and soils however; insufficient information was available to estimate aquatic exposures that would result in acute or chronic toxicity. The purpose of this review is to identify which areas are lacking in data to perform either regional or site specific ecological risk assessments. While estimates can be made for releases from use as a diesel fuel additive, and predicted toxicity is low in most terrestrial species tested to date, estimates for releases from other uses are difficult at this stage. We recommend that future studies focus on environmentally realistic exposures that take into account potential environmental transformations of the nanoceria surface as well as chronic toxicity studies in benthic aquatic organisms, soil invertebrates and microorganisms.
Nano impactThis critical review identifies the most critical data gaps that should be filled before comprehensive ecological risk assessments for nanoceria can be performed. It provides a review of the sources and sinks of nanoceria in the environment, detection and characterization methods, fate and transport processes and a review of the toxicity literature. |
Introduction
Due to their increasing use in a wide variety of beneficial industrial and consumer applications, ranging from use as a fuel catalyst, to chemical and mechanical planarization media, there have been increasing concerns about the potential environmental health and safety aspects of manufactured ceria (CeO2) nanomaterials.1,2 Ce is among the most abundant of the rare earth elements making up approximately 0.0046% of the Earth's crust by weight (similar in abundance to Cu).3 For example, Ce concentration in soils range from 2 to 150 mg kg−1.4 In Europe, the median concentrations of Ce were 48.2 mg kg−1 in soils, 66.6 mg kg−1 in sediment and 55 ng l−1 in water (http://www.gsf.fi/publ/foregsatlas/). There are many naturally occurring Ce containing minerals include rhabdophane, allanite, cerite, cerianite, samarskite, zircon, monazite and bastnasite.5,6 The existence of naturally occurring ceria nanoparticles is also likely and may play a key role in controlling dissolved Ce concentrations,6 but precisely how the properties of naturally occurring ceria nanoparticles compare to manufactured ceria (CeO2) nanomaterials (nanoceria) is unclear. There is concern that nanoceria, due to its small particle size and enhanced reactivity by design, may present unique hazards to ecological receptor species. Of critical importance are the redox properties of ceria which enables it to transition between Ce(III) and Ce(IV), which are the key to understanding its potential toxicity.7 While there has been somewhat extensive investigation into the mammalian toxicity of ceria (as well as it's therapeutic uses),8 based on the present review, there has been considerably less effort invested into investigation of the environmental fate and effects of nanoceria. In this critical review, we discuss the likely points of environmental release along product life-cycles and resulting environmental exposure to nanoceria, methods of detection in the environment, fate and transport, as well as the available toxicity literature for ecological receptor species. We identify key the data gaps that need to be filled in order to proceed with meaningful ecological risk assessments, whether they are more global/regional in nature, or for site specific assessments. Finally, we attempt to draw conclusions from the literature about the relative sensitivity of different model organisms, as well as the importance of particle properties on fate, transport and effects.Production, use and environmental releases of ceria
Estimation of production volume
The European Commission estimates the global production of nanoceria to be around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.9 Similarly, a comprehensive market study provides an estimate of 7500 to 10
000 tons.9 Similarly, a comprehensive market study provides an estimate of 7500 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons for the year 2011.10 According to the US Geological Survey (USGS), over 80% of the global CeO2 supply originates from China.11 The major nanoceria producers are located in Asia, Australia, and Europe. It is estimated that only 35–700 tons are produced per year in the US.12
000 tons for the year 2011.10 According to the US Geological Survey (USGS), over 80% of the global CeO2 supply originates from China.11 The major nanoceria producers are located in Asia, Australia, and Europe. It is estimated that only 35–700 tons are produced per year in the US.12
Applications
Nanoceria is used in electronic and optical devices, polishing agents for glass and of silicon wafers, exterior paints, metallurgy, and diesel fuel additives.9 Additionally, nanoceria is used in automotive catalytic converters.13 It is also used in catalysts in petroleum refining, in the fluid catalytic cracking process (FCC). Based on the amount of total global CeO2 annual production and global nanoceria production rates,10 roughly 15–25% of total CeO2 production is nano (unverified industry sources). Cerium oxide is used in these applications in both nano and non-nano form and quantitative estimates of cerium oxide use within specific applications do not distinguish between nanoceria and its bulk counterpart.Catalytic converters
Nanoceria is used to improve catalytic reactions in catalytic converters.11 However, studies of CeO2 use in catalytic converters do not distinguish between nanoceria and its bulk counterpart. According to the USGS, approximately 80 g of CeO2 are contained in an average catalytic converter, and roughly 85% of cars and light-duty trucks are equipped with catalytic converters.11 By combining these estimates with global automotive sales reports13 the global demand for CeO2 for use in catalytic converted was estimated to be roughly 4900 tons per year.Fuel additives
Park, et al. projects as much as 1255 tons of CeO2 will be used as a combustion enhancement additive in diesel fuel in the EU.14 According to a global oil industry report, Europe diesel consumption accounts for 29% of world consumption.15 Assuming that the use of CeO2 as a diesel fuel additive is proportional to regional diesel use, global consumption of CeO2 as a fuel additive was estimated to be 4400 tons per year with 15–25% of CeO2 being present as nanoceria.Release during use
There are few studies that quantify the release of engineered nanomaterials during use, and even less nanoceria specific studies. One of the few studies by Park et al., indicates that 6–100% of CeO2 will be released during the use phase of diesel fuel additives.14 This has not yet been validated by other researchers. In laboratory conditions, particles filters from diesel cars removed 99.9% of Ce present in fuel additives.16 However the manuscript does not specify whether the Ce additive was in the nanoscale. Considering the applications and the likelihood that the nanomaterials are released to the environment, the following assumptions were made. For example, nanoceria in batteries is enclosed within a protective casing, which is likely to minimize release during use. If the batteries are disposed of improperly, the most likely environmental compartment would be soil, with negligible release to air, water or wastewater treatment plants (WWTP). Similar assumptions were made for metallurgical products, catalysts in FCC, polishing powders used in industry (which may be released to air or in wastewater), and other applications. Experimental studies have been conducted to measure the release of various manufactured nanoparticles from surface paints on exterior facades. Kaegi et al. measured concentrations as high as 600 μg L−1 of nano-TiO2 in runoff from newly-painted building facades,17 and estimated that as much as 30% of nano-Ag is released from surface paints within a year of paint application.18 However, no data exist on nanoceria released from paints.Based on similar information, estimated nanoceria concentrations in treated WWTP effluent discharged to waterbodies are expected to be in the range of 0.003–1.17 μg L−1.19 In biosolids, nanoceria concentrations are expected to be around 0.53–9.10 mg kg−1.19 These estimated concentrations are expected to increase as nanoceria is used more widely, and there will likely be accumulation of CeO2 in soils and sediments, further increasing exposure concentrations in these media.
Detection and characterization of nanoceria in complex media
The detection and characterization of nanoceria under conditions relevant to environmental, toxicological and biological systems remains a challenging, and frequently impossible, task. However, there is little or nothing that is ceria-specific, but applies to all nanomaterials. However, aspects of characterization are included here since it is fundamental to understanding of all nanomaterials, including nanoceria. Essential general aspects are listed below:i) In environmental systems, the specific and accurate detection and characterization of manufactured nanoceria remains essentially impossible,20,21 due to the gap between metrology and analysis and the complexity of the system (low concentrations, background Ce in many forms, presence of natural colloids and nanoparticles, spatial and temporal variability etc.). Total Ce detection is useful as it acts an upper limit of nano-ceria concentrations for risk assessment, but is not synonymous with manufactured nanoceria. The discussion below applies primarily to spiked materials, mainly in the laboratory or mesocosm.
ii) As with other nanomaterials, nanoceria should be fully characterized using suitable preparation methods and a multi-method metrological approach. In a multi-method approach, independent techniques operating on independent measuring principles provide cross-validation of measured properties. The source of the nanomaterial also needs to be fully reported, given the likely effects on properties. Fuller discussion is given elsewhere.21–24
iii) A number of properties require characterization which can be grouped as size, shape, morphology, aggregation/agglomeration, surface charge and dissolution (and related parameters). These groups, or classes, contain several individual properties. For instance, for size, an average size (mean or median) should be reported, along with some measure of spread (standard deviation, polydisperity).25
iv) Given the changes that are well known to occur upon storage or changing media,26–29 it is essential to perform appropriate measurement over temporal and spatial scales which adequately capture the dynamics of the nanomaterial system.
Although, none of the points above are ceria specific, nanoceria is capable of oxygen storage, which is size and shape dependent.30 Nanoceria is generally thought to have low solubility in water,31 although this is size and oxidation state dependent. Where dissolution and solubility are low, study is rendered simpler because dissolved ions should have little impact on toxicity. However, recent work has shown potential effects of even low level dissolution.32 Nano-ceria has two stable oxidation states (Ce(III) and Ce(IV)) under environmental conditions33 and cerium has the ability to transition readily between these two states.34–36 This redox activity gives nanoceria some of its key properties.37 However, oxidation state and morphology are usually poorly controlled or defined and spatially variable within an individual particle,38 giving rise to poorly reproducible data and uncertainties in understanding toxicity or exposure. These uncertainties, along with dynamic changes that occur in complex media, could explain the variable environmental and toxicological results that are seen in the literature for nanoceria.27,39
Table 1 shows a non-definitive selection of studies of nanoceria in a variety of different environmental, toxicological and standard complex media. These studies are examples of some of the most complete characterization in the literature, although there is still little consistency between studies and it is often not clear which nanomaterial properties require analysis because it is not well understood how each affects biological or environmental processes. Lastly, because of logistical or other constraints, characterization is often not performed as fully as necessary to interpret such processes.
| Pristine particles | Media | Measurements made | Measurands | Study purpose | Comments | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Size | Surface | Chemistry | ||||||
| *Particles brought in characterization from manufacturer stated, +particles brought in characterized in house. TEM – transmission electron microscopy, STEM – scanning transmission electron microscopy, DLS – dynamic light scattering, FFF – field flow fractionation, AFM – atomic force microscopy, FCS – fluorescence correlation spectroscopy, NTA – nanosight tracking analysis, LD – laser diffraction, TGA – thermogravimetric analysis, BET, Zeta – zeta potential, XRD – X-ray diffraction, XPS – X-ray photoelectron spectroscopy, XANES – X-ray absorption near edge structure, EELS – electron energy loss spectroscopy, EDS – energy dispersive X-ray spectroscopy, ICP-MS – inductively coupled plasma mass spectrometry, FT-IR – Fourier transform infrared spectroscopy, UV-vis – ultraviolet-visible spectroscopy. | ||||||||
| TEM | Zeta | XRD | 1/4 strength hoagland solution | ICP-MS | Ceria uptake, | Uptake and transformation of nanoceria into plants. | Characterization of the pristine NPS and final products were undertaken. Studies to try and pinpoint the transformation process were performed. | 40 |
| DLS | BET | XANES | Chemical | |||||
| FT-IR | Transformations | |||||||
| TEM/EDS | ||||||||
| DLS | ||||||||
| Zeta | ||||||||
| DLS | ZETA | XANES | Aquarium water | DLS | Size | Determine the distribution of ceria in a model aquatic environment. | No chemical transformations were measured. | 41 |
| TEM | EDS | Zeta potential | Surface charge. | |||||
| Radiotracer. | ||||||||
| ICP-MS | Algae | DLS | Size | Algae growth inhibition over time. | Size and aggregation are measured throughout exposures. Cerium attachment to algae is shown but EDX can only show co-habitation not chemical interactions. | 42 | ||
| TEM | LD | Surface potential | ||||||
| TEM | Algae morphology cerium attachment | |||||||
| Zeta | ||||||||
| E-SEM/EDS | ||||||||
| TEM | ZETA | XRD | Natural waters (seawater, lagoon, ground, river, effluent and storm). | UV-vis/DLS | Aggregation | Stability of nanoceria in complex media. Determination of aggregation and settling rates. | Techniques used are ensemble techniques which are biased towards larger NPs. | 43 + |
| TGA | BET | Artificial sea water | Sedimentation | |||||
| DLS | ||||||||
| BET | BET | Algae | NTA | Size | Effect of different NOM on the suspension of nanoceria in media | Techniques used are ensemble techniques which are biased towards larger NPs. | 44* | |
| Zeta | Surface potential | |||||||
| ICP-MS | Concentration | |||||||
Some of the most powerful techniques for the visualization of nanoparticles are transmission electron microscopy (TEM), atomic force microscopy (AFM) and scanning electron microscopy (SEM). These techniques not only provide direct visual images but can be used to quantify other properties such aggregation, dispersion, sorption, size, structure and shape of the nanoparticles,45 although the sample preparation (e.g. the drying) may alter considerably the sample. These techniques have been extensively applied to nanoceria, occasionally in complex media. Van Hoecke et al.46 and Rodea-Palomares et al.47 used TEM to visualize the interaction between the nanoceria and algal cells in order to test whether the nanoparticles are taken up or adsorbed by the algal cell wall. Zhang et al.40 used TEM to further investigate the needle like clusters on the epidermis and in the intercellular spaces of cucumber roots after treatment with nanoceria over 21 days. In some cases, TEM has been coupled with spectroscopy, for instance TEM coupled with EDS was used to determine the elemental composition of ceria clusters on both the root epidermis and in the intercellular regions of the cucumber plant.40 Merrifield et al.38 used AFM to image and quantify the size of PVP-coated nanoceria while compared them using TEM and DLS in toxicology exposure media. TEM confirmed that the larger particles (ca. 20 nm) are aggregates composed of smaller individual particles (4–5 nm), but that nanoceria properties did not measurably change in the exposure media tested. In the same study, EELS was used to quantify the oxidation states showing that the smallest and the largest samples were composed of entirely Ce(III), with only small amounts of Ce(IV) present in the largest sample. Such spectroscopy is essential to microscopy imaging in complex media and is required to unambiguously identify the nanoparticles of interest in the presence of materials with similar sizes, shapes and electron densities/tip interactions. Microscopy, although a powerful single particle method, remains challenging when attempting to provide statistically meaningful measurements. Much data reported in the literature is pictorial and non-quantitative; careful design and time consuming analysis are required to be able to determine representative parameters with confidence.
Nanoparticle tracking analysis (NTA) is another widely used characterization technique which utilises microscopy to determine size distributions and number concentration of nanoparticles in liquid samples. NTA has been infrequently used for nanoceria, for instance to determine the mean size of nanoceria in green alga and crustaceans46 and to better understand the effect of natural organic matter (NOM) on the particle-size distribution of nanoceria settling in model fresh water as a function of time.44 However, the methodology has some limitations in complex and realistic media.22
X-ray photoelectron spectroscopy (XPS) has been used in only one relevant study, to our knowledge, in this case to understand the antioxidant capacity of nanoceria to DNA. The calculation of Ce(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce(IV) ratios was performed,48 in an analogous manner to EELS, within a multi-method approach. Similarly, synchrotron-based X-ray spectroscopy has been used in several studies to assess Ce speciation. Studies using micro X-ray fluorescence (μXRF) coupled with X-ray absorption near edge structure (XANES) in natural matrices have been conducted concluding that nanoceria can undergo biotransformations within a matrix, so the modifications, the mechanism and extent of these transformations should be fully addressed.2,40,49 Scanning transmission X-ray microscopy (STXM) is an analytical microscopy which, with extended X-ray absorption fine structure (EXAFS) spectroscopy, provided 2D quantitative maps of chemical species at concentrations which are environmentally relevant.50 X-ray microscopy can in principle provide a spatial resolution down to ~30 nm while imaging the specimen in the aqueous state without the need for sample preparation.51,52 Synchrotron-based techniques provide direct structural information regarding the nanoparticles and their interaction with the environment.53–55 It is clear that X-ray spectroscopy, XPS and EELS are complementary methods for oxidation state analysis and combination may prove fruitful.
Ce(IV) ratios was performed,48 in an analogous manner to EELS, within a multi-method approach. Similarly, synchrotron-based X-ray spectroscopy has been used in several studies to assess Ce speciation. Studies using micro X-ray fluorescence (μXRF) coupled with X-ray absorption near edge structure (XANES) in natural matrices have been conducted concluding that nanoceria can undergo biotransformations within a matrix, so the modifications, the mechanism and extent of these transformations should be fully addressed.2,40,49 Scanning transmission X-ray microscopy (STXM) is an analytical microscopy which, with extended X-ray absorption fine structure (EXAFS) spectroscopy, provided 2D quantitative maps of chemical species at concentrations which are environmentally relevant.50 X-ray microscopy can in principle provide a spatial resolution down to ~30 nm while imaging the specimen in the aqueous state without the need for sample preparation.51,52 Synchrotron-based techniques provide direct structural information regarding the nanoparticles and their interaction with the environment.53–55 It is clear that X-ray spectroscopy, XPS and EELS are complementary methods for oxidation state analysis and combination may prove fruitful.
Field flow fractionation (FFF) has also been used on nano-ceria to measure the size distribution of nanoceria in synthesized samples30 as well as to understand the aggregation behavior of other nanoparticles (such as TiO2 and ZnO) in the presence and absence of humic substances.22 ICP-MS can be used as a detector for FFF, but has not been for environmental or toxicological studies of nano-ceria, to our knowledge. Preliminary studies56 have shown the feasibility of ICP-MS for nanoceria analysis in single particle mode, although its further application in real systems has yet to be demonstrated. Infrared spectroscopy (IR) has also been used40 to study biotransformations in plants by comparing the molecular environment of the sample before and after exposure hence concluding that cerium speciation changes after incubation of nanoceria in different exposure media over 21 days. Ultraviolet-visible spectroscopy (UV-vis) has been used43 to monitor the dynamic aggregation process of nanoceria in various waters with time along with DLS and TEM
Fate and transformations of nanoceria in the environment
Nanoparticles properties are altered by the water chemistry such as pH, ionic strength, nature of electrolytes or presence of NOM. One of the most important changes may be aggregation of nanoparticles: between the same nanoparticle, homoaggregation, or between nanoparticles and an environmental particle, heteroaggregation. The increase in size of the aggregates affects their transport, behavior, reactivity, uptake by organisms, and toxicity. In pure water, the stability of non-coated nanoparticles in solution depends on their surface charge. Nanoparticles brought into close contact via Brownian diffusion processes will repel each other if the charge is strong enough to overcome attractive forces. Nanoceria surface charge is dependent of the pH; nanoceria are positively charged at low pH, negatively charged at high pH and have an isoelectric point at approximately pH 8.21 The methods of synthesis and the cleanup of nanoceria have been shown to play a role in affecting the experimental point of zero net charge (PZC) for nanoceria suspensions, which range from 6.5 to 8.1.29,57,58 Differences in the reported PZC may also come from differences in the method applied to determine the PZC, the order of titration process, and sorption of anions used in the titration.21 The presence of monovalent (Na+) or divalent (Ca2+) cations controlled the stability of non-coated nanoceria in aquatic system.29 These authors measured the aggregation kinetic of nanoceria and compared to the theoretical prediction of Derjaguin–Landau–Verwey–Overbeek (DLVO). At pH 11, the experimental critical coagulation concentration (CCC) was higher for the monovalent (NaCl), than the divalent (CaCl2) salts, 80 mM and 16 mM respectively. They showed that DLVO theory could predict quite well the stability of nanoceria at this pH. However, this model fails to explain aggregation behavior as solution conditions become more environmentally relevant and non- DLVO forces may also play important roles between particles.29,59 In a water-saturated column packed with sand, water composition has also been shown to control the transport and deposition of nanoceria.60 Transport was significantly hindered at acidic conditions (pH 3) and high ionic strengths (10 mM and above), and the deposited nanoceria may not have been re-entrained by increasing the pH or lowering the ionic strength of water. At neutral and alkaline conditions (pH 6 and 9), and lower ionic strengths (below 10 mM), partial breakthrough of nanoceria was observed and particles could be partially detached and re-entrained from porous media by changing the solution chemistry.60In a more complex system, heteroaggregation, i.e. between a nanoparticle and another particle in the environment, is more likely to occur due to the greater concentration of environmental particles.27 It has been shown that in various solutions, the agglomeration and sedimentation rate of nanoceria were dependent on NOM content and ionic strength.43,44 In freshwater, with a high TOC, and low ionic strength, nanoceria dispersion were stable with a low rate of sedimentation.43 In algae medium, Quick et al.44 showed that the sedimentation decreased with increasing NOM content. The fraction of nanoceria that remained suspended in algae medium increased with increasing NOM content. The main mechanism explaining the increased stability is the adsorption of NOM to the particle surface. Recently, Li and Chen61 measured and modeled the aggregation kinetic of nanoceria in the presence of humic acid (HA), in monovalent and divalent solutions. HA has been shown to stabilize nanoceria in all KCl concentration. However at high CaCl2 concentration HA enhanced the aggregation of nanoceria probably owing to the bridging attraction between nanoceria, which is induced by the HA aggregates formed through intermolecular bridging via Ca2+ complexation. The stability and mobility of nanoceria in dilute NaCl solution was also greatly enhanced in the presence of humic acid, fulvic acid, citric acid, alginate and CMC due to electrostatic effect.62
Even in the presence of NOM in the media, homoaggregation was measured in several studies. Keller et al.43 measured >500 nm aggregates formed in sea water (low TOC and high ionic strength conditions) whereas ~300 nm aggregates were stable in suspension for a high TOC. Van Hoecke et al.63 measured nanoceria aggregation in algal test media, between 200 and 1000 nm but the extend of the agglomeration was dependent on pH, NOM, IS. Increasing pH and ionic strength enhanced aggregation, while NOM decreased mean aggregate sizes. Organic molecules that can adsorb onto the particle surfaces provide a barrier to aggregation but were not able to overcome the van der Waals forces holding small nanoparticles aggregates together.63
Available reports on the behavior of nanoceria in complex natural ecosystem are scarce. In a simulated freshwater ecosystem in laboratory, sediments were measured as the major sink of nanoceria with a recovery of 75.7% of total nanoceria after 15 days.41 In several types of soil, Cornelis et al.64 showed, by investigated the retention (Kr) of nanoceria, that nanoceria retention in soil is low. The retention of nanoceria in soils was proposed to be associated with naturally occurring colloids, such as Al, Si, and Fe oxides.64
Contrary to some other manufactured nanoparticles (such as Ag, ZnO, CuO), nanoceria have an inherently low solubility. Negligible solubility was reported; e.g. in freshwater system over 72 h,65 in moderately hard reconstituted water for 48 h2 or in algal medium for 3 days.46 Similarly, Röhder et al. measured a low dissolved Ce concentration in different algae exposure media ranging from 0.01 to 0.11% total Ce, and 0.47 to 1.13% in the presence of EDTA. However, they show that the dissolved Ce may be responsible for the observed toxicity in Chlamydomonas reinhardtii.32 The dissolution of nanoceria (20 nm) has been shown to be very low in 16 different types of soil spiked with nanoceria.64 Dissolution of nanoceria studied in an artificial soil solution was only significant at pH 4 and was less than 3.1% of total Ce.
Ce redox state is affected by environmental transformation. A reduction of Ce(IV) to Ce(III) in nanoceria has been observed during the contact between nanoceria and E. coli,49 in C. elegans,2 in cucumber plants,40 and to a lesser extent in corn66 and soybean.67 The Ce reduction may explain the toxicity induced by these nanoparticles by suggesting oxidative damage of macromolecules or generation of ROS.2 The reduction of Ce was not observed in all studies: Ce was found as Ce(IV) in the roots seedlings of cucumber, alfalfa, tomato, corn and soybean seedling exposed to 4000 mg l−1 of nanoceria.68,69 However, nanoceria interaction with HA (Suwannee River Humic Acid) and with biological media induced a decrease of Ce(III) proportion measured by EELS.70 This may indicate that nanoceria had been oxidized in the presence of humic substances and biological media.
The presence of phosphate in media can modify nanoceria properties. Zhang et al.40 identified the formation of cerium phosphate from a nanoceria suspension, KH2PO4 and a reducing substance (ascorbic acid). Singh et al.71 suggested that the interaction of nanoceria with phosphate may have caused the formation of cerium phosphate at the particle surface, in which cerium is mainly present as Ce(III). They showed that binding of phosphate anions to nanoceria leads to the complete disappearance of superoxide dismutase (SOD) activity and concomitant increase in catalase mimetic activity.71
To summarize, the few available studies showed that the properties of environmental media modifies the stability and the chemical state of nanoceria. But we lack sufficient knowledge to understand and predict the extent of transformations in the environment and the risks associated with the release of nanoceria on biological systems.
Transformation and toxicity in waste water treatment plant
Wastewater treatment plants (WWTP) are an important intermediate pathway for NP to soil and water.72 NPs may undergo transformations before being discharged with treated effluent or biosolids. Transformations of two varieties of nanoceria, pristine and citrate-functionalized, were followed in an aerobic bioreactor simulating wastewater treatment by conventional activated sludge.73 This study indicates that the majority of nanoceria (>90%) was associated with the solid phase where a reduction of the Ce(IV) NPs to Ce(III) occurred. After 5 weeks in the reactor, 44 ± 4% reduction was observed for the pristine nanoceria and 31 ± 3% for the citrate-functionalized nanoceria, illustrating surface functionality dependence. The authors suggest that the likely Ce(III) phase generated would be Ce2S3. At maximum, 10% of the CeO2 will remain in the effluent and be discharged as CeO2, a Ce(IV) phase.73Nanoceria can also be toxic and/or provoke changes in the microbial communities involved in wastewater treatment therefore affecting the performance of the wastewater treatment process. Garcia et al.74 evaluated the effect of nanoceria on the activity of the most important microbial communities of a WWTP: ordinary heterotrophic organisms, ammonia oxidizing bacteria, and thermophilic and mesophilic anaerobic bacteria. A great inhibition in biogas production (nearly 100% at 640 mg l−1) and a strong inhibitory action of other biomasses were caused by nanoceria coated with hexamethylenetetramine (HMT). On the contrary, the study of Limbach et al., 2008,75 showed that an ordinary heterotrophic organisms biomass from a municipal WWTP in Switzerland was not affected by 1000 mg l−1 of non-coated nanoceria. This discrepancy could be related to differences in the characteristics of the bacterial community and the nanoparticles properties (such as coating) used in both studies.
Bioavailability and toxicity in terrestrial organisms
The literature assessing the fate and effects of nanoceria on terrestrial plants is not extensive, and far less work has been done with other terrestrial organisms such as soil invertebrates. The existing work will be reviewed in terms of three separate parameters or endpoints; toxicity, translocation, and transformation. Papers reporting findings under hydroponic exposure in plants will be covered first, followed by plant studies done under soil conditions.Hydroponic exposures in plants
Ma et al.76 were among the first to investigate potential nanoceria phytotoxicity. The authors reported that the seed germination of 7 different species (radish, canola, tomato, wheat, lettuce, cabbage, cucumber) was completely unaffected by 2000 mg l−1 of nanoceria suspension. Similarly, subsequent root elongation tests with these plant species was largely unaffected by nanoceria; only lettuce root growth was suppressed by 34% at this concentration. Lopez-Moreno et al.69 also showed that nanoceria at 2000–4000 mg l−1 had no overt toxicity on soybean, although particles were detected within root tissue by synchrotron X-ray absorption spectroscopy (XAS). The authors did report genotoxicity as measured by random amplified polymorphic DNA assay; however, the precise nature of the molecular effects is not known. In a follow up study, the same group reported the effects of 0–4000 mg l−1 nanoceria exposure on alfalfa, corn, cucumber and lettuce growth.68 The germination and root elongation of several of the species were enhanced at lower concentrations but were significantly inhibited (20–30%) at 2000 and 4000 mg l−1. Interestingly, shoot elongation was enhanced in nearly all cases. ICP-OES was used to confirm ceria presence within the seedlings, although root and shoot tissues appear to not have been separated prior to analysis. After dilute acid rinsing, XAS confirmed that the oxidation state was unaltered in the root tissues of these four plant species. Zhang et al.40 reported a concentration-dependent sorption of nanoceria to cucumber roots in a 14 day hydroponic exposure. Most of the adsorbed nanoceria were only loosely bound to the root surface and more than 85% of the nanoparticles could be washed off with deionized water. Translocation of the particles to shoot tissue was minimal but measurable, and interestingly, 7 nm size particles were found at significantly higher amounts than 25 nm nanoceria. In a follow up 21 day hydroponic study, exposure to 2000 mg l−1 bulk CeO2 and nanoceria resulted in no toxicity to cucumber.40 Although minimal root to shoot translocation was noted, soft X-ray scanning transmission microscopy (STXM) and XANES were used to show measurable biotransformation to CePO4 in roots and cerium carboxylates in shoot tissue. Notably, the authors hypothesize that root exudate mediated dissolution of nanoparticles precedes ion uptake, subsequently followed by in planta reduction to nanoceria and/or biotransformed products. Similarly, Schwabe et al.77 observed plant exudate induced changes in solution pH, nanoceria agglomeration and particle size. However, they reported no phytotoxicity to pumpkin and wheat after 8 day exposure at 100 mg l−1 nanoceria; no cerium was detected in wheat shoots but minimal translocation in pumpkin yielded tissue levels of 15 mg kg−1 (60–450 times less than root content). Interestingly, the association of cerium with the roots of both plant species was reduced in the presence of NOM. Rice exposed to nanoceria at 63–500 mg l−1 experienced no visible signs of phytotoxicity, although altered lipid peroxidation, electrolyte leakage, and other enzyme activity suggested possible oxidative stress.78 Wang et al.79 noted that tomato seeds harvested from plants previously exposed to nanoceria yielded a “second generation” of individuals that produced less biomass, transpired less water, possessed differential root morphology, and exhibited overall higher levels of reactive oxygen species that did seeds from unexposed plants.Soil exposures in plants
Birbaum et al.80 were the first to report on nanoceria exposure to terrestrial plants (corn) under soil conditions. The authors reported that after 14 day exposure with the nanoceria in the irrigation water (50 ml of 10 μg ml per day), no ceria was found in the leaves or sap of corn plants. However, no mention was made of toxicity or of root ceria content. Interestingly, the authors included an aerial exposure on leaves and although nanoceria could not be washed from the tissue, the particles were not internalized or transferred to new growth. Similarly, Wang et al.81 grew tomato in the presence of nanoceria-amended (0.1–10 mg l−1) irrigation water and reported either no impact or slight enhancements in plant growth and yield. The authors did observe ceria in the shoots, including edible tissues, which suggests translocation, but the mechanism and form of element transfer is unknown. Zhao et al.66 observed that after one month of growth in soil, corn roots accumulated significantly greater quantities of alginate coated nanoceria than uncoated particles but no mention was made of toxicity. These authors also noticed that soils with high organic matter generally enhanced the association of nanoceria with roots but reduced the translocation to shoots, regardless of the surface properties of nanoceria. However, the effect of soil organic matter was more significant on uncoated nanoceria than alginate coated nanoceria. Although translocation in general was low, μXRF did confirm the presence of nanoparticles within vascular tissues, as well as in epidermal and cortex cell walls, suggesting an apoplastic uptake pathway. A separate study with cucumber showed that up to 800 mg nanoceria/kg soil did not demonstrate any adverse effect on a suite of plant physiological indictors such as the net photosynthesis rate, leaf stomatal conductance, but nanoceria at this concentration did lower the yield of cucumber by 31.6%. The authors also observed nanoceria in the vasculature of leaf veins, providing further evidence that nanoceria may be transported from roots to shoots with water through vascular tissues.82Priester et al.83 noted that soybean exposed to 100–1000 mg kg−1 nanoceria had root ceria content of up to 200 mg kg−1 but that translocation was minimal. Plant growth and yield were modestly reduced but importantly, nitrogen fixation was almost entirely eliminated. Nodule content of ceria approached 11 mg kg−1 in some instances and electron microscopy confirmed the complete absence of symbiotic bacteria. Similarly, Hernandez-Viezcas et al.67 used synchrotron μXRF and μXANES to observe nanoceria within soybean root nodules and pods, although up to 20% had been transformed from Ce(IV) to Ce(III). However, the inhibition of bacterial nitrogen fixation did not necessarily result in nitrogen shortage for the plants; soybeans exposed to high doses of nanoceria actually grew better those exposed to low doses of nanoceria in the Priester study,83 suggesting that the plants successfully used an alternative source of nitrogen for growth. In a related study, Bandyopadhyaya et al.84 observed that nanoceria at 31–125 mg l−1 significantly inhibited the growth of Sinorhizobium meliloti, the primary symbiotic nitrogen fixing bacteria of alfalfa. The authors reported that the negative impact of nanoceria on nitrogen fixing bacteria resulted from nanoparticle adsorption on the extracellular surface and the subsequent alteration of certain surface protein structures. These changes could potentially affect colonization of symbiotic bacteria on root surfaces and therefore negatively impact plant nitrogen cycling. Notably, this study was conducted in cell culture and more investigation in soil-based systems will be needed. In a final soil study, Morales et al.85 noted that nanoceria at concentrations up to 500 mg kg−1 had no impact on cilantro shoot biomass and in some instances, increased root growth. However, the authors did report FTIR-detected changes in carbohydrate chemistry, which raises the potential for altered nutritional content in edible tissues. A recent study with rice confirmed that exposure of 500 mg nanoceria/kg soil throughout the life cycle of rice substantially altered the nutritional values of rice grains.86 For examples, the authors reported that nanoceria generally reduced the sulfur and iron content of rice grains and the extent of reduction depended upon the variety of rice types. The authors also reported the alteration of macromolecule contents (e.g. fatty acid or proteins) in rice grains by nanoceria exposure, providing the first direct evidence on the mitigation of nutritional values of agricultural grains by nanoceria.
Soil microbial toxicity
Due to their small sizes, nanoparticles can move through the macro and microporosity of the soil and be detrimental for soil microbial communities.87 Currently very little information is available on how nanoparticles affect the soil microbial community. They may have an impact on soil microorganisms via a direct effect (toxicity), changes in the bioavailability of toxicants or nutrients, indirect effects resulting from their interaction with natural organic compounds and interaction with toxic organic compounds which would amplify or alleviate their toxicity.87 In two soils contaminated with nanoceria at 100 mg Ce/kg of dry soil, no significant effect on both microbial biomass C and N were observed after 60 days.88 However nanoceria decreased microbial C/N ratio and increased the metabolic quotient (qCO2), probably due to microbial stress and changes in the composition of microbial communities inhabiting soil. They found that nanoceria were associated to small aggregates rich in both labile organic C, microbial biomass and clays, suggesting that nanoparticles can interact with most of microbial communities inhabiting soil.Terrestrial invertebrates
So far, the only two terrestrial organism to have been used to assess nanoceria soil toxicity are the earthworm Eisenia fetidia and the nematode Caenorhabditis elegans. Lahive et al.89 compared the toxicity of cerium salt (ammonium cerium nitrate) and three different nanoceria to E. fetida in exposed in standard Lufa 2.2 soil. While median lethal concentration (LC50) and effective concentration (EC50) values of 317.8 and 294.6 mg Ce kg−1 were found for survival (at day 28) and reproduction (at day 56), respectively, neither of these endpoints were affected by even the highest Cerium particle concentrations of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg Ce kg−1. The three nanoceria used varied in size ranges (5–80 nm), with one larger particle (300 nm) and the cerium salt used as controls. However, there was a dose-dependent increase in cerium in the organisms at all exposure concentrations, and for all material types. With earthworms exposed to CeO2 particles interestingly having higher concentrations of total cerium compared to those exposed to ionic cerium, without exhibiting the same toxic effect. Additionally, histological observations in earthworms exposed to the particulate forms of CeO2 showed cuticle loss from the body wall and some loss of gut epithelium integrity. The data overall suggesting that while nanoceria do not affect survival or reproduction in E. fetida over the relatively short standard test period, then there were histological changes that could indicate possible deleterious effects over longer-term exposures. In contrast to E. fetida, then C. elegans is most often exposed in aquatic media rather than soil and so it is also often considered an aquatic toxicity testing organism.90 Roh et al.91 assessed the interaction between nanoceria and C. elegans and encountered a marked size-dependent effect on the fertility and survival of C. elegans. Zhang et al.92 evaluated the in vivo effects of a positively charged coated nanoceria (8.5 nm) on C. elegans at low concentrations (from 0.172 to 17.2 μg l−1). The results indicated that nanoceria induced ROS accumulation and oxidative damage in C. elegans, and finally lead to a significant decreased lifespan even at the exposure level of 0.172 μg l−1. Collin et al.2 showed that the toxicity and bioaccumulation of coated nanoceria in C. elegans were dependent on the surface charge of the nanoceria. The positively charged nanoceria were significantly more toxic to C. elegans and bioaccumulated to a greater extent than the neutral and negatively charged nanoceria. They measured a LC50 of 15.5 mg l−1 for L1 stage C. elegans exposed during 24 h to the positively charged coated nanoceria.
000 mg Ce kg−1. The three nanoceria used varied in size ranges (5–80 nm), with one larger particle (300 nm) and the cerium salt used as controls. However, there was a dose-dependent increase in cerium in the organisms at all exposure concentrations, and for all material types. With earthworms exposed to CeO2 particles interestingly having higher concentrations of total cerium compared to those exposed to ionic cerium, without exhibiting the same toxic effect. Additionally, histological observations in earthworms exposed to the particulate forms of CeO2 showed cuticle loss from the body wall and some loss of gut epithelium integrity. The data overall suggesting that while nanoceria do not affect survival or reproduction in E. fetida over the relatively short standard test period, then there were histological changes that could indicate possible deleterious effects over longer-term exposures. In contrast to E. fetida, then C. elegans is most often exposed in aquatic media rather than soil and so it is also often considered an aquatic toxicity testing organism.90 Roh et al.91 assessed the interaction between nanoceria and C. elegans and encountered a marked size-dependent effect on the fertility and survival of C. elegans. Zhang et al.92 evaluated the in vivo effects of a positively charged coated nanoceria (8.5 nm) on C. elegans at low concentrations (from 0.172 to 17.2 μg l−1). The results indicated that nanoceria induced ROS accumulation and oxidative damage in C. elegans, and finally lead to a significant decreased lifespan even at the exposure level of 0.172 μg l−1. Collin et al.2 showed that the toxicity and bioaccumulation of coated nanoceria in C. elegans were dependent on the surface charge of the nanoceria. The positively charged nanoceria were significantly more toxic to C. elegans and bioaccumulated to a greater extent than the neutral and negatively charged nanoceria. They measured a LC50 of 15.5 mg l−1 for L1 stage C. elegans exposed during 24 h to the positively charged coated nanoceria.
Influence of NOM on nanoceria bioavailability and toxicity on terrestrial invertebrate
The presence of NOM has been shown to influence the bioavailability and toxicity of other nanoparticles.93,94 The presence of humic acid (HA) in the exposure media had been shown to influence Ce bioaccumulation in C. elegans exposed to positively charged coated nanoceria.2 Ce bioaccumulation was influenced by the ratio between HA and nanoceria. For a relevant scenario, i.e. when the concentration of HA was higher than the nanoceria concentration, Ce bioaccumulation decreased. Interestingly, for all tested concentration, the presence of HA in the exposure media significantly decreased the toxicity of nanoceria to C. elegans. The decrease of toxicity was explained by the profound modifications induced by the adsorption of humic acid such as a change of the ZP or the formation of μ size aggregates, which were too large to be absorbed by C. elegans.Bioavailability and toxicity in aquatic organisms
This section presents few studies carried out on aquatic microorganisms and macroorganisms in order to highlight some of the bioavailability and ecotoxicity mechanisms of nanoceria. Table S1† summarizes the published toxicity data in aquatic and terrestrial organisms along with the nanoceria characterization data.First of all, the aggregation state appears to be an important parameter to consider when dealing with exposure of aquatic organisms to nanoceria due to their low solubility. On a large scale, aggregation/sedimentation of nanoceria in aquatic environments will leave a small portion of the total mass of nanoceria available for direct uptake by planktonic organisms (micro- or macro-), while the majority will be in contact with benthic organisms (micro- or macro-). In this case, sediments should be regarded as a sink for nanoceria discharged to the aquatic environment. Not only can the exposure pathway be different upon aggregation, but the mechanisms of internalization can also vary.
Like the aggregation, the chemical stability of nanoceria can change in environmental biological pH/Eh conditions. Metals such as Ce exhibit various possible redox states (Ce(III), Ce(IV)) for which stability is a function of Eh and pH values. Intracellular Eh is controlled by metabolic processes as the oxidative phosphorylation (respiration) in mitochondria. It is based on a series of redox reactions at near circumneutral pH for which potentials are in a – 0.32 (NAD+/NADH) to 0.29 V (cytochromes). Extracellular Eh is generally controlled by thiol/disulfide redox systems (mainly GSH/GSSH and Cys/CySS) for which Eh vary in a – 0.140/–0.08 V range. In such intra- and extra-cellular Eh conditions, Ce can be redox unstable which lead to electron exchange between nanoparticle surface and surrounding media. This could be the starting point of disequilibrium of the redox balance and then to oxidative stress toward micro- and macro-organisms.
Regarding microorganisms, up to now, no undisputable evidence of nanoceria uptake by cells has been obtained. The nanoceria were either found in direct contact with the bacterial wall47,49 or trapped in the exopolysaccharides (EPS) layer surrounding the microorganisms.95 For instance, studies have shown that Escherichia coli exposed to nanoceria in a simplified exposure media were covered by a thin and regular monolayer of nanoceria surrounding the cell wall. But for Synechocystis, nanoparticles could not form a shell at the cell surface because they were adsorbed onto the protecting layer of EPS bound to cell membranes. These nanoparticles-trapping EPS likely explains the higher level of nanoceria adsorption onto Synechocystis as compared to E. coli.
Several studies have been conducted investigating toxicity in microorganisms. The toxicity of nanoceria was found to be strain- and size-dependent for E. coli and B. subtilis, whereas they did not affect S. oneidensis growth and survival.96 EC50 was near 5 mg l−1 for E. coli49 and ranged from 0.27 to 67.5 mg l−1 for Anabaena in pure water.47 Chronic toxicity to algae P. subcapitata with 10% effect concentrations (EC10) between 2.6 and 5.4 mg l−1 was observed. Van Hoecke et al.63 observed that the presence of NOM decreased the toxicity of nanoceria to P. subcapitata. They assumed that the adsorption of NOM to the nanoceria surface prevented the particle from directly interacting with algal cells thereby decreasing the bioavailability of the particles. Under exposure to nanoceria, N. europaea cells show larger sedimentation coefficient than the control.97 The toxicity of nanoceria was either exerted by direct contact with cells,47,49,95 membrane damage,97 cell disruption,47 release of free Ce(III).95 No oxidative stress response was detected with E. coli or B. subtilis, but nanoceria and CeCl3 alter the electron flow, and the respiration of bacteria.96 Pelettier et al.96 also observed the disturbance of genes involved in sulfur metabolism, and an increase of the levels of cytochrome terminal oxidase (cydAB) transcripts known to be induced by iron limitation. Rodger et al.65 also monitored the growth inhibition of P. subcapitata and reported EC50 value of 10.3 mg l−1 of a 10- to 20 nm nanoceria. This inhibitory mode of action was mediated by a cell-particle interaction causing membrane damage and likely photochemically induced. Even if free Ce(III) is toxic, release of Ce(III) from the nanoceria did not explain by itself the toxicity observed in these studies (e.g.ref. 2, 46 and 47). However, the reduction of the Ce(IV) into Ce(III) at the surface of the nanoceria correlates with the toxicity. Using XANES at Ce L3-edge, Thill et al.49,95 and Auffan et al.98 showed that the cytotoxicity/genotoxicity of nanoceria could be related to the reduction of surface Ce(IV) atoms to Ce(III). But, further research is needed to find out whether the oxidative activity of ceria could be responsible.
Regarding inverterbrates, one of the most favorable routes for nanoceria uptake by aquatic organisms is ingestion. For instance, ingestion via food chain was the main route of nanoceria uptake by the microcrustaceans Daphnia pulex.99 The adsorption of nanoceria on algae (Chlorella pseudomonas) during the exposure to sub-lethal doses of nanoceria enhanced by a factor of 3 the dry weight concentration of Ce on the whole D. pulex. Nanoparticles were localized in the gut content, in direct contact with the peritrophic membrane,99 and on the cuticle.99,100 Interestingly, the depuration (24 h) was not efficient to remove the nanoceria from the organisms. From 40% to 100% (depending on the feeding regime during exposure) of the nanoceria taken up by D. pulex was not release after the depuration process. However, the authors demonstrated that the shedding of the chitinous exoskeleton was the crucial mechanism governing the released of nanoceria regardless of the feeding regime during exposure.99 Moreover, interspecific toxic effects of nanoceria toward daphnia were explained by morphological differences such as the presence of reliefs on the cuticle and a longer distal spine in D. similis acting as traps for the nanoceria aggregates. Acute ecotoxicity testings showed that D. similis was 350 times more sensitive to nanoceria than D. pulex with 48 h EC50 for D. similis about of 0.3 mg l−1.100 In addition, D. similis has a mean swimming velocity twice as fast as D. pulex and thus initially collide with twice more nanoceria aggregates. The effect of the exposure methods, direct and through sorption to phytoplankton was tested on the mussel Mytilus galloprovincialis.101 Ce uptake was enhanced by the ingestion via the phytoplankton in the first 5 days of exposure but was similar to a direct exposure after 2 weeks. The authors showed that with increasing nanoceria concentration, mussels increased their clearance rates as well as the pseudofeces production in order to prevent the ingestion of nanoceria. Due to these responses Ce concentrations in the tissue and pseudofeces remain constant with increasing exposure concentrations.
Studies on nanoceria toxicity and uptake on fish are really scarce. Nanoceria has been shown to be accumulated in the liver on the zebrafish Danio rerio exposed to 500 μg l−1 during 14 days, however no significant uptake were measured for a higher concentration (5000 μg l−1).102 No cerium was detected in gill, brain and skin. Nanoceria was found to be non toxic for Danio rerio embryos exposed up to 200 mg l−1 nanoceria during 72 h.46
Table S1† illustrates the diversity in the measured effect concentration of nanoceria. Even for a given species, the results varied widely between studies. For example, Lee et al. showed significant mortality of D. magna after 96 h of exposure to 1 mg l−1 of 15 and 30 nm nanoceria103 while no toxicity was measure in D. magma after the same duration at 10 mg l−1 (ref. 104) or a 48 h exposure at 1000 mg l−1 nanoceria.46 Van Hoecke et al. exposed D. magna to higher concentrations of 14, 20, and 29 nm nanoceria for 21 days, and found an LC50 of approximately 40 mg l−1 for the two smaller particles and 71 mg l−1 for the 29 nm particles.46 When combining all aquatic toxicity data, including C. elegans (Table S1†), no trends were observed between the nanoparticle size and the toxicity. We observed one extreme value, which is a report of reduction in life span of C. elegans at a concentration of 0.172 μg L−1.92 Some have suggested that the toxicity at low concentration can be explained by differences in the aggregation state as a function of concentration. NPs may be less aggregated at lower concentration.105 However, the nanoceria used in this study were positively charged, coated with hexamethyleneteramine (HMT). It is possible that this coating rendered nanoceria much more toxic. Another Fig. 1 depicts the median of the lowest observed effect concentration (LOEC) and the EC10 or LC10 toward different species. This figure illustrates the high variability of the observed LOEC/EC10 between studies for a same organism (e.g. Daphnia magna). Based on the LOEC/EC10, the more sensitive species is the cyanobacterium Anabaena, while the least sensitive is Daphnia magna. No toxicity was observed up to 5000 mg Ce/L for the zebrafish Danio rerio and Thamnocephalus platyurusFig. 1.
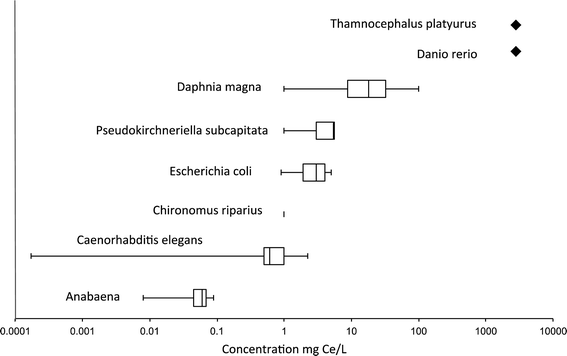 | ||
| Fig. 1 Boxplots of published aquatic toxicity data (LOEC and LC10/EC10). The diamonds represents the HONEC (highest observed no effect concentration). Each box represents the lower and the upper quartiles, the middle bar represents the median, and the end of the whiskers indicates the minimum and maximum values. Available LOEC or LC10/EC10 of all the studies reported in Table S1† were included. Only one value is available for Chironomus riparius. | ||
It is noteworthy that exposure models predict concentrations significantly lower than those for which ecotoxicity investigations have encountered toxic effects. Therefore, nanoceria might not have any impact at environmental concentrations, despite the fact that some results are more worrying. Moreover, most of the nano-ecotoxicology performed on aquatic organisms used a single species or a short trophic links and do not take into account important parameters such as the colloidal destabilization (hetero- vs. homo-aggregation) of the nanoceria, their interactions with (in)organic molecules/particles naturally occurring or bio-excreted, or the flux between compartments of the ecosystems (aqueous phase, sediments, biota). To work under more realistic scenario of exposure, few nano-ecotoxicological studies are now performed in aquatic mesocosms with low doses of nanoceria, chronic and long-term exposure. Such studies will allow obtaining reliable exposure and impact data and their integration into an environmental risk assessment model that is currently missing.
Potential acute risks to the environment for selected environmental release pathways: United Kingdom as a case study
Although the data on environmental effects are far from complete, it is useful to consider case studies in order to gain knowledge about key data gaps and to give a first impression of relative risks based on current knowledge. While this case study is useful to point out areas where research is most needed, it is critical to point out the limitations of this case study. First, nanoceria have not yet been detected or measured in environmental media, and the actual environmental concentrations are not known. Second, very little is known about the fate and transport of nanoceria in the environment. Third, the toxicity data base is still very limited. Only a select few ecological receptor species have been tested to date and few if any sub-chronic or chronic exposures have been performed in longer lived organisms or in environmentally realistic exposure scenarios. The following case study characterizes the likely exposure concentrations and compares them to toxicity values for soil and water based on emissions due to combustion of fuels containing nanoceria additives and for discharge of chemical mechanical planarization media into sanitary sewers.Acute exposures in aquatic environments
Based on Table S1,† with the exception of HMT coated nanoceria, which do not apply to this case study and for which coating controls are lacking, the lowest EC10 value measured so far is 8000 ng l−1 for luminescence inhibition in cyanobacteria.47 Previous estimates have been made for nanoceria used as a fuel catalyst and arriving in soil and water following atmospheric discharge106 in the UK based on known market size for this product.Clearly there is a wide disparity between concentrations likely to occur due to fuel catalyst combustion106 and the lowest toxicity values observed so far (Table 2). However, there remains concern that nanoceria may enter water courses through its uses in specialized industrial polishing or chemical/mechanical planarization.107 Without specialized local knowledge on where these industrial concerns are located, the quantities of nanoceria used, that are disposed of from the premises, and the capacity of the associated sewage treatment plant, the local receiving water concentrations cannot be predicted. Unfortunately, knowing global or national consumption of nanoceria in the polishing industry would not allow us to predict water concentrations. This is because the use of the product would not be evenly geographically spaced, or linked directly to human population density. However, it is possible to ask: what discharge would be needed to exceed the 8000 ng L−1 toxicity threshold for aquatic exposures?
| Loss route | Water concentration (ng l−1) | Proximity to 8000 ng l−1 effect level |
|---|---|---|
| General aerial deposition direct to water courses | 0.003–0.023 | 5-Order of magnitude difference |
| Loss from landmass to water courses assuming 1% entrainment in runoff | 0.001–0.008 | 6-Order of magnitude difference |
| Loss from landmass to water courses assuming loss through soil erosion | 0.0005–0.004 | 6-Order of magnitude difference |
| Direct loss to adjacent ditch from contaminated road surface | 40–293 | 27-Fold difference |
The dilution factor for sewage effluent recommended by EU risk assessment is 10. So effluent would need to contain 80 μg L−1 nanoceria. However, it is estimated that on entering an WWTP 95% of the nanoceria would enter sludge and only 5% pass through into the effluent.75 In that case the influent concentration would need to be 1.6 mg l−1 nanoceria. WWTPs are designed around population equivalents (PE) which tend to be around 160–200 L per PE per day in the UK108,109 so a PE unit would need to discharge 256–320 mg Ce per day to receiving waters. Given the current uses of nanoceria, this only seems likely to occur if a large industrial facility is directly discharging wastewater containing high concentrations of nanoceria directly into a sanitary sewer. Note that a population equivalent is a unit describing a given biodegradable load as measured by its biological oxygen demand.
Acute soil exposures
Growth inhibition in the nematode C. elegans has been noted down to a level of 2.5 mg l−1 which could be considered as a pore water concentration equivalent to 2.5 mg kg−1. As a conservative assumption this may be used as a lower effect level for soil.110Previous estimates have been made for nanoceria used as a fuel catalyst and arriving in soil following atmospheric discharge106 in the UK based on known market size for this product. The highest soil concentration assumed all the particles would be deposited within a band of 20 m distant from UK roads and that over 7 years (since the application started in the UK) would be 0.016 mg kg−1. This is over 2 orders of magnitude below the effect level of concern. There is evidence to suggest that when nanoceria particles enter the soil they will not remain permanently fixed but form new charged heterocoagulated colloids giving them some mobility in the pore water.64,111 Thus, assuming a year on year accumulation in topsoil could be seen as an overly conservative assumption.
The other scenario to consider is an industrial facility which discharges nanoceria particles to the sewer. This may occur where factories use nanoceria particles for polishing. What level of nanoceria particles in sewage sludge would be needed to exceed the 2.5 mg kg−1 threshold in soil given that the majority of these particles are likely to partition to sludge?75 Good agricultural practice advises limiting total N applications to 250 kg ha−1 per year N, so as sludge is considered to contain a minimum of 3% N by dry weight (DW)112 up to 8.3 tonnes DW ha−1 sludge may be applied. This is the same as applying 830 g DW sludge m−2 of soil. In the UK the mean soil bulk density is considered to be 1.28 g cm−3.113 It is reasonable to assume that sewage sludge applied to land would be incorporated into the top 20 cm of soil. Thus, a 1 m2 of block of soil that is 20 cm deep would contain 256 kg of soil in the UK.
Thus, for the soil to receive an exposure of 2.5 mg kg−1 nanoceria the 1 m2 block of soil would need to receive 640 mg nanoceria in the sewage sludge application of 830 g DW sludge m−2 of soil. This would require a presence of 771 mg kg−1 nanoceria in sludge DW, or almost 1 g kg−1. Whilst this appears to be technically possible, to put this in some context back in 1997 the median metal content of UK sewage sludge was 792 mg kg−1 Zn, 568 mg kg−1 Cu, 221 mg kg−1 Pb, 157 mg kg−1 Cr, 3 mg kg−1 Cd, and 2 mg kg−1 Hg.114 So to reach a level of 771 mg kg−1 from a single application nanoceria would make Ce almost the most abundant metal in sewage sludge. Given the toxicities of the other metals, it seems that nanoceria would not be the most hazardous element of sewage sludge, even if it did reach that concentration. Generally speaking, so far the application of sludge or compost to soils, even with the relatively high metal content, appears to generally stimulate soil microbial processes.115
Conclusions and recommendations for further research
We have comprehensively reviewed what is known for nanoceria about the environmental releases, methods for detection and characterization, fate and transport, toxicity and likelihood of toxicity in soil and water from acute exposures. Initial estimates of releases suggest that the majority of nanoceria will ultimately end up in landfills, with lesser amounts emitted to air, soil and water in that order. Once nanoceria enters the environment, it has been shown that NOM will have a major impact on their fate, transport and toxicity. As with other nanomaterials, aggregation is a key consideration and this has been shown to be influenced by water chemistry and interactions with natural coatings such as NOM. An important feature of nanoceria with respect to its behavior and toxicity is its valence state. There are several techniques that can characterize this property in environmental and biological media, such as XAS, but most require relatively high concentrations. While we didn't identify studies that detected nanoceria in natural environments or environmental media, a suite of techniques have been used to detect and characterize them in complex toxicity testing media and in controlled laboratory studies. Thus, a major data gap and area for future research is the prediction and measurement of actual nanoceria concentrations in the environment, either from point sources or non-point sources.As a whole nanoceria appears to exhibit similar aquatic toxicity values other commonly studied manufactured nanomaterials. For example, a recent review found that species average LC50 values for Ag nanoparticles ranged from 0.01 mg L−1 to 40 mg L−1 while species mean LC50 values for ZnO ranged from 0.1–500 mg L−1.116 The range of EC50 values reported for Ce are similar to those for ZnO. Although reported toxicity data here uses LC10 and LOEC values, the range of species means 0.05–25.9 mg L−1 and many of the reported LC50 values are within the range of 0.1–100 mg L−1, suggesting similar acute toxicity to ZnO NPs in aquatic exposures. This is of course based on the available data, which are predominantly on the toxicity of nanoceria to aquatic organisms, with sediment and terrestrial organism data severely lacking. For example, few if any studies have investigated toxicity in sediment dwelling organisms, which are likely to be exposed to nanoceria in the aquatic environment due to aggregation, settling and accumulation of nanoceria in sediment. Given the persistence of nanoceria, chronic studies are lacking as we are aware of only the C. elegans study.92 Equally important, very few species (aquatic and terrestrial) from few taxonomic groups have been tested. Large taxonomic groups such as insects and gastropods have not been tested and only one non-mammalian vertebrate species has been tested (zebrafish). Another difficulty is that most of the studies were performed with different nanoparticles, doses, duration, organisms, exposure media, and their results are not directly comparable. Perhaps due to these differences, there are no apparent patterns to suggest that, as a whole, particle size has a major impact on toxicity. A problem in conducting realistic toxicity studies is the likely transformation of the free particles into homo or heteroaggregates or even organic complexes in the real environment. There have been few studies that investigated the impact of size across a wide range of systematically varied particle sizes within a single study. Such studies are needed to definitively establish weather size is important. On the other hand coating may be an important variable given the extreme sensitivity seen with HMT coated particles in C. elegans.92 Coating was demonstrated to be a major determinant of toxicity in a more well controlled study that systematically varied coating properties and used coating controls.2
Of all of the taxonomic groups, toxicity is most well studied in vascular terrestrial plants. Overt phytoxicity of nanoceria seems minimal and, while root to shoot translocation of these particles is often measurable it is generally quite low. In summary, although the literature on nanoceria impacts on terrestrial plants is not extensive, it is clear that overt phytotoxicity is minimal, even at excessive exposure concentrations. The data do suggest accumulation of nanoceria within plant tissues, although the precise form of the element that crosses into the plant and the mechanism driving that process remains unknown. The potential transgenerational effects noted in the literature,79 as well as the complete lack of information on trophic transfer, are areas of concern. In addition, studies investigating environmentally relevant concentrations, potentially secondary effects from nanoceria exposure, including impacts on symbiotic microorganisms or on edible tissue nutritional quality, certainly warrant further investigation.
As a whole, the aquatic and terrestrial toxicity testing data for animals and microorganisms spans multiple orders of magnitude for acute toxicity values (EC10 and LOECs). This large variation can be exhibited within a single species exposed to similar nanoceria. For example, toxicity values for D. magna range from around 1–100 mg l−1 for fairly similar particles. Based on the overall toxicity database, it appears that C. elegans is the most sensitive animal and Anabaena is the most sensitive microorganism tested to date, although an important caveat is that the same endpoints were not compared across all species and that exposure systems varied. Interestingly no toxicity was observed in the fish species that has been tested (D. rerio) even at extremely high exposure concentrations (Fig. 1). Unfortunately, only two fish studies have been reported in the literature. There is a complete lack of toxicity testing data for sediment dwelling organisms, and extremely limited data for soil invertebrates. As a whole the data suggest that acute toxicity is possible at low μg L−1 concentrations in the water column. Data are lacking on soils and sediments, but toxicity values are likely to be far lower.
One study indicated toxicity at lower concentrations than these values (at 172 ng L−1) when 8 nm nanoceria were coated with HMT. Since no coating controls were used, it is critical that the influence of this coating and other similar positively charged coatings be studied using a similar endpoint (lifespan) and suitable controls. The use and disposal of any nanoceria containing products with this coating should also be evaluated. It is not clear whether the chronic nature of this exposure or the influence of the coating on uptake and toxicity explain why this toxicity threshold is so low. Although this coating may not persist on the particles in the environment, what is clear is that the effects of chronic dosing and the effects of coating are critical data gaps that should be evaluated. Also completely lacking are more environmentally realistic exposure scenarios, such as ones using natural waters and soils and also multispecies microcosm or mesocosm studies, although such studies are underway. These studies will bring the importance of environmental transformations and indirect ecological impacts into light. It is possible that community or ecosystem level impacts may be more sensitive than individual level effects. Also more chronic and food chain transfer studies should be encouraged to deal with the possible long term effects from, or accumulations of, the likely persistent nanoceria entities.
The current available data do not suggest an immediate risk from acute exposures to nanoceria from use as a fuel additive or mechanical/chemical polishing or planarization. However, the data gaps we have discussed should be addressed before a comprehensive ecological risk assessment can be performed for ceria for chronic exposures or for other exposure pathways. This review lays the foundation for such assessments and clearly identifies the areas where research is most critically needed.
Acknowledgements
This material is based upon work supported by U.S. Environmental Protection Agency (EPA) Science to Achieve Results (STAR) grant R834857, The National Science Foundation (NSF) and Agency (EPA) under grants DBI-1266252 and DBI-0830117, the SmartState Center for Environmental Nanoscience, the Natural Environment Research Council (NE/H013148/1; NE/H008764/1; NE/I008314/1), USDA AFRI grant 2011-67006-30181, the CNRS funding for GDRi-iCEINT, and the French ANR funding for ANR-10-NANO-0006/MESONNET, and ANR-3-CESA-0014/NANOSALT. The NERC – CEH portion of work was conducted in the context of NanoFATE, Collaborative Project CP-FP 247739 (2010–2014) under the European Commission (FP7-NMP-ENV-2009, Theme 4). The Authors gratefully acknowledge R. Yokel for his helpful comments on earlier versions of the manuscript. This perspective is a product of a workshop on nanoceria held November 2, 2013 at Fess Parker's Doubletree Resort, Santa Barbara, California which was made possible by financial support from the Sustainable Nanotechnology Organization; NSF grant CBET-1343638 to UCSB; and the Tracy Farmer Institute for Sustainability and the Environment, Department of Pharmaceutical Sciences, Office of the Vice President for Research, and Associate Dean for Research of the College of Pharmacy, University of Kentucky.References
- R. A. Yokel, R. L. Florence, J. M. Unrine, M. T. Tseng, U. M. Graham, P. Wu, E. A. Grulke, R. Sultana, S. S. Hardas and A. Butterfield, Nanotoxicology, 2009, 3, 234–248 CrossRef CAS.
- B. Collin, E. Oostveen, O. V. Tsyusko and J. M. Unrine, Environ. Sci. Technol., 2014, 48, 1280–1289 CrossRef CAS PubMed.
- Z. Hu, S. Haneklaus, G. Sparovek and E. Schnug, Commun. Soil Sci. Plant Anal., 2006, 37, 1381–1420 CrossRef CAS.
- J. Emsley, Nature's Building Blocks: An A-Z Guide to the Elements, Oxford University Press, 2001 Search PubMed.
- C. R. Hammond, in Handbook of chemistry and physics, 81st edn, C. press, 2000 Search PubMed.
- J. Cervini-Silva, D. A. Fowle and J. Banfield, Am. J. Sci., 2005, 305, 711–726 CrossRef CAS.
- M. Auffan, J. Rose, M. R. Wiesner and J.-Y. Bottero, Environ. Pollut., 2009, 157, 1127–1133 CrossRef CAS PubMed.
- R. A. Yokel, S. Hussain, S. Garantziotis, P. Demokritou, V. Castranova and F. R. Cassee, Environ. Sci.: Nano, 2014, 1(5), 406–428 RSC.
- European Commission, Commission Staff Working Paper: Types and Uses of Nanomaterials, Including Safety Aspects, http://ec.europa.eu/nanotechnology/index_en.html, 2012 Search PubMed.
- The Global Market for Nanomaterials 2002–2016: Production volumes, revenues and end user markets, Future Markets, Inc., http://www.futuremarketsinc.com, (accessed Jan 29, 2013), 2012 Search PubMed.
- D. I. Bleiwas, Potential for recovery of cerium contained in automotive catalytic converters, U.S. Geological Survey, Open-File Report 2013–1037, 2013 Search PubMed.
- C. O. Hendren, X. Mesnard, J. Droge and M. R. Wiesner, Environ. Sci. Technol., 2011, 45, 2562–2569 CrossRef CAS PubMed.
- C. Gomes, Global Auto Report, Scotiabank Economics, Toronto, Ontario Canada, 2013 Search PubMed.
- B. Park, K. Donaldson, R. Duffin, L. Tran, F. Kelly, I. Mudway, J. P. Morin, R. Guest, P. Jenkinson, Z. Samaras, M. Giannouli, H. Kouridis and P. Martin, Inhalation Toxicol., 2008, 20, 547–566 CrossRef CAS PubMed.
- World oil outlook, Organization of the Petroleum Exporting Countries, Vienna, Austria, 2011 Search PubMed.
- A. Ulrich and A. Wichser, Anal. Bioanal. Chem., 2003, 377, 71–81 CrossRef CAS PubMed.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS PubMed.
- R. Kaegi, B. Sinnet, S. Zuleeg, H. Hagendorfer, E. Mueller, R. Vonbank, M. Boller and M. Burkhardt, Environ. Pollut., 2010, 158, 2900–2905 CrossRef CAS PubMed.
- A. Lazareva and A. A. Keller, ACS Sustainable Chem. Eng., 2014, 2, 1656–1665 CrossRef CAS.
- M. Baalousha, Y. Ju-Nam, P. A. Cole, B. Gaiser, T. F. Fernandes, J. A. Hriljac, M. A. Jepson, V. Stone, C. R. Tyler and J. R. Lead, Environ. Toxicol. Chem., 2012, 31, 983–993 CrossRef CAS PubMed.
- M. Baalousha, Y. Ju-Nam, P. A. Cole, J. A. Hriljac, I. P. Jones, C. R. Tyler, V. Stone, T. F. Fernandes, M. A. Jepson and J. R. Lead, Environ. Toxicol. Chem., 2012, 31, 994–1003 CrossRef CAS PubMed.
- R. F. Domingos, M. A. Baalousha, Y. Ju-Nam, M. M. Reid, N. Tufenkji, J. R. Lead, G. G. Leppard and K. J. Wilkinson, Environ. Sci. Technol., 2009, 43, 7277–7284 CrossRef CAS PubMed.
- D. R. Baer, M. H. Engelhard, G. E. Johnson, J. Laskin, J. F. Lai, K. Mueller, P. Munusamy, S. Thevuthasan, H. F. Wang, N. Washton, A. Elder, B. L. Baisch, A. Karakoti, S. Kuchibhatla and D. Moon, J. Vac. Sci. Technol., A, 2013, 31 Search PubMed.
- M. Baalousha, Y. Nur, I. Roemer, M. Tejamaya and J. R. Lead, Sci. Total Environ., 2013, 454, 119–131 CrossRef PubMed.
- M. Baalousha and J. R. Lead, Colloids Surf., A, 2013, 419, 238–247 CrossRef CAS.
- A. S. Karakoti, N. A. Monteiro-Riviere, R. Aggarwal, J. P. Davis, R. J. Narayan, W. T. Self, J. McGinnis and S. Seal, JOM, 2008, 60, 33–37 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- M. Tejamaya, I. Roemer, R. C. Merrifield and J. R. Lead, Environ. Sci. Technol., 2012, 46, 7011–7017 CrossRef CAS PubMed.
- K. M. Buettner, C. I. Rinciog and S. E. Mylon, Colloids Surf., A, 2010, 366, 74–79 CrossRef CAS.
- H.-X. Mai, L.-D. Sun, Y.-W. Zhang, R. Si, W. Feng, H.-P. Zhang, H.-C. Liu and C.-H. Yan, J Phys. Chem. B, 2005, 109, 24380–24385 CrossRef CAS PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Acc. Chem. Res., 2013, 46, 854–862 CrossRef CAS PubMed.
- L. A. Röhder, T. Brandt, L. Sigg and R. Behra, Aquat. Toxicol., 2014, 152, 121–130 CrossRef PubMed.
- S. Kuchibhata, A. S. Karakoti, D. R. Baer, S. Samudrala, M. H. Engelhard, J. E. Amonette, S. Thevuthasan and S. Seal, J. Phys. Chem. C, 2012, 116, 14108–14114 Search PubMed.
- T. Suzuki, I. Kosacki, H. U. Anderson and P. Colomban, J. Am. Ceram. Soc., 2001, 84, 2007–2014 CrossRef CAS.
- G. S. Herman, Surf. Sci., 1999, 437, 207–214 CrossRef CAS.
- J. Conesa, Surf. Sci., 1995, 339, 337–352 CrossRef CAS.
- S. Turner, S. Lazar, B. Freitag, R. Egoavil, J. Verbeeck, S. Put, Y. Strauven and G. Van Tendeloo, Nanoscale, 2011, 3, 3385–3390 RSC.
- R. C. Merrifield, Z. W. Wang, R. E. Palmer and J. R. Lead, Environ. Sci. Technol., 2013, 47, 12426–12433 CrossRef CAS PubMed.
- D. R. Baer, M. H. Engelhard, G. E. Johnson, J. Laskin, J. F. Lai, K. Mueller, P. Munusamy, S. Thevuthasan, H. F. Wang, N. Washton, A. Elder, B. L. Baisch, A. Karakoti, S. Kuchibhatla and D. Moon, J. Vac. Sci. Technol., A, 2013, 31, 34 Search PubMed.
- P. Zhang, Y. Ma, Z. Zhang, X. He, J. Zhang, Z. Guo, R. Tai, Y. Zhao and Z. Chai, ACS Nano, 2012, 6, 9943–9950 CrossRef CAS PubMed.
- P. Zhang, X. He, Y. H. Ma, K. Lu, Y. L. Zhao and Z. Y. Zhang, Chemosphere, 2012, 89, 530–535 CrossRef CAS PubMed.
- N. Manier, A. Bado-Nilles, P. Delalain, O. Aguerre-Chariol and P. Pandard, Environ. Pollut., 2013, 180, 63–70 CrossRef CAS PubMed.
- A. A. Keller, H. T. Wang, D. X. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. X. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- J. T. K. Quik, I. Lynch, K. V. Hoecke, C. J. H. Miermans, K. A. C. D. Schamphelaere, C. R. Janssen, K. A. Dawson, M. A. C. Stuart and D. V. D. Meent, Chemosphere, 2010, 81, 711–715 CrossRef CAS PubMed.
- D. Mavrocordatos, W. Pronk and M. Boller, Water Sci. Technol., 2004, 50, 9–18 CAS.
- K. Van Hoecke, J. T. K. Quik, J. Mankiewicz-Boczek, K. A. C. De Schamphelaere, A. Elsaesser, P. Van der Meeren, C. Barnes, G. McKerr, C. V. Howard, D. Van De Meent, K. Rydzynski, K. A. Dawson, A. Salvati, A. Lesniak, I. Lynch, G. Silversmit, B. De Samber, L. Vincze and C. R. Janssen, Environ. Sci. Technol., 2009, 43, 4537–4546 CrossRef CAS PubMed.
- I. Rodea-Palomares, K. Boltes, F. Fernández-Piñas, F. Leganés, E. García-Calvo, J. Santiago and R. Rosal, Toxicol. Sci., 2011, 119, 135–145 CrossRef CAS PubMed.
- Y. Xue, Y. Zhai, K. Zhou, L. Wang, H. Tan, Q. Luan and X. Yao, Chem. – Eur. J., 2012, 18, 11115–11122 CrossRef CAS PubMed.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS PubMed.
- G. A. Johansson, T. Tyliszczak, G. E. Mitchell, M. H. Keefe and A. P. Hitchcock, J. Synchrotron Radiat., 2007, 14, 395–402 CAS.
- S. Jearanaikoon and J. V. Abraham-Peskir, J. Microsc., 2005, 218, 185–192 CrossRef CAS PubMed.
- J. Thieme, I. McNult, S. Vogt and D. Paterson, Environ. Sci. Technol., 2007, 41, 6885–6889 CrossRef CAS PubMed.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS PubMed.
- G. V. Lowry, B. P. Espinasse, A. R. Badireddy, C. J. Richardson, B. C. Reinsch, L. D. Bryant, A. J. Bone, A. Deonarine, S. Chae, M. Therezien, B. P. Colman, H. Hsu-Kim, E. S. Bernhardt, C. W. Matson and M. R. Wiesner, Environ. Sci. Technol., 2012, 46, 7027–7036 CrossRef CAS PubMed.
- R. Ma, C. Levard, S. M. Marinakos, Y. Cheng, J. Liu, F. M. Michel, G. E. Brown and G. V. Lowry, Environ. Sci. Technol., 2012, 46, 752–759 CrossRef CAS PubMed.
- R. B. Reed, C. P. Higgins, P. Westerhoff, S. Tadjiki and J. F. Ranville, J. Anal. At. Spectrom., 2012, 27, 1093–1100 RSC.
- M. Nabavi, O. Spalla and B. Cabane, J. Colloid Interface Sci., 1993, 160, 459–471 CrossRef CAS.
- L. A. De Faria and S. Trasatti, J. Colloid Interface Sci., 1994, 167, 352–357 CrossRef CAS.
- D. Grasso, K. Subramaniam, M. Butkus, K. Strevett and J. Bergendahl, Rev. Environ. Sci. Bio/Technol., 2002, 1, 17–38 CrossRef CAS.
- Z. Li, E. Sahle-Demessie, A. A. Hassan and G. A. Sorial, Water Res., 2011, 45, 4409–4418 CrossRef CAS PubMed.
- K. G. Li and Y. S. Chen, J. Hazard. Mater., 2012, 209, 264–270 CrossRef PubMed.
- X. Liu, G. Chen and C. Su, Environ. Sci. Technol., 2012, 46, 6681–6688 CrossRef CAS PubMed.
- K. Van Hoecke, K. A. C. De Schamphelaere, P. Van der Meeren, G. Smagghe and C. R. Janssen, Environ. Pollut., 2011, 159, 970–976 CrossRef CAS PubMed.
- G. Cornelis, B. Ryan, M. J. McLaughlin, J. K. Kirby, D. Beak and D. Chittleborough, Environ. Sci. Technol., 2011, 45, 2777–2782 CrossRef CAS PubMed.
- N. J. Rogers, N. M. Franklin, S. C. Apte, G. E. Batley, B. M. Angel, J. R. Lead and M. Baalousha, Environ. Chem., 2010, 7, 50–60 CrossRef CAS.
- L. J. Zhao, J. R. Peralta-Videa, A. Varela-Ramirez, H. Castillo-Michel, C. Q. Li, J. Y. Zhang, R. J. Aguilera, A. A. Keller and J. L. Gardea-Torresdey, J. Hazard. Mater., 2012, 225, 131–138 CrossRef PubMed.
- J. A. Hernandez-Viezcas, H. Castillo-Michel, J. C. Andrews, M. Cotte, C. Rico, J. R. Peralta-Videa, Y. Ge, J. H. Priester, P. A. Holden and J. L. Gardea-Torresdey, ACS Nano, 2013, 7, 1415–1423 CrossRef CAS PubMed.
- M. L. López-Moreno, G. de la Rosa, J. A. Hernández-Viezcas, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2010, 58, 3689–3693 CrossRef PubMed.
- M. L. López-Moreno, G. de la Rosa, J. A. Hernandez-Viezcas, H. Castillo-Michel, C. E. Botez, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Environ. Sci. Technol., 2010, 44, 7315–7320 CrossRef PubMed.
- M. Baalousha, P. Le Coustumer, I. Jones and J. R. Lead, Environ. Chem., 2010, 7, 377–385 CrossRef CAS.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753 CrossRef CAS PubMed.
- A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1–17 CrossRef.
- L. E. Barton, M. Auffan, M. Bertrand, M. Barakat, C. Santaella, A. Masion, D. Borschneck, L. Olivi, N. Roche, M. R. Wiesner and J. Y. Bottero, Environ. Sci. Technol., 2014, 48, 7289–7296 CrossRef CAS PubMed.
- A. Garcia, L. Delgado, J. A. Tora, E. Casals, E. Gonzalez, V. Puntes, X. Font, J. Carrera and A. Sanchez, J. Hazard. Mater., 2012, 199, 64–72 CrossRef PubMed.
- L. K. Limbach, R. Bereiter, E. Müller, R. Krebs, R. Gälli and W. J. Stark, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS PubMed.
- Y. Ma, L. Kuang, X. He, W. Bai, Y. Ding, Z. Zhang, Y. Zhao and Z. Chai, Chemosphere, 2010, 78, 273–279 CrossRef CAS PubMed.
- F. Schwabe, R. Schulin, L. K. Limbach, W. Stark, D. Burge and B. Nowack, Chemosphere, 2013, 91, 512–520 CrossRef CAS PubMed.
- C. M. Rico, J. Hong, M. I. Morales, L. Zhao, A. C. Barrios, J. Y. Zhang, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Environ. Sci. Technol., 2013, 47, 5635–5642 CrossRef CAS PubMed.
- Q. Wang, S. D. Ebbs, Y. Chen and X. Ma, Metallomics, 2013, 5, 753–759 RSC.
- K. Birbaum, R. Brogioli, M. Schellenberg, E. Martinoia, W. J. Stark, D. Günther and L. K. Limbach, Environ. Sci. Technol., 2010, 44, 8718–8723 CrossRef CAS PubMed.
- Q. Wang, X. Ma, W. Zhang, H. Pei and Y. Chen, Metallomics, 2012, 4, 1105–1112 RSC.
- L. Zhao, Y. Sun, J. A. Hernandez-Viezcas, A. D. Servin, J. Hong, G. Niu, J. R. Peralta-Videa, M. Duarte-Gardea and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2013, 61, 11945–11951 CrossRef CAS PubMed.
- J. H. Priester, Y. Ge, R. E. Mielke, A. M. Horst, S. C. Moritz, K. Espinosa, J. Gelb, S. L. Walker, R. M. Nisbet, Y.-J. An, J. P. Schimel, R. G. Palmer, J. A. Hernandez-Viezcas, L. Zhao, J. L. Gardea-Torresdey and P. A. Holden, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E2451–E2456 CrossRef CAS PubMed.
- S. Bandyopadhyay, J. R. Peralta-Videa, G. Plascencia-Villa, M. Jose-Yacaman and J. L. Gardea-Torresdey, J. Hazard. Mater., 2012, 241–242, 379–386 CrossRef CAS PubMed.
- M. I. Morales, C. M. Rico, J. A. Hernandez-Viezcas, J. E. Nunez, A. C. Barrios, A. Tafoya, J. P. Flores-Marges, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2013, 61, 6224–6230 CrossRef CAS PubMed.
- C. M. Rico, M. I. Morales, A. C. Barrios, R. McCreary, J. Hong, W.-Y. Lee, J. Nunez, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2013, 61, 11278–11285 CrossRef CAS PubMed.
- R. Dinesh, M. Anandaraj, V. Srinivasan and S. Hamza, Geoderma, 2012, 173, 19–27 CrossRef.
- L. V. Antisari, S. Carbone, A. Gatti, G. Vianello and P. Nannipieri, Soil Biol. Biochem., 2013, 60, 87–94 CrossRef.
- E. Lahive, K. Jurkschat, B. J. Shaw, R. D. Handy, D. J. Spurgeon and C. Svendsen, Environ. Chem., 2014, 11, 268–278 CrossRef CAS.
- P. L. Williams and D. B. Dusenbery, Environ. Toxicol. Chem., 1990, 9, 1285–1290 CrossRef CAS.
- J. Y. Roh, Y. K. Park, K. Park and J. Choi, Environ. Toxicol. Pharmacol., 2010, 29, 167–172 CrossRef CAS PubMed.
- H. Zhang, X. He, Z. Zhang, P. Zhang, Y. Li, Y. Ma, Y. Kuang, Y. Zhao and Z. Chai, Environ. Sci. Technol., 2011, 45, 3725–3730 CrossRef CAS PubMed.
- S. Lee, K. Kim, H. Shon, S. Kim and J. Cho, J. Nanopart. Res., 2011, 13, 3051–3061 CrossRef CAS.
- D. H. Lin, J. Ji, Z. F. Long, K. Yang and F. C. Wu, Water Res., 2012, 46, 4477–4487 CrossRef CAS PubMed.
- O. Zeyons, A. Thill, F. Chauvat, N. Menguy, C. Cassier-Chauvat, C. Orear, J. Daraspe, M. Auffan, J. Rose and O. Spalla, Nanotoxicology, 2009, 3, 284–295 CrossRef CAS.
- D. A. Pelletier, A. K. Suresh, G. A. Holton, C. K. McKeown, W. Wang, B. H. Gu, N. P. Mortensen, D. P. Allison, D. C. Joy, M. R. Allison, S. D. Brown, T. J. Phelps and M. J. Doktycz, Appl. Environ. Microbiol., 2010, 76, 7981–7989 CrossRef CAS PubMed.
- X. H. Fang, R. Yu, B. Q. Li, P. Somasundaran and K. Chandran, J. Colloid Interface Sci., 2010, 348, 329–334 CrossRef CAS PubMed.
- M. Auffan, J. Rose, T. Orsiere, M. De Meo, A. Thill, O. Zeyons, O. Proux, A. Masion, P. Chaurand, O. Spalla, A. Botta, M. R. Wiesner and J.-Y. Bottero, Nanotoxicology, 2009, 3, 161–171 CrossRef CAS.
- M. Auffan, D. Bertin, P. Chaurand, C. Pailles, C. Dominici, J. Rose, J. Y. Bottero and A. Thiery, Water Res., 2013, 47, 3921–3930 CrossRef CAS PubMed.
- E. Artells, J. Issartel, M. Auffan, D. Borschneck, A. Thill, M. Tella, L. Brousset, J. Rose, J. Y. Bottero and A. Thiery, PLoS One, 2013, 8, 11 Search PubMed.
- J. R. Conway, S. K. Hanna, H. S. Lenihan and A. A. Keller, Environ. Sci. Technol., 2014, 48, 1517–1524 CrossRef CAS PubMed.
- B. D. Johnston, T. M. Scown, J. Moger, S. A. Cumberland, M. Baalousha, K. Linge, R. van Aerle, K. Jarvis, J. R. Lead and C. R. Tyler, Environ. Sci. Technol., 2010, 44, 1144–1151 CrossRef CAS PubMed.
- S. W. Lee, S. M. Kim and J. Choi, Environ. Toxicol. Pharmacol., 2009, 28, 86–91 CrossRef CAS PubMed.
- B. K. Gaiser, A. Biswas, P. Rosenkranz, M. A. Jepson, J. R. Lead, V. Stone, C. R. Tyler and T. F. Fernandes, J. Environ. Monit., 2011, 13 Search PubMed.
- N. B. Hartmann, C. Engelbrekt, J. Zhang, J. Ulstrup, K. O. Kusk and A. Baun, Nanotoxicology, 2013, 7, 1082–1094 CrossRef CAS PubMed.
- A. C. Johnson and B. Park, Environ. Toxicol. Chem., 2012, 31, 2582–2587 CrossRef CAS PubMed.
- A. Garcia, R. Espinosa, L. Delgado, E. Casals, E. Gonzalez, V. Puntes, C. Barata, X. Font and A. Sanchez, Desalination, 2011, 269, 136–141 CrossRef CAS.
- A. C. Johnson, R. J. Williams, P. Simpson and R. Kanda, Environ. Pollut., 2007, 147, 194–202 CrossRef CAS PubMed.
- R. J. Williams, J. H. Churchley, R. Kanda and A. C. Johnson, Environ. Toxicol. Chem., 2012, 31, 892–898 CrossRef CAS PubMed.
- M. C. Arnold, A. R. Badireddy, M. R. Wiesner, R. T. Di Giulio and J. N. Meyer, Arch. Environ. Contam. Toxicol., 2013, 65, 224–233 CrossRef CAS PubMed.
- G. Cornelis, J. K. Kirby, D. Beak, D. Chittleborough and M. J. McLaughlin, Environ. Chem., 2010, 7, 298–308 CrossRef CAS.
- F. Hogan, M. McHugh and S. Morton, Environ. Technol., 2001, 22, 1347–1353 CrossRef CAS PubMed.
- S. H. Hallett, PhD thesis, Cranfield University, 2008 Search PubMed.
- A. Gendebien, C. Carlton-Smith, M. Izzo and J. E. Hall, UK sewage sludge survey - National presentation, Report R and D Technical Report P165, Environment Agency, Bristol, UK, 1999 Search PubMed.
- S. R. Smith, Environ. Int., 2009, 35, 142–156 CrossRef CAS PubMed.
- O. Bondarenko, K. Juganson, A. Ivask, K. Kasemets, M. Mortimer and A. Kahru, Arch. Toxicol., 2013, 87, 1181–1200 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4en00149d |
| This journal is © The Royal Society of Chemistry 2014 |
