Applications and implications of nanoceria reactivity: measurement tools and environmental impact
Daniel
Andreescu
,
Gonca
Bulbul
,
Rifat Emrah
Özel
,
Akhtar
Hayat
,
Naimish
Sardesai
and
Silvana
Andreescu
*
Department of Chemistry and Biomolecular Science, Clarkson University, Potsdam, NY 13699-5810, USA. E-mail: eandrees@clarkson.edu
First published on 11th July 2014
Abstract
Cerium oxide nanoparticles or nanoceria have a unique structure and interesting and unusual redox and catalytic properties that vary with the size, shape, charge, surface coating and chemical reactivity. This paper highlights applications and environmental implications of nanoceria, and describes methodologies for the assessment of the reactivity and potential toxicological effects of these particles. The physical and chemical properties in the particle design that are responsible for their reactivity and transformation in environmental and biological conditions are described. Processes such as surface oxidation, formation of surface complexes and potential interaction with redox active components of the environment are discussed. An overview of analytical characterization methods for study of nanoceria properties, reactivity and impact, highlighting methodological challenges and limitations is presented. Examples discussed include strategies to determine physicochemical properties, cytotoxicity and antioxidant or pro-oxidant activity in various exposure environments. Development of new measurement tools to facilitate rapid assessment and accelerate screening of these particles for their reactivity and effects is discussed. Future research needs for environmental assessment of benefits and potential risks associated with the use of nanoceria are also provided.
Nano impactNanoceria particles have great promise as active materials in biomedicine, microelectronics, environmental remediation, catalysis and sensing. The many useful properties of nanoceria are derived from the unique nanosize structure, surface reactivity and redox activity, which also pose significant environmental toxicological challenges. This paper provides a critical overview of the environmental implications of the reactivity of nanoceria. We survey research exploring the chemistry of nanoceria particles, their benefits and potential risks for the environment. We describe the role of the physicochemical and surface properties that are unique to their nanoparticulate form and discuss analytical methodologies for assessing and screening these particles. Finally, we provide an outlook of future research needs for study and environmental assessment of nanoceria. |
1. Introduction
Nanoceria particles (or cerium oxide nanoparticles, CeO2 NPs) are used in a variety of applications including chemical mechanical polishing, catalysis, solid oxide fuel cells, environmental remediation, sensing and more recently biomedicine.1 The popularity of these particles is a consequence of the many interesting mechanical, spectroscopic, catalytic and oxidant/antioxidant properties of these particles enabling them to be used as polishing agents for electronics, oxygen buffers, UV blockers, sorbets for environmental contaminants, colorimetric dyes for quantifying redox reactions and as synthetic antioxidants.2–5 Ceria NPs are also used as diesel fuel additives, resulting in release of these particles in the atmosphere. In spite of the many uses of these particles, little is known about their potential impact on the environment and human health.The useful properties of nanoceria are a consequence of the physical and chemical characteristics of these particles, and their reactivity, and can vary greatly with the size, shape, charge and surface coating. Many of the unique features are linked with the dual oxidation state of cerium at the NP surface enabling them to act as both oxidizing and reducing agents. The environmental and health effects are related with the aforementioned characteristics. Changes in both the oxidation state (switching between Ce3+ and Ce4+) and surface adsorption (e.g. through hydroxyl groups) have been identified as major contributing parameters to the reactivity and environmental behavior of these particles.6–8 Particle composition and surface chemistry as a consequence of different synthetic pathways and surface ligands are also important when assessing biological and environmental effects. Both toxic9,10 and beneficial effects of nanoceria11–13 obtained by different procedures have been related with these characteristics and the results are often contradictory.14,15 Published research indicates that the surface properties and reactivity can change depending upon the environment and exposure conditions, and can vary with time (particle aging), storage conditions, particle concentration and composition (pH, ionic strength, presence of redox species).16 Therefore, assessing these parameters and predicting changes in various exposure scenarios are critical for the safe implementation and practical use of nanoceria.
This review highlights applications and environmental implications of nanoceria particles and describes methodologies for rapid assessment of the impact of these particles in the environment and biological systems. The first part of the paper highlights the chemistry and surface reactivity of nanoceria in various exposure scenarios. The second part describes development of analytical methods for the assessment of the physicochemical properties and reactivity of these particles with their advantages and limitations. Classical, commonly used methods for measuring nanoceria properties are compared with novel tools for screening and predicting chemical reactivity, catalytic behavior and surface changes in various environments. Electrochemical measurements with surface-modified electrode probes as predictive tools for screening nanoceria reactivity and for evaluating cytotoxic effects as a result of nanoceria exposure are highlighted as an example of emerging technology. Current state-of-the-art detection methods and future research needs for study and environmental assessment of nanoceria are discussed.
2. The chemistry and surface reactivity of nanoceria: applications and implications
Nanoceria particles can be distinguished among other metal oxides by their oxygen storage and release characteristics17 and by the dual oxidation state allowing them to participate in redox reactions both as an oxidizing and a reducing agent. While ceria NPs contain a mixture of Ce4+ and Ce3+ cations, their ratio varies with the size of the particle with smaller particles having a higher Ce3+ content due to the high surface-to-volume ratio and a higher concentration of defects and oxygen vacancies.18 The oxygen mobility and the ability to store and release oxygen play critical roles in the catalytic activity and reactivity of nanoceria, on the basis of quantum processes of electron delocalization of the Ce 4f.19 In an oxidative or reductive environment, structural and physicochemical features of the particles such as surface area, quantum confinement, lattice parameter and symmetry can change. For example, the crystal lattice strain increases with the decrease in the particle size due to increase in the Ce3+/C4+ ratio.20 This strain occurs due to hydrolysis of cerium ions to form hydroxides on the surface when the hydrogen atoms in the solution bind with the oxygen atoms within the lattice.21 The hydroxyl species act as precursors to remove oxygen during a reduction process that generates Ce3+ cations. In small-sized particles (~3 nm) loss of even one oxygen atom creates a high lattice strain leading to an increase in the lattice parameter favoring formation of oxygen vacancies.20 Formation of more oxygen vacancies was found to promote reducibility and reactivity of nanosized ceria.22 Therefore efforts to control the morphological parameters such as the size, shape, crystal plane and symmetry during synthesis are essential for controlling reactivity of these particles.22,23 Reviews describing various synthetic strategies such as alcohothermal, hydrothermal and thermolysis methods of crystalline nanoceria particles with high surface area have been published.23,24 Self-assembly systems such as micelles, vesicles, and water-in-oil microemulsions have also been employed to achieve better control over properties such as surface area, porosity, shape, size distribution, reactivity and dispersion stability through changes in compositions, treatments and thermodynamic variables.24 However tailored synthesis of nanoceria with precisely controlled shape and size, desired surface oxidation states is difficult due to different nucleation growth, and hydrolysis behavior of these particles.23 Due to the large number of variability between structures and resulting effects, strategies for high-throughput screening to evaluate the reactivity of these particles are highly desired.Most environmental and biological exposure scenarios involving these particles are at room or physiological temperatures and in aqueous medium when the particles are hydroxylated and can easily form surface complexes. Particle surface (e.g. surface charge and coating) are sensitive to the presence of different complexing agents, proteins, oxidizing or reducing agents and pH.6,8,25 Such changes affect aggregation behavior which determines the fate and transport of these particles. The stability phase diagram of cerium species in aqueous systems is characterized by a number of hydroxylated ionic cerium species depending on the pH and the environment. These include CeO2·2H2O [or Ce(OH)4] at pH > 10, Ce(OH)2+ and Ce(OH)2+ at pH < 10 as well as a number of Ce(OH)3+, Ce(OH)22+, Ce(OH)3+ at pH < 3.26 The particle concentration in the exposure environment is also important.27 The surface chemistry of nanoceria in aqueous and physiological environments is not well understood. Since these processes take place at room or physiological temperature (~37 °C), the existing knowledge of ceria catalysis at high temperatures is not directly applicable to understanding environmental and biological effects. The following sections summarize the current understanding of the chemical reactivity of nanoceria in aqueous environments relevant to environmental exposure.
2.1. Interaction with reactive oxygen species
Most studies on nanoceria in aqueous solutions focus on assessment of the interaction of these particles with H2O2, often selected as a model reactive oxygen species to determine their antioxidant or pro-oxidant activity. H2O2 can act both as a reducing and oxidizing agent on ceria28,29 depending on the concentration and reaction time.30 This chemistry has been often associated with a superoxide dismutase (SOD) and catalase activity of these particles,31 represented by the following reactions: reaction (1) – corresponding to a SOD like activity12,30 and reaction (2) – corresponding to a catalase like activity:32| Ce2O3 + O2˙− + 2H+ → 2CeO2 + H2O | (1) |
| CeO2 + H2O2 → Ce2O3 + O2 + H2O | (2) |
In addition to surface oxidation/reduction, surface complexation with superoxide (O2˙−) type ligands and formation of surface bound peroxo (Ce–O22−) and superoxo-like complexes (Ce–O2−) have been observed during reaction with H2O2, together with partial loss of crystallinity, indicating a ‘catalytic sponge’ type inactivation mechanism for reactive oxygen species.7 Recent studies of the catalytic decomposition of H2O2 by nanoceria (3 nm) on a quantum chemical level show an increase of the interatomic distances between the Ce and O with a delocalized redistribution of charge density over the entire particle structure when oxygen vacancies are formed, rather than a localized surface reduction of Ce4+ to Ce3+ suggesting an ‘electron sponge’ mechanism.33 Adsorption of O2˙− was found energetically more favorable on reduced (Ce3+) ceria rather than on unreduced (Ce4+) using density functional calculations (DFT).34 Thus oxygen species are likely to react with surface oxygen vacancies and Ce3+ ions on nanoceria, resulting in fully oxidized Ce4+ and O2˙− bound species (Ce–O2−) of charge transfer or electrostatic nature.16 This is confirmed by the immediate change in color of ceria from light yellow to dark brown after exposure to H2O2. This property has been used to develop colorimetric ceria based sensors in which the particles provide a colorimetric readout for the detection of H2O2 and oxidase enzyme substrates.35 The presence of these complexes has been confirmed by Raman and FTIR spectroscopy.35 These complexes are reversible and the particles can recover their activity after several weeks at room temperature, or within minutes by heating at temperatures of ~80–100 °C to decompose the bound peroxide and O2˙− species.35 The high reactivity of nanoceria for H2O2 was observed for particles of various sizes and also for particles coated with surface stabilizers. In a comparative study, small 3.8 nm ceria particles with a thin stabilizer layer of poly(lactic acid) or oleic acid coating were found to be more reactive than particles of 8.2 nm with a thicker polymer (e.g. polyethylene imine or polymaleicanhydride-alt-1-octadecene).36 This reactivity is specific to nanoceria.
The reversibility of these processes and the variability among the particles studied (different sizes, coatings and surface charge) in addition to the concentration dependency could explain the diverse, often contradictory results reported in the literature with respect to the antioxidant or pro-oxidant activity of these particles. The diversity of the exposure environment and biological models used add to this controversy. More systematic studies of the effect of surface area, size, coatings, surface complexation reactions and particle concentration are needed to more comprehensively assess the effect of the physicochemical parameters to their oxidant/antioxidant activity and predict such reactivity.
2.2. Interaction with redox active compounds
With the exception of H2O2, little is known about the interaction of nanoceria with other redox active components of the environment and biological systems. Species such as ascorbic acid and citric acid as well as a variety of phenolic compounds can act as reducing agents and react with the Ce4+ on the particle surface converting it to Ce3+. These species can form strong chemical bonds with the surface exposed hydroxyl species and with the Ce3+ generating surface complexes. We have used this chemistry to design detection schemes and portable database37 for phenolic compounds25 and for the detection of alkaline phosphatase activity.38,39 Such processes have been investigated in the chemical mechanical polishing literature but have received significantly less attention in the environmental and biomedical fields. In physiological environments the highly oxidizing nature of nanoceria can induce oxidation of monoamine neurotransmitters such as dopamine, serotonin (5-HT) and noradrenaline, as well as of the natural antioxidant ascorbic acid.39–41 Such reactivity is obvious from Fig. 1 showing immediate color changes of nanoceria before and after interaction with dopamine (Fig. 1B) or 5-HT (Fig. 1D) and validated by the corresponding UV-Vis spectra of dopamine (Fig. 1A) and 5-HT (Fig. 1C) respectively. A possible reaction mechanism for dopamine is shown in Fig. 2 and may include changes in the oxidation state and formation of charge transfer complexes on the NPs surface. The UV-Vis spectra show strong shifts in the nanoceria absorption peak. Similar reactivity profile occurs with other types of commercially available nanoceria particles indicating that potential oxidation and surface binding take place irrespective of the particle (and potentially synthetic conditions) used.39 Interaction of nanoceria with these compounds, many of which have physiological relevance and are widely present in living organisms, is important as their oxidation or complexation by nanoceria might affect metabolic and oxidative processes, change their physiological levels or generate toxic metabolites with neurotoxic potential. Such changes could affect the neural and antioxidant system function. Recent studies of the interaction of nanoceria with dopamine and serotonin suggest occurrence of these processes in physiological environments.40,42 Exposure to nanoceria induced a decrease in the physiological level of these compounds in human serum42 and an in vivo system (the intestine of zebrafish).40 These preliminary findings indicate the need for a more comprehensive assessment of the interaction of nanoceria with redox active components that are present in biological and environmental systems. Since these particles are actively studied as therapeutic agents11–13 for in vivo applications, DNA/RNA biosensors,43 metal-ion detection and speciation analysis44 and can be found in inhaled air (e.g. from uses as fuel additives)45 thus freely entering biological systems, further work should be dedicated to evaluate potential neurotoxic consequences resulting from intended or unintended exposure to these particles.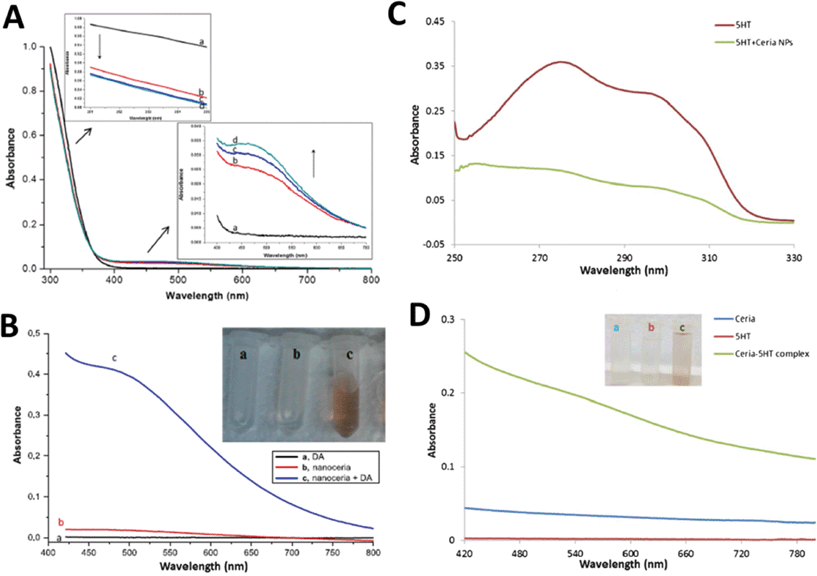 | ||
| Fig. 1 (A) UV-Vis spectra of 22.5 ppm ceria NPs in the absence (a) and presence of 12.5 μM dopamine after 0 (b), 5 (c) and 10 (d) minutes incubation. The insets show enlarged spectra of the 300–305 nm and 400–700 nm regions. Arrows indicate increase or decrease in absorbance. (B) Absorption spectra and color change of dopamine (DA) (a), ceria NPs (b) and dopamine with ceria NPs (c) UV spectra of 3 mM 5-HT in the presence and absence of 50 ppm ceria NPs dispersion. (C). VIS spectra of 5-HT, ceria NPs, and 5-HT after incubation with ceria NPs in E3 medium (D) (from ref. 40 and 42 with permission). | ||
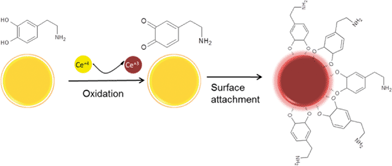 | ||
| Fig. 2 Possible reaction mechanism of the chemical reactivity of ceria against dopamine indicating oxidation followed by chemisorption on the NP surface (with permission from ref. 42). We note that these processes are concentration and size dependent. | ||
2.3. Physicochemical changes of nanoceria in environmental exposure conditions
Most environmental exposure scenarios take place in aqueous conditions and in media containing different ionic composition, pH, natural organic matter and microorganisms. Many of the constituents of a ‘real’ environmental or biological matrix can interact or surface adsorb onto nanoceria changing the charge and surface chemistry of these particles, also affecting dispersity, reactivity and mobility which determine environmental and biological impact. Particles that appear highly dispersed in optimized conditions may aggregate (or be stabilized) once they are applied to a complex aqueous environment. This process is strongly dependent on the surface coating and composition, i.e. isoelectric point and presence of stabilizers. Determination of these parameters is essential and should be considered when performing cytotoxicity and ecotoxicity studies.Keller's group found that electrophoretic mobility of metal oxides including ceria NPs was determined by the presence of natural organic matter and ionic strength rather than pH.46 Interestingly, adsorption of natural organic matter was found to stabilize the particles and reduce aggregation in ambient water conditions. In a recent work by Unrine et al. addition of humic acid significantly reduced nanoceria toxicity in Caenorhabditis elegans as a result of particle coating with the organic matter.47 Studies of the transport characteristics in biological environments pose different challenges due to the presence of high concentrations of proteins that have been shown to attach to particles forming a protein corona48 and affecting their transport characteristics within in vivo and in vitro systems, accumulation and uptake. Seal et al. reported that positively charged nanoceria adsorbed more bovine serum albumin than negatively charged particles, which showed little or no protein adsorption and consequently preferential cellular uptake.49
The ionic composition can alter the surface properties and reactivity of nanoceria through surface complexation reactions with anionic constituents. In particular, anions such as phosphate, citrate and ascorbate can bind to the particle surface as Ce-phosphate, ascorbate and citrate complexes (e.g. Ce(C6H5O7), Ce(C6H5O7)23− with citric acid). These species are commonly used in physiological buffers and are present in biological systems. Thus, surface adsorption studies are essential and should be considered when designing experiments to assess antioxidant activity or cytotoxicity of nanoceria in biological environments rich in these ions. In a study by Seal's group the binding of phosphate anions to nanoceria have affected the catalytic properties of the particles50 but did not cause aggregation or alteration in the crystal structure. On the other hand, sulfates and carbonates did not change the catalytic properties of these particles. Another study with oleic acid coated nanoceria indicate that the organic coating does not affect the antioxidant capacity of these particles, which showed an antioxidant activity nine fold higher than that of a standard antioxidant (Trolox).36
Other parameters such as aging and dissolution characteristics are also important when determining environmental impact. Synthesis parameters, e.g. temperature and surfactants, sample storage and handling, impurities and residues from the synthetic process determine the stability of these particles over time.15,51 Dissolution of nanoceria as a function of environmental and exposure conditions is not well studied. In principle, ceria particles are sparingly soluble in aqueous media and do not dissolve appreciably over time.52 However, some dissolution may be expected in the presence of reducing agents such as ascorbic acid especially at low pH values and in the presence of catalytic materials, as these can reducibly dissolve ceria generating free Ce3+.53–55 This process is commonly used in chemical mechanical polishing (CMP) in microelectronics applications for post CMP cleaning of ceria containing slurries. Changes in the oxidation state and pH can change solubility status. The purification of the particles also plays an important role in determining the surface properties.56 Testing for residual cerium ions (from the synthesis) is highly recommended as these can be weakly adsorbed on the particle surface or can be present in the dispersion environment.56 Changes in the particle surface characteristics due to aging varies with the synthetic procedure and is most likely to occur for particles synthesized at room temperature and stored in aqueous solutions.51 An example of an aging study where the particle size and chemical state changed over time has been reported for nanoceria prepared at room temperature from aqueous Ce(NO3)3 6H2O precursor by reaction with H2O2 with hydrolysis and formation of ceria NPs.15 Crystalline particles synthesized at high temperatures should be less susceptible to such changes. Stabilization of nanoceria particles is possible by using complexation agents that can form strongly adsorbed surface chelates, generating stable NP dispersions. For example, aminopolycarboxylic acids such as nitrilotriacetic acid have been used to stabilize nanoceria through ionic interaction and inner-sphere complexation.57 Further studies are necessary to determine surface and structural changes in various environmental conditions due to particle aging, especially in the presence of reducing agents.
2.4. Environmental applications
Questions related to the presence of nanoceria in the environment were raised since 1999, after the first commercial application of ceria NPs in diesel particulate filters. Most environmental uses of nanoceria are related to its catalytic and sorption properties. Ceria or composites of ceria based oxides are used as chemical oxidation catalysts or catalyst support in three–way engine catalysts, low-temperature water-gas shift reactions, oxygen permeation membranes and fuel cells58 and more recently in environmental remediation as sorbents for the removal of toxic compounds.59 Applications of ceria in the CMP process in the microelectronics industry are widely reported.60 The oxygen buffering capacity was used to remove exhaust emission pollutants by simultaneous oxidation of CO and hydrocarbon from automobile engine combustion. The ceria based engine catalysts have shown high performances in the reduction of NOx and in the selective catalytic oxidation of NH3 from engine exhaust components.61 Applications of nanoceria as active sensing component in the development of portable assays are rapidly emerging.35,38,39,62In the environmental remediation field, ceria based materials have been used for wet catalytic oxidation of different pollutants. For example, CeO2, CuO/CeO2 and Pt modified MnO2/CeO2 NPs have shown high catalytic activity for the wet oxidation of phenol and phenol intermediates.63–65 More recently, ceria NPs have demonstrated high adsorption and desorption capacity for the removal of arsenic in aqueous solutions.59,66 Nanoceria supported on carbon nanotubes showed increased adsorption capacity for As(V)8 and Cr(VI),6 due to their large surface area, small size, excellent electron transfer ability, and easy surface-modification. Positively charged nanoceria-CNTs adsorb these ions by coulombic attraction. The adsorption is pH dependent. Therefore increase in pH decreases adsorption of these ions by the nanoceria-CNTs surface.6,8 Nanocomposites of carbon nanotubes/TiO2–CeO2 have been tested for the photodegradation of the organic content of agro-industrial wastewaters, showing detoxification rates of up to 88.2 % and reutilization of up to five consecutive photooxidation cycles.67 Another application is for the desulfurization of biomass effluents by adsorption of H2S on CeO2-lanthanide mixed oxides by a process involving oxygen vacancy formation followed by H2S adsorption and dissociation with subsequent oxygen vacancy regeneration.68 In another work, ceria modified MnO/TiO2 demonstrated high adsorption capacity of both Hg(0) and Hg(II) from flue gas69 demonstrating that these materials can be used for the environmental remediation of Hg.
3. Characterization tools for assessing nanoceria reactivity
Appropriate assessment of the reactivity of nanoceria particles relies on proper characterization of their physicochemical parameters including size, shape, morphology, surface coatings and chemical reactivity. The large number of uncontrolled experimental variables and the complexity of environmental and biological samples pose significant challenges. The myriad of nanoceria particle types of various sizes, composition and surface coatings that are being produced by different synthetic methods and the lack of standardized protocols add to the complexity of the system. Many studies assess ceria reactivity by indirectly measuring biological effects (e.g. cells and tissue response – lipid pro-oxidation, DNA damage) resulting from exposure to these particles. In this case physicochemical characterization is combined with a study of particles behavior in the exposure medium (e.g. cell culture) followed by the evaluation of biochemical processes related to oxidant/pro-oxidant mechanisms. The effect of secondary reaction mechanisms and proper controls should be considered for accurate data interpretation. In this section, we highlight conventional characterization methods of the physicochemical characteristics and reactivity of nanoceria and propose alternative or complementary tools for rapid screening of particle reactivity, including measurements of their antioxidant / pro-oxidant activity. Strategies for direct real-time assessment of reactive mediators in biological environments are also provided. Advantages and limitations of these methods are discussed.3.1. Assessing physicochemical properties of nanoceria
A primary requirement for environmental nanotoxicology studies is to define a minimum set of characterization techniques to reliably assess the physicochemical properties of synthesized or commercially purchased nanoceria particles and their transformation in the environment. Environmental reactivity depends upon the particle shape, size, chemical composition (Ce3+/Ce4+ ratio) and surface chemistry, pH, particle surface charge and surface functionality.15,47 An example of comprehensive physicochemical characterization of nanoceria including size and size related properties as well as shape, morphology, chemical and structural characteristics of two commercial types of ceria (Sigma Aldrich: a <25 nm size and a microsize ceria particle prepared in 10 mM NaCl) can be found in ref. 70 and 71. The authors emphasize the dependence of the results on the method used (each functioning based on a different principle) and sample preparation protocol and recommend the use of a multimethod approach, as well as the need to develop novel analytical tools to characterize particles in environmental relevant conditions.70 Sample preparation protocols play an important role in this analysis. In a study comparing the effect of sorption and ultracentrifugation on the quality of the atomic force microscopy (AFM) analysis it was found that results are dependent on the interaction forces between the mica surface and the particles.72,73 Recorded data varied with the method used, surface charge of the particles, pH and ionic strength of the suspension. AFM analysis was recommended to be used in conjunction with transmission electron microscopy (TEM) and scanning electron microscopy (SEM) for assessment of particle size. Particle size and crystallinity can be evaluated using X-ray diffraction (XRD) by measuring broadening of the diffraction peaks resulting from crystal size and lattice strain. The method may not provide ‘true’ particle size values in the case of polydisperse samples that contain amorphous components.70 The total surface area can be estimated by Brunauer–Emmett–Teller (BET) analysis. Potential loss of surface area due to inter-particle contact could lead to an underestimation of the surface area and overestimation of particle diameter. In addition, the effect of shape and porosity as well as the pressure applied during the BET-N2 can further complicate surface area calculations.70 The chemical composition and surface chemistry can be characterized by X-Ray energy dispersive spectroscopy (X-EDS), electron energy loss spectroscopy (EELS) and X-ray photoelectron spectroscopy (XPS).71 The X-EDS data for nanoceria show characteristic peaks corresponding to both cerium and oxygen. Peaks corresponding to surface adsorbed species and stabilizing agents as well as Cu from the Cu TEM grid could also be observed. The EELS spectrum shows two peaks at approximately 880 to 883 for smaller particles (<25 nm) corresponding to cerium M5 and M4 edges with higher M5 peak intensity suggesting a mixture of Ce3+ and Ce4+. The XPS of Ce 3d of these particles is characterized by two main peaks occurring at kinetic energies of about 884 and 900.5. The Ce 3d XPS photoemission spectra is the method of choice for assessing the contribution of Ce4+ to Ce3+ species in both pure and mixed oxide samples.74 Surface charge and the dependence of surface charge on the exposure environment (e.g. pH, anions, cell culture, serum, buffers, and natural organic macromolecules) can be determined by zeta potential studies. Defining the relationship between pH and surface charge is needed to understand NP transport.71 The technique has limitations when used in complex environments containing ions or chelating agents that can induce particle aggregation. Zeta potential data obtained from the electrophoretic mobility measurements can be used to characterize the electrical charge densities on the particle surface as a function of pH.75 At present there is no single best technique for particle characterization, and therefore multiple complementary methods should be used to evaluate physicochemical properties of these particles.703.2. Methods for studying nanoceria reactivity and effects in vitro and in vivo
The antioxidant capacity of nanoceria can be evaluated in vitro using conventional antioxidant activity assays designed for soluble natural antioxidants.87 Widely used methods are the ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) assay, the Trolox equivalent antioxidant activity (TEAC), the 2,2-diphenylpicrylhydrazyl (DPPH) radical scavenging assay and the oxygen radical absorbance capacity (ORAC) assay, among others.87 In general, these assays assess the scavenging ability of an antioxidant compound against a specific radical (e.g. ABTS, peroxyl, O2˙−). Radicals are generated in situ using either chemical or enzymatic procedures. Nanoceria particles have different reactivity profiles with each radical (that depend on their physicochemical and surface properties) and thus these methods can be used to establish a link between the physicochemical properties and the scavenging ability of these particles against a specific radical. Assessing the scavenging ability of ceria using multiple methods is recommended in order to obtain a general reactivity profile against different radicals. Although the results can vary significantly among the various methods (due to the different methodologies and radicalic species), in vitro tests are still useful to obtain predictive values of the antioxidant activity in controlled conditions. Effect of parameters such as particle size, coating or synthetic procedure can be established using these methods. Data can be used as a screening tool of particles reactivity prior performing more extensive cell or animal experiments. Few studies have taken advantage of these methods to assess the antioxidant activity of inorganic NPs, most of which are with metal oxides other than ceria. In one example, the TEAC assay has been used to establish the radical scavenging capacity of Fe3O4, CoFe2O4 and MnO2 stabilized by alginate. With the exception of MnO2, Fe3O4 and CoFe2O4 have shown efficient radical scavenging activity against H2O2.88 Given their wide use and well established procedures in the field of natural antioxidants, these assays can be easily adapted to study the scavenging activity of various types of nanoceria particles against various radicals, relate the activity to their physicochemical properties and compare the scavenging activity with that of conventional antioxidants such as Trolox or gallic acid.
In addition to cell work, in vivo whole animal exposure studies are commonly used to assess the physiological impact of nanoceria and determine biodistribution and particle localization within organs. In a brain slice study, treatment with nanoceria decreased ischemia-induced 3-nitrotyrosine levels, a modification to tyrosine residues in proteins induced by the peroxynitrite radical as a result of scavenging of this radical by particles.13 Indirect oxidative modification of protein carbonyl groups from the oxidation of protein amino acid chains with products of lipid peroxidation is another commonly used procedure to assess the extent of oxidative damage by nanoceria.92 On the other hand, production of large concentrations of ROS and RNS can cause lipid peroxidation and induce damage to proteins and nucleic acids and therefore the biological testing is based on measurements of the resulting damage.93 Several studies report a free radical scavenging activity with a number of nanoceria particles of various sizes including commercial ~15–20 nm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and pegylated 5 nm particles prepared by a reverse micelle method.11 Other studies report a pro-oxidant effect for 5 nm nanoceria synthesized by a hydrothermal process.10 Pro and antioxidant effects in brain after 30 days administration were evaluated by measuring levels of protein carbonyls, 3-nitrotyrosine, protein-bound-4-hydroxy-2-trans-nonenal in the hippocampus, cortex and cerebellum, glutathione reductase, glutathione peroxidase, SOD, catalase and inducible nitric oxide.10,92 ROS accumulation and oxidative damage was also observed in a study on Caenorhabditis elegans for low concentration nanoceria exposure (< 1 nM with 8.5 nm particles), assessed using an ABTS assay.9
13 and pegylated 5 nm particles prepared by a reverse micelle method.11 Other studies report a pro-oxidant effect for 5 nm nanoceria synthesized by a hydrothermal process.10 Pro and antioxidant effects in brain after 30 days administration were evaluated by measuring levels of protein carbonyls, 3-nitrotyrosine, protein-bound-4-hydroxy-2-trans-nonenal in the hippocampus, cortex and cerebellum, glutathione reductase, glutathione peroxidase, SOD, catalase and inducible nitric oxide.10,92 ROS accumulation and oxidative damage was also observed in a study on Caenorhabditis elegans for low concentration nanoceria exposure (< 1 nM with 8.5 nm particles), assessed using an ABTS assay.9
Potential causes for the large variability of reported results include variations in particle coating, size, exposure conditions, biological model used and dose. Methodological limitations should also be considered. In spite of well-established procedures, these methods have a series of limitations when applied to analysis of samples containing colloidal particles. Many experiments are carried out with nanoceria dispersed in complex media, some of which contain oxidizing or reducing agents that may interact with the particles, or with the induced or generated ROS/RNS species, affecting data accuracy and interpretation.94 Moreover, proteins of the medium can bind to particles forming a protein coating that may change the surface chemistry and potentially alter reactivity or induce NP aggregation.49 Little work has been done to establish how dispersion of nanoceria in cell culture medium affects particle reactivity with respect to ROS/RNS species and composition of the medium. Most of these assays involve the use of redox dyes to provide absorbance or fluorescence signals related to ROS/RNS species.95 Potential interactions and cross-reactivity between these dyes and the particles should be tested.96 Some biochemical assays (e.g. SOD activity tests) use enzymes that when added to a nanoceria dispersion can induce aggregation. In some cases samples measured by these assays are extremely complex containing mixture of redox active nanoceria, enzyme, reactive ROS/RNS species (or products associated with oxidative damage) and organic dyes all of which can enter in competitive redox reactions, making proper quantification particularly challenging.39,96 Particle concentrations and incubation times can also affect spectroscopic readings. The variability can be due not only to the use of different assays but also to variability among experimental parameters within the same procedure. Unfortunately variability of analysis on these parameters is not discussed in published literature. Even if particles are not aggregated and there is no cross-reactivity, both spectroscopic and fluorescence measurements are carried out with colloidal particle dispersions where there could be significant light scattering. Control experiments should be carefully run to determine background changes and identify potential interferences to prevent artifactual interpretation. Systematic studies of such effects would be relevant for future research activities in this area.
3.3. Real time quantitative assessment of localized oxidative and neurotoxic response
Most conventional methodologies used to assess reactivity and impact of ceria NPs do not provide real time quantitative information and have limitations in terms of sensitivity, selectivity as well as spatial and temporal resolution. A powerful, yet underutilized method that can be used to assess impact of environmental exposure to NPs is electrochemistry.82 Advantages of electrochemical methods include real-time detection at low cost, using relatively simple and inexpensive procedures and with minimum disturbance of the sample. Electrochemical measurements can be used to determine redox active reagents that are directly involved in the reaction mechanism of nanoceria, or can be used to study biochemical and cellular events (e.g. cell or tissue response) involved in the NP exposure. Such measurements can be adapted to a variety of environmental samples, biological media, single cells and tissues as well as whole animals. Measurements can be performed at a predetermined location and sensors can be inserted at localized regions within intact organisms. For example, a Pt modified microeloectrode with a diameter of ∼125 μm, a linear range between 0.01 μM and 600 μM and a detection limit of 1 nM allowed real time quantitative monitoring of NO in a brain slice model over a period of three hours.76 A carbon fiber microelectrode with a diameter of 5 μm inserted in the intestine of zebrafish embryos allowed quantitative assessment of changes in the physiological NO level.82 Similar methodologies with electrodes modified with catalytic materials for signal amplification and semipermeable membranes to allow passage of the small radicals and prevent interferences from larger molecules to measure H2O2 ,97 O2˙−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 and ONOO−
77 and ONOO−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 have been reported.99,100 This section summarizes examples of recent and potential future uses of these methods to study nanoceria reactivity and effects, with focus on oxidative stress response (e.g. quantification of ROS/RNS species) and neurotoxic effects.
98 have been reported.99,100 This section summarizes examples of recent and potential future uses of these methods to study nanoceria reactivity and effects, with focus on oxidative stress response (e.g. quantification of ROS/RNS species) and neurotoxic effects.
ROS/RNS species such as NO, H2O2 and O2˙− can be oxidized or reduced at their characteristic potentials and therefore can be measured directly by electrochemical means. Such measurements can provide a real time concentration profile of the evolution of these species with high sensitivity.76 Other species present in the physiological environment such as catecholamine neurotransmitters can also be determined using direct or indirect electrochemical procedures, thus allowing direct assessment of potential neurotoxic effects. Electrochemical probes to measure the temporal and spatial concentration profile of these species and potentially of other markers of NPs exposure have been developed and can be used to analyze nanoceria effects. Electrochemical sensors for the detection of O2˙−, H2O2, NO and ONOO− in various environments have been reported76,101 and can be adapted to nano-exposure studies. These sensors are miniaturized, with sizes as low as 5 microns and can be customized for a particular environment and sample size. In particular, microelectrodes can be extremely useful for direct measurements of oxidative stress markers. ROS/RNS species are known to have a very short life time and dynamic levels and their dynamic profile is difficult to quantify by other methods. These methods can be used to obtain a quantitative response and a kinetic profile and can replace or complement conventional spectroscopic methodologies that are currently used for the detection of these species. In general direct electrochemical measurement of these species does not involve addition of reagents as most signal amplification and recognition reagents are immobilized onto the surface of the probe. Therefore, limitations of spectroscopic methods such as light scattering and cross-reactivity from organic chromophores are prevented.
In a recent study we have used NO specific carbon fiber microelectrodes inserted in the intestine of zebrafish embryos to obtain quantitative measurements of changes in the NO level resulting from exposure to nanoceria to assess changes in the oxidative system and intestinal physiology. Low nanoceria concentration exposure (1 ppm) decreased the physiological NO level indicating a scavenging effect. However, higher doses of NPs (>10 ppm) increased the NO level indicating potential for nitrosative damage.82 These results have provided a quantitative relationship between the level of nanoceria exposure and the intestinal NO, demonstrating strong concentration dependent changes. In another work we have used a cytochrome c biosensor to monitor real time release and inactivation of O2˙− radicals over a period of 4 h in acutely prepared brain slices exposed to commercial nanoceria with an average diameter of 15 nm. The procedure allowed us to determine both the amount and the kinetic profile of the generation and inactivation of O2˙− radicals and estimate the free radical scavenging activity of these particles directly in the biological tissue.102 A O2˙− anion radical scavenging activity of the 15 nm nanoceria equivalent to 527 U of SOD for each 1 μg ml−1 of nanoceria added was determined using this experimental set up.102 These results demonstrated a O2˙− scavenging activity and suggested possible neuroprotective effects in a brain slice model of ischemia (for the level of nanoceria concentration tested). Such measurements can be used to provide a greater understanding of the role of NO, O2˙− and potentially of other oxidative species such as ONOO− on organ system functions exposed to nanoceria and determine the role of oxidative stress in nanoceria exposure studies.
In other works, differential pulse voltammetry (DPV) with a 5-HT specific carbon fiber microelectrode (CFME) inserted in the zebrafish intestine has been used to determine changes in the physiological 5-HT level as a result on nanoceria exposure.40Fig. 3 shows examples of electrochemical DPV readings of intestinal 5-HT in embryos exposed to various NP concentrations. The peak at 0.39 V corresponds to the oxidation potential of 5-HT. The intensity of the current at this potential was used to determine the level of 5-HT. Exposure of embryos to 20 and 50 ppm ceria NPs (10–30 nm Sky Spring Nanomaterials Inc., Houston, TX) decreased the 5-HT level in the intestine to 20.5 (±1.3) and 5.3 (±1.5) nM respectively as compared to 30.8 (±3.4) nM for unexposed embryos, indicating depletion of the 5-HT level for exposure periods longer than three days (Fig. 3). However, exposure to 100 ppm ceria NPs had little effect at this particle concentration, which was attributed to increased aggregation of the NPs in the exposure medium. The results, combined with spectroscopic and surface characterization methods demonstrate that nanoceria interacts with 5-HT and forms a surface adsorbed 5-HT-nanoceria complex (Fig. 1). Such findings suggest that nanoceria could have potential neurotoxic effects and highlights the need for future studies to assess long term neurophysiological effects of nanoceria exposure and their impact in living organisms.
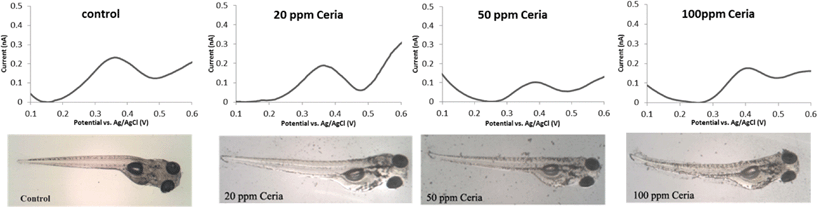 | ||
| Fig. 3 Differential pulse voltammograms and microscope images of embryos embryos treated with 0, 20, 50 and 100 ppm nanoceria. Microelectrodes were implanted to mid-intestine of live embryos fixed on an agar plate and electrochemical measurements were carried in E3 medium (with permission from ref. 40). | ||
3.4. Predictive tools for screening and assessing nanoceria reactivity
Although a number of characterization methods are available, there is a need to develop methodologies to accelerate screening and assess surface reactivity of the large number of nanoceria particles that are being developed. Many of the published studies involve extensive cell culture or animal work that are expensive and time consuming. Since both the reactivity and environmental impact varies with the surface properties and reactivity of these particles, and some change with the exposure and storage conditions, the development of predictive tools for assessing nanoceria reactivity in various environments using rapid and simple procedures would be highly valuable. Such methods would also be useful in the identification of parameters that contribute to reactivity changes (e.g. pH, ionic strength, storage conditions, and constituents of environmental matrices) and for the screening of large libraries of synthesized nanoceria candidates of various particle sizes and surface coatings for a particular application. In the biomedical field for example, rapid strategies to assess the antioxidant response of synthesized particles can help in the identification of suitable NP candidates to be utilized in protection of cells against ROS/RNS and in drug delivery systems for the treatment of oxidative stress related diseases. This approach would reduce the number of costly animal experiments. In the environmental field, assessing changes of surface properties and reactivity as a result of exposure to various environmental conditions and constituents would accelerate study of fundamental chemical processes and predict impact.An example of screening method is a recently developed zebrafish high throughput screening (HTS) assay. The method is based on bright-field and fluorescence images to determine observable defects such as reduction in hatching rate, vertebral malformation, and viability and accelerate nanotoxicity assessment of various NP types.103 Further developments of HTS methods can provide a rapid preliminary evaluation of the potential toxic effects of nanomaterials.
Fundamental electrochemical study of redox processes of the surface of nanoceria particles is another method that can provide rapid assessment of nanoceria reactivity and facilitate rapid screening using an easy-to-use procedure and inexpensive instrumentation. Recent work in electrochemistry has illustrated the applicability of this method to assess physicochemical and catalytic properties, particle size, particle reactivity and surface coatings through a newly developed electrochemical collision technique.104,105 The method is based on monitoring redox processes between a NP and a microelectrode as the particle interacts and collide with the microelectrode. Changes in the collision signals have been associated with the NPs size and coating, agglomeration degree and concentration. The method has been applied for study of catalytic and surface properties of various types of catalytic NPs.104,106–111 We have recently reported electrochemical collision studies of the interaction of nanoceria with ROS species.7 Using Pt microelectrodes, we were able to measure the presence of surface bound superoxo and peroxo anions on nanoceria following exposure to H2O2. Fig. 4 shows the method principle and provides an example of representative electrochemical collision recordings. The ability of the particles to bind and inactivate ROS was correlated with the collision profile. The electrochemical collision spike used for quantification originates from the reduction of surface bound-oxygen species. An increase in spike current frequency indicates a higher number of surface-adsorbed ROS on the NP surface. The specificity of measurements for the adsorbed oxygen species was assessed with resveratrol that has a high inactivation behavior against ROS. Ceria NPs exposed to H2O2 and treated with resveratrol had significantly lower number of spikes suggesting inactivation of surface bound O2˙− (Fig. 4C). The results suggest that the collision current can be used to assess surface adsorbed ROS. The results showed good correlation with conventional SOD reactivity tests of O2˙− scavenging activity of these particles. This method can be applied to screen particles for their ability to inactivate (or release) ROS and assist with prior selection of ceria NPs candidates before more extensive experiments are being performed. Since both antioxidant and pro-oxidant effects of nanoceria vary largely with the concentration and surface coatings of the NPs, this method can be particularly useful to assess these parameters and provide evaluation of physicochemical properties including size, surface coating and reactivity in various environmental conditions.
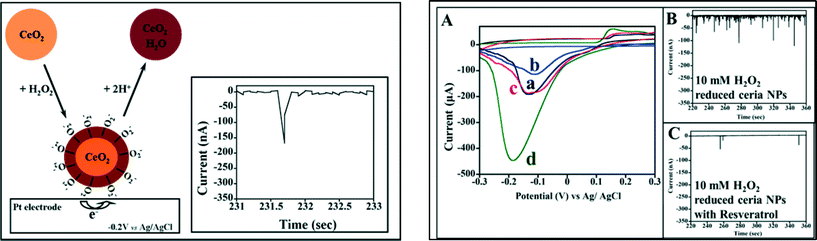 | ||
| Fig. 4 Left – schematic illustration of single ceria NP collision event and the reduction current “spike” for Ce–O2− NPs in contact with a Pt microelectrode with a diameter of 125 μm. Right – A) CVs of PtME at a scan rate of 100 mV s−1 in absence of ceria NPs (a) and presence of: ceria NPs (b), H2O2 reduced ceria NPs treated with 50 mM resveratrol (c), and H2O2 reduced ceria NPs (d) (B, C) chronoamperometric plots of NP collisions for particles treated with 10 mM H2O2 (30 min) without (B) and with (C) resveratrol treatment (from ref. 7 with permission). | ||
4. Conclusions and future outlook
Nanoceria particles have already had a major impact in the biomedical and environmental fields. The many beneficial uses of nanoceria derive from their unusual physicochemical properties and surface reactivity which are unique to their nanoparticulate form. At the same time, while useful, these properties pose significant challenges as both the surface reactivity and catalytic properties can interfere with environmental and biological processes. Although a great deal has already been learned about nanoceria, studies of the chemical and surface reactivity, fate, transport, bioavailability and toxicity in relation with their physicochemical properties are still in their infancy.Several trends are beginning to be observed including: (1) the dependency of the chemical reactivity on the particle size with smaller particles being more reactive,36 (2) the presence of natural organic matter and ionic strength determine electrophoretic mobility, transport characteristics and toxicity of these particles in the environment,49 (4) particle coatings are needed to prevent aggregation;57 coated particles seem to conserve reactivity against H2O2,90 (5) uptake and cellular toxicity depend on physicochemical properties and surface modification,21 (6) the radical scavenging effect is a common feature for a variety of nanoceria particles; the effects are dependent on the particle concentration and size,26,82 (7) several possible mechanisms have been postulated including: ionic contribution, creation of oxygen vacancies, surface complexation, charge delocalization,7,16,20,30,33 (8) there is no single best technique for nanoceria characterization; a complementary multi-method approach is recommended for physicochemical characterization and assessment of the reactivity and effects of these particles.70
Fundamental and systematic studies are needed in the future to identify key factors in the synthetic design that can be used to predict reactivity, permit targeted screening and allow generation of safer particles of desired properties and controlled environmental impact. In the biological field, development of novel synthetic pathways and surface chemistry to prevent aggregation and facilitate biological transport for more efficient delivery, as well as design of novel assays which can more accurately and reliably determine their antioxidants or pro-oxidant effects are needed. As more knowledge of structure-activity relationships is gained, rational engineering of more powerful synthetic nanoceria antioxidants might be possible. In the environmental field, transformations of nanoceria in relevant environmental conditions and the interaction of these particles with environmental constituents need to be studied in greater detail. Several specific needs can be pointed out. (1) Work is still needed to determine the origin of nanoceria reactivity (e.g. changes in the oxidation state, oxygen vacancies, surface complexation or a combination mechanism). (2) Before further applications are explored, there needs to be studies of the interaction of nanoceria with redox active components (beyond ROS/RNS species) and define potential effects. (3) Along the same line, it is still not clear whether and in what conditions nanoceria is a ROS scavenger versus a promoter of ROS generation. A quantitative relationship between the Ce(3+/4+) ratio, surface area and the concentration of ROS is needed to define such reactivity. (4) Formation of surface ligands with environmental and biological components and their contribution to the overall surface reactivity must be studied in both standard and environmentally realistic conditions. (5) The toxicological impact of these particles should be further assessed in relation to the physicochemical parameters and exposure conditions. (6) Development of new tools and methodologies for rapid assessment of nanoceria reactivity would be highly valuable to allow screening and accelerate study of these particles and their toxicological impact.
In summary, it is increasingly clear that nanoceria particles have great promise as active material in many practical applications in biomedicine, environmental remediation, catalysis, sensing, etc. As applications of nanoceria continues to increase and more particles are being found in the environment there is also a need to develop detection methodologies to assess exposure and occupational risks associated with the presence of these particles in the environment. Removal of nanoceria from the environment is another area that is lacking today and should be explored in future research activities. In summary, the use of nanoceria particles should be considered with full understanding of all their properties in order to balance antioxidant versus pro-oxidant effects, reduce environmental toxicity risks and ensure the safe implementation of these particles.
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grants 1336493, 1200180 and 0954919. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. SA acknowledges all past and present members of Andreescu's group for their contribution to the study of various aspects of nanoceria chemistry.References
- C. W. Sun, H. Li and L. Q. Chen, Energy Environ. Sci., 2012, 5, 8475–8505 CAS.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- F. Gottschalk, R. W. Scholz and B. Nowack, Environ. Modell. Softw., 2010, 25, 320–332 CrossRef PubMed.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15 Search PubMed.
- Z. C. Di, J. Ding, X. J. Peng, Y. H. Li, Z. K. Luan and J. Liang, Chemosphere, 2006, 62, 861–865 CrossRef CAS PubMed.
- N. P. Sardesai, D. Andreescu and S. Andreescu, J. Am. Chem. Soc., 2013, 135, 16770–16773 CrossRef CAS PubMed.
- X. J. Peng, Z. K. Luan, J. Ding, Z. H. Di, Y. H. Li and B. H. Tian, Mater. Lett., 2005, 59, 399–403 CrossRef CAS PubMed.
- H. F. Zhang, X. A. He, Z. Y. Zhang, P. Zhang, Y. Y. Li, Y. H. Ma, Y. S. Kuang, Y. L. Zhao and Z. F. Chai, Environ. Sci. Technol., 2011, 45, 3725–3730 CrossRef CAS PubMed.
- S. S. Hardas, R. Sultana, G. Warrier, M. Dan, R. L. Florence, P. Wu, E. A. Grulke, M. T. Tseng, J. M. Unrine, U. M. Graham, R. A. Yokel and D. A. Butterfield, NeuroToxicology, 2012, 33, 1147–1155 CrossRef CAS PubMed.
- C. K. Kim, T. Kim, I. Y. Choi, M. Soh, D. Kim, Y. J. Kim, H. Jang, H. S. Yang, J. Y. Kim, H. K. Park, S. P. Park, S. Park, T. Yu, B. W. Yoon, S. H. Lee and T. Hyeon, Angew. Chem., Int. Ed., 2012, 51, 11039–11043 CrossRef CAS PubMed.
- M. Das, S. Patil, N. Bhargava, J. F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS PubMed.
- A. Y. Estevez, S. Pritchard, K. Harper, J. W. Aston, A. Lynch, J. J. Lucky, J. S. Ludington, P. Chatani, W. P. Mosenthal, J. C. Leiter, S. Andreescu and J. S. Erlichman, Free Radical Biol. Med., 2011, 51, 1155–1163 CrossRef CAS PubMed.
- J. M. Dowding, S. Das, A. Kumar, T. Dosani, R. McCormack, A. Gupta, T. X. T. Sayle, D. C. Sayle, L. von Kalm, S. Seal and W. T. Self, ACS Nano, 2013, 7, 4855–4868 CrossRef CAS PubMed.
- A. S. Karakoti, P. Munusamy, K. Hostetler, V. Kodali, S. Kuchibhatla, G. Orr, J. G. Pounds, J. G. Teeguarden, B. D. Thrall and D. R. Baer, Surf. Interface Anal., 2012, 44, 882–889 CrossRef CAS PubMed.
- G. Preda, A. Migani, K. M. Neyman, S. T. Bromley, F. Illas and G. Pacchioni, J. Phys. Chem. C, 2011, 115, 5817–5822 CAS.
- C. T. Sun and D. F. Xue, Phys. Chem. Chem. Phys., 2013, 15, 14414–14419 RSC.
- C. Loschen, A. Migani, S. T. Bromley, F. Illas and K. M. Neyman, Phys. Chem. Chem. Phys., 2008, 10, 5730–5738 RSC.
- N. V. Skorodumova, S. I. Simak, B. I. Lundqvist, I. A. Abrikosov and B. Johansson, Phys. Rev. Lett., 2002, 89 Search PubMed.
- S. Deshpande, S. Patil, S. V. N. T. Kuchibhatla and S. Seal, Appl. Phys. Lett., 2005, 87 Search PubMed.
- H. Zou, Y. S. Lin, N. Rane and T. He, Ind. Eng. Chem. Res., 2004, 43, 3019–3025 CrossRef CAS.
- X. W. Liu, K. B. Zhou, L. Wang, B. Y. Wang and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 3140–3141 CrossRef CAS PubMed.
- Q. Yuan, H. H. Duan, L. L. Li, L. D. Sun, Y. W. Zhang and C. H. Yan, J. Colloid Interface Sci., 2009, 335, 151–167 CrossRef CAS PubMed.
- A. Bumajdad, J. Eastoe and A. Mathew, Adv. Colloid Interface Sci., 2009, 147–148, 56–66 CrossRef CAS PubMed.
- E. Sharpe, T. Frasco, D. Andreescu and S. Andreescu, Analyst, 2013, 138, 249–262 RSC.
- S. A. Hayes, P. Yu, T. J. O'Keefe, M. J. O'Keefe and J. O. Stoffer, J. Electrochem. Soc., 2002, 149, C623–C630 CrossRef CAS PubMed.
- R. E. Ozel, R. S. J. Alkasir, K. Ray, K. N. Wallace and S. Andreescu, Small, 2013, 9, 4250–4261 CrossRef PubMed.
- P. Yu, S. A. Hayes, T. J. O'Keefe, M. J. O'Keefe and J. O. Stoffer, J. Electrochem. Soc., 2006, 153, C74–C79 CrossRef CAS PubMed.
- C. Ispas, J. Njagi, M. Cates and S. Andreescu, J. Electrochem. Soc., 2008, 155, F169–F176 CrossRef CAS PubMed.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- I. Celardo, J. Z. Pedersen, E. Traversa and L. Ghibelli, Nanoscale, 2011, 3, 1411–1420 RSC.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058 RSC.
- J. D. Cafun, K. O. Kvashnina, E. Casals, V. F. Puntes and P. Glatzel, ACS Nano, 2013, 7, 10726–10732 CrossRef CAS PubMed.
- Y. M. Choi, H. Abernathy, H. T. Chen, M. C. Lin and M. L. Liu, ChemPhysChem, 2006, 7, 1957–1963 CrossRef CAS PubMed.
- M. Ornatska, E. Sharpe, D. Andreescu and S. Andreescu, Anal. Chem., 2011, 83, 4273–4280 CrossRef CAS PubMed.
- S. S. Lee, W. S. Song, M. J. Cho, H. L. Puppala, P. Nguyen, H. G. Zhu, L. Segatori and V. L. Colvin, ACS Nano, 2013, 7, 9693–9703 CrossRef CAS PubMed.
- E. Sharpe, R. Bradley, T. Frasco, D. Jayathilaka, A. Marsh and S. Andreescu, Sens. Actuators, B, 2014, 193, 552–562 CrossRef CAS PubMed.
- A. Hayat and S. Andreescu, Anal. Chem., 2013, 85, 10028–10032 CrossRef CAS PubMed.
- A. Hayat, B. Gonca and S. Andreescu, Biosens. Bioelectron., 2014, 56, 334–339 CrossRef CAS PubMed.
- R. E. Ozel, A. Hayat, K. N. Wallace and S. Andreescu, RSC Adv., 2013, 3, 15298–15309 RSC.
- R. E. Ozel, K. N. Wallace and S. Andreescu, Anal. Chim. Acta, 2011, 695, 89–95 CrossRef CAS PubMed.
- A. Hayat, D. Andreescu, G. Bulbul and S. Andreescu, J. Colloid Interface Sci., 2014, 418, 240–245 CrossRef CAS PubMed.
- R. Pautler, E. Y. Kelly, P. J. Huang, J. Cao, B. Liu and J. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 6820–6825 CAS.
- P. J. Huang, J. Lin, J. Cao, M. Vazin and J. Liu, Anal. Chem., 2014, 86, 1816–1821 CrossRef CAS PubMed.
- F. R. Cassee, E. R. van Balen, C. Singh, D. Green, H. Muijser, J. Weinstein and K. Dreher, Crit. Rev. Toxicol., 2011, 41(3), 213–229 CrossRef PubMed.
- A. A. Keller, H. T. Wang, D. X. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. X. Ji, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- B. Collin, E. Oostveen, O. V. Tsyusko and J. M. Unrine, Environ. Sci. Technol., 2014, 48, 1280–1289 CrossRef CAS PubMed.
- C. Gunavan, M. Lim, C. P. Marquis and R. Amal, J. Mater. Chem. B, 2014, 2, 2060–2083 RSC.
- S. Patil, A. Sandberg, E. Heckert, W. Self and S. Seal, Biomaterials, 2007, 28, 4600–4607 CrossRef CAS PubMed.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753 CrossRef CAS PubMed.
- S. V. N. T. Kuchibhata, A. S. Karakoti, D. R. Baer, S. Samudrala, M. H. Engelhard, J. E. Amonette, S. Thevuthasan and S. Seal, J. Phys. Chem. C, 2012, 116, 14108–14114 Search PubMed.
- R. C. Merrifield, Z. W. Wang, R. E. Palmer and J. R. Lead, Environ. Sci. Technol., 2013, 47, 12426–12433 CrossRef CAS PubMed.
- S. Jakab, S. Picart, B. Tribollet, P. Rousseau, H. Perrot and C. Gabrielli, Anal. Chem., 2009, 81, 5139–5145 CrossRef CAS PubMed.
- S. Tamilmani, V. Lowalekar, S. Raghavan and R. Small, Solid State Phenom., 2005, 103–104, 283–286 CrossRef CAS PubMed.
- M. Virot, T. Chave, D. Horlait, N. Clavier, N. Dacheux, J. Ravaux and S. I. Nikitenko, J. Mater. Chem., 2012, 22, 14734–14740 RSC.
- D. Andreescu, E. Matijevic and D. V. Goia, Colloids Surf., A, 2006, 291, 93–100 CrossRef CAS PubMed.
- E. J. Salazar-Sandoval, M. K. G. Johansson and A. Ahniyaz, RSC Adv., 2014, 4, 9048–9055 RSC.
- Z. A. Qiao, Z. Wu and S. Dai, ChemSusChem, 2013, 6, 1821–1833 CrossRef CAS PubMed.
- R. H. Li, Q. Li, S. Gao and J. K. Shang, Chem. Eng. J., 2012, 185, 127–135 CrossRef PubMed.
- Z. F. Zhang, L. Yu, W. L. Liu and Z. T. Song, Appl. Surf. Sci., 2010, 256, 3856–3861 CrossRef CAS PubMed.
- L. Zhang, J. Pierce, V. L. Leung, D. Wang and W. S. Epling, J. Phys. Chem. C, 2013, 117, 8282–8289 CAS.
- J. Njagi, C. Ispas and S. Andreescu, Anal. Chem., 2008, 80, 7266–7274 CrossRef CAS PubMed.
- A. F. Santiago, J. F. Sousa, R. C. Guedes, C. E. Jeronimo and M. Benachour, J. Hazard. Mater., 2006, 138, 325–330 CrossRef CAS PubMed.
- L. Chang, I. P. Chen and S. S. Lin, Chemosphere, 2005, 58, 485–492 CrossRef CAS PubMed.
- S. S. Lin, C. L. Chen, D. J. Chang and C. C. Chen, Water Res., 2002, 36, 3009–3014 CrossRef CAS.
- Q. Z. Feng, Z. Y. Zhang, Y. H. Ma, X. He, Y. L. Zhao and Z. F. Chai, Nanoscale Res. Lett., 2012, 7, 1–8 CrossRef PubMed.
- D. Wilson, W. Wang and R. J. G. Lopes, Appl. Catal., B, 2012, 123, 273–281 CrossRef PubMed.
- A. D. Mayernick, R. Li, K. M. Dooley and M. J. Janik, J. Phys. Chem. C, 2011, 115, 24178–24188 CAS.
- J. He, G. K. Reddy, S. W. Thiel, P. G. Smirniotis and N. G. Pinto, J. Phys. Chem. C, 2011, 115, 24300–24309 CAS.
- M. Baalousha, Y. Ju-Nam, P. A. Cole, B. Gaiser, T. F. Fernandes, J. A. Hriljac, M. A. Jepson, V. Stone, C. R. Tyler and J. R. Lead, Environ. Toxicol. Chem., 2012, 31, 983–993 CrossRef CAS PubMed.
- M. Baalousha, Y. Ju-Nam, P. A. Cole, J. A. Hriljac, I. P. Jones, C. R. Tyler, V. Stone, T. F. Fernandes, M. A. Jepson and J. R. Lead, Environ. Toxicol. Chem., 2012, 31, 994–1003 CrossRef CAS PubMed.
- M. Baalousha, M. Motelica-Heino, S. Galaup and P. Le Coustumer, Microsc. Res. Tech., 2005, 66, 299–306 CrossRef CAS PubMed.
- K. J. Wilkinson, E. Balnois, G. G. Leppard and J. Buffle, Colloids Surf., A, 1999, 155, 287–310 CrossRef CAS.
- E. Beche, P. Charvin, D. Perarnau, S. Abanades and G. Flamant, Surf. Interface Anal., 2008, 40, 264–267 CrossRef CAS.
- J. F. L. Duval, K. J. Wilkinson, H. P. Van Leeuwen and J. Buffle, Environ. Sci. Technol., 2005, 39, 6435–6445 CrossRef CAS.
- J. Njagi, J. S. Erlichman, J. W. Aston, J. C. Leiter and S. Andreescu, Sens. Actuators, B, 2010, 143, 673–680 CrossRef CAS PubMed.
- M. Ganesana, J. S. Erlichman and S. Andreescu, Free Radical Biol. Med., 2012, 53, 2240–2249 CrossRef CAS PubMed.
- Y. Xue, Q. F. Luan, D. Yang, X. Yao and K. B. Zhou, J. Phys. Chem. C, 2011, 115, 4433–4438 CAS.
- J. M. Dowding, S. Seal and W. T. Self, Drug Delivery Transl. Res., 2013, 3, 375–379 CrossRef CAS PubMed.
- Y. Q. Guo, K. U. Kim, I. J. Lee, J. I. Yoder and D. H. Shin, Allelopathy J., 2008, 22, 371–378 Search PubMed.
- J. M. Dowding, T. Dosani, A. Kumar, S. Seal and W. T. Self, Chem. Commun., 2012, 48, 4896–4898 RSC.
- R. E. Özel, X. Liu, R. S. Alkasir and S. Andreescu, TrAC, Trends Anal. Chem., 2014, 59, 112–120 CrossRef PubMed.
- S. Babu, A. Velez, K. Wozniak, J. Szydlowska and S. Seal, Chem. Phys. Lett., 2007, 442, 405–408 CrossRef CAS PubMed.
- F. A. Villamena and J. L. Zweier, Antioxid. Redox Signaling, 2004, 6, 619–629 CrossRef CAS PubMed.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. S. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- Y. Y. Tsai, J. Oca-Cossio, S. M. Lin, K. Woan, P. C. Yu and W. Sigmund, Nanomedicine, 2008, 3, 637–645 CrossRef CAS PubMed.
- D. J. Huang, B. X. Ou and R. L. Prior, J. Agric. Food Chem., 2005, 53, 1841–1856 CrossRef CAS PubMed.
- C. I. Covaliu, C. Matei, S. Litescu, S. A. M. Eremia, N. Stanica, L. Diamandescu, A. Ianculescu, I. Jitaru and D. Berger, Mater. Plast., 2010, 47, 5–10 CAS.
- H. J. Eom and J. Choi, Toxicol. Lett., 2009, 187, 77–83 CrossRef CAS PubMed.
- M. S. Lord, M. S. Jung, W. Y. Teoh, C. Gunawan, J. A. Vassie, R. Amal and J. M. Whitelock, Biomaterials, 2012, 33, 7915–7924 CrossRef CAS PubMed.
- S. S. Lee, H. G. Zhu, E. Q. Contreras, A. Prakash, H. L. Puppala and V. L. Colvin, Chem. Mater., 2012, 24, 424–432 CrossRef CAS.
- R. A. Yokel, R. L. Florence, J. M. Unrine, M. T. Tseng, U. M. Graham, P. Wu, E. A. Grulke, R. Sultana, S. S. Hardas and D. A. Butterfield, Nanotoxicology, 2009, 3, 234–248 CrossRef CAS.
- J. W. Zmijewski, A. Landar, N. Watanabe, D. A. Dickinson, N. Noguchi and V. M. Darley-Usmar, Biochem. Soc. Trans., 2005, 33, 1385–1389 CrossRef CAS PubMed.
- V. Stone and K. Donaldson, Nat. Nanotechnol., 2006, 1, 23–24 CrossRef CAS PubMed.
- A. Arya, N. K. Sethy, S. K. Singh, M. Das and K. Bhargava, Int. J. Nanomed., 2013, 8, 4507–4519 Search PubMed.
- B. Kalyanaraman, V. Darley-Usmar, K. J. A. Davies, P. A. Dennery, H. J. Forman, M. B. Grisham, G. E. Mann, K. Moore, L. J. Roberts and H. Ischiropoulos, Free Radical Biol. Med., 2012, 52, 1–6 CrossRef CAS PubMed.
- M. Cortina-Puig, A. C. H. Scangas, Z. S. Marchese, S. Andreescu, J. L. Marty and C. Calas-Blanchard, Electroanalysis, 2010, 22, 2429–2433 CrossRef CAS.
- S. F. Peteu, T. Bose and M. Bayachou, Anal. Chim. Acta, 2013, 780, 81–88 CrossRef CAS PubMed.
- M. I. Song, F. F. Bier and F. W. Scheller, Bioelectrochem. Bioenerg., 1995, 38, 419–422 CrossRef CAS.
- V. Lvovich and A. Scheeline, Anal. Chim. Acta, 1997, 354, 315–323 CrossRef CAS.
- F. Bedioui and S. Griveau, Electroanalysis, 2013, 25, 587–600 CrossRef CAS.
- M. Ganesana, J. S. Erlichman and S. Andreescu, Free Radical Biol. Med., 2012, 53, 2240–2249 CrossRef CAS PubMed.
- S. Lin, Y. Zhao, T. Xia, H. Meng, Z. Ji, R. Liu, S. George, S. Xiong, X. Wang, H. Zhang, S. Pokhrel, L. Mädler, R. Damoiseaux, S. Lin and A. E. Nel, ACS Nano, 2011, 5, 7284–7295 CrossRef CAS PubMed.
- X. Y. Xiao and A. J. Bard, J. Am. Chem. Soc., 2007, 129, 9610–9612 CrossRef CAS PubMed.
- E. J. Stuart, K. Tschulik, D. Omanovic, J. T. Cullen, K. Jurkschat, A. Crossley and R. G. Compton, Nanotechnology, 2013, 24, 444002 CrossRef CAS PubMed.
- H. J. Zhou, J. H. Park, F. R. F. Fan and A. J. Bard, J. Am. Chem. Soc., 2012, 134, 13212–13215 CrossRef CAS PubMed.
- S. J. Kwon and A. J. Bard, J. Am. Chem. Soc., 2012, 134, 7102–7108 CrossRef CAS PubMed.
- S. J. Kwon, F. R. F. Fan and A. J. Bard, J. Am. Chem. Soc., 2010, 132, 13165–13167 CrossRef CAS PubMed.
- F. R. F. Fan and A. J. Bard, Nano Lett., 2008, 8, 1746–1749 CrossRef CAS PubMed.
- S. E. F. Kleijn, S. C. S. Lai, T. S. Miller, A. I. Yanson, M. T. M. Koper and P. R. Unwin, J. Am. Chem. Soc., 2012, 134, 18558–18561 CrossRef CAS PubMed.
- J. M. Kahk, N. V. Rees, J. Pillay, R. Tshikhudo, S. Vilakazi and R. G. Compton, Nano Today, 2012, 7, 174–179 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |
