Behavior of nanoceria in biologically-relevant environments
Amit
Kumar
a,
Soumen
Das
b,
Prabhakaran
Munusamy
c,
William
Self
d,
Donald R.
Baer
c,
Dean C.
Sayle
e and
Sudipta
Seal
*abf
aAdvanced Materials Processing and Analysis Centre, University of Central Florida, Orlando, FL, USA. E-mail: Sudipta.Seal@ucf.edu
bNanoScience Technology Centre, University of Central Florida, Orlando, FL, USA
cEnvironmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA, USA
dBurnett School of Biomedical Sciences, University of Central Florida, Orlando, FL, USA
eSchool of Physical Sciences, University of Kent, Canterbury, Kent CT2 7NZ, UK
fCollege of Medicine, University of Central Florida, Orlando, FL, USA
First published on 8th September 2014
Abstract
Cerium oxide nanoparticles (nanoceria) have gained considerable attention in biological research due to their anti-oxidant like behaviour and regenerative nature. The current literature on nanoceria reports many successful attempts on harnessing the beneficial therapeutic properties in biology. However studies have also shown toxicity with some types of nanoceria. This article discusses issues associated with the behaviours of nanoceria in biological systems and identifies key knowledge gaps. We explore how salient physico-chemical properties (size, surface chemistry, surface stabilizers) of nanoceria corresponds to its behaviour in biological relevant buffers and cell culture media, and this can provide guidelines for potential positive and negative aspects of nanoceria in biological systems. Based on variations of results reported in the literature, important issues need to be addressed. Are we really studying the same particles with slight variations in size and physico-chemical properties or do the particles being examined have fundamentally different behaviours? Are the variations observed the result of differences in the initial properties of the particles or the results of downstream effects that emerge as the particles are prepared for specific studies and they interact with biological or other environmental moieties? How should particles be appropriately prepared for relevant environmental/toxicology/safety studies? It is useful to recognize that nanoparticles encompass some of the same complexities and variability associated with biological components.
Nano impactThe redox based regenerative anti-oxidant property of cerium oxide nanoparticles (nanoceria, CeO2) has generated deep interest among researchers as a potential tools for medical applications. However, the observed therapeutic properties of nanoceria can be significantly influenced by several environmental factors including its interaction with the biological system. In this report, we discuss the environmental factors which can alter the physicochemical properties of nanoceria and modify its behavior within biological systems. This article provides insight to identify and in many cases control the environmental effects of nanoceria for any biologically relevant application including regenerative nanomedicine. |
Introduction
The advancement of nanotechnology has provided new or alternative solutions to many issues facing society. One such field happens to be health. Nanoparticles are being extensively researched for use in drug delivery, diagnostic and therapeutic imaging, as biosensors, and to enable high-resolution neural interfacing, and photoactivated interfaces.1–5 Understanding the fate of nanoparticles in the environment and their interactions with and within biological system is the key to both useful application and regulation of nanomaterials.6–9 Like pharmaceuticals, various engineered nanomaterials are known to be advantageous in controlling disease but can still be observed to affect human health adversely. Nanomaterials, with potential application as bio-medicinal agents, may exploit the chemical properties characteristic of a solid, but have the ability to be transported – like a large molecule – to a variety of bodily compartments.10 This opens several new approaches for functional materials design, (i) exploit change in properties of a nanoscale in comparison to properties at bulk material; (ii) take advantage of the ability to tune material properties as a function of composition, structure and size.There are an unprecedented number of potential nanomaterials that can manifest as potential therapeutic/toxic agents. Typical (molecular) therapeutic agents, for example aspirin (acetylsalicylic acid) are normally structurally invariant. Conversely, nanoceria as a potential therapeutic agent can exhibit wide structural distributions, which will influence their physicochemical properties with implications for their therapeutic potentials.11–13 In this article we present the current state of understanding and ability to control cerium oxide nanoparticles (nanoceria) properties which will impact their role as in biological system.
The behaviour of any type of nanoparticle depends upon the physical and chemical characteristics of the particles. There are several issues needed to be addressed or at least acknowledged:
i. Nanoparticles that are thought to be “equivalent” may have significantly different properties because of major or subtle differences in their synthesis or processing history.
ii. Often the particles have one set of characteristics and properties when they are synthesized, but these characteristics often evolve with time as the particles are stored, processed or released into a biological or other environment.
iii. The biological system used also plays a major role on how the nanoparticle behaves. For example, the transport and fate of a nanoparticle via oral ingestion in a zebrafish is likely quite different than the absorption and distribution of an aerosolized nanoparticle in the lung.
Many lists of important particle characteristics have been generated describing characteristics of importance for nanoparticles.14,15 Here we highlight that the size, shape, composition (bulk, phase, impurities) and nature of the particle surface can significantly alter particle behaviours. The cumulative physical and chemical properties of nanoparticles arise from the grain/particle properties, bulk and elemental composition, surface features, and surface charge. It is important to note that the physical and chemical properties of a nanoparticle may be entangled and difficult to distinguish. A specific morphology might result from a specific synthesis route that involves chemicals, temperatures and surfactants each of which might contribute to the resultant particle properties.
In many cases particles are not stable as stored, handled or processed for application. Particles purchased from vendors may no longer have the initial “ideal” properties after shipping and storage.16,17 One reason that nanoparticles are often different from when synthesized is that they generally respond to changes in their environment and may change in several ways.11,18 Therefore, it is important to recognize that particles are likely to change during biological interactions and understanding doses and biological impact may require knowing the types (and rates) of transformations that particles undergo during such processes.
For clarity in addressing nanoceria properties and their behaviours within biological systems, it is wise to understand not only the starting state of the particles, but what may happen to nanoparticles in any of the wide variety of environments that the particles may encounter in association with the preparation, delivery and transport within a biological system. Issues of importance include movement (forces), attachment (forces, binding, coatings), and transformation (dissolution, aggregation, reformation).
Nanoparticles chemistry within biological environment
Knowledge of how various environments affect nanoparticle surface chemistry is required to understand how nanoparticles interact with living systems. Just as nanoparticles are dynamic (especially those 10 nm or smaller), the cell surface is dynamic. Therefore the interaction of a cell with the outside environment may change the cell surface and impact the colloidal chemistry of nearby nanoparticles. Some of the complexity of nanomaterial physicochemical behavior may arise from the responses from cells which may further modify the microenvironment of nanoparticles, for example, TGFβ signalling in tumour cells modifies the micro-environment.19,20Cellular responses from the cellular uptake of nanoparticles will contribute further to the environment of nanoparticles outside the cell in addition to placing the captured particles into a new environment. The types of forces through which a nanoparticle interacts with its environment including van der Waals, electrostatic, and steric forces may be alternated by the dynamic environment around the nanoparticles (including the growth of surface layers such as protein coronas). Such altered states of nanoparticles before and with cell interaction can result in nanoparticle agglomeration and dissolution.21 It has been argued that nanoparticles are as responsive to their environment as proteins.22 Thus the physico-chemical properties of nanoparticles at biological interfaces are transient and heterogeneous in nature.
Although nanoparticles are often thought to be small and round, heterogeneity in nanoparticles arises from defects, edges, and crystalline facets.23 The morphology, surface features and surface active species of the nanoparticles determine the dynamic interaction with cell entities, tissues, membranes, proteins, endocytic vesicles, phospholipids organelles, DNA and biological fluids within the living system. Such interaction may lead to desirable or adverse outcomes with respect to biocompatibility or therapeutic applications.
Recent literature24 identifies three different nano–bio interfaces or zones: the nanoparticle surface, the solid–liquid interface around the nanoparticle, and the solid–liquid interface's contact zone with biological substrates. Considering only the nanoparticles to determine the initial or core characteristic properties, such as chemical composition, crystallinity, porosity, heterogeneity, and roughness is often essential. However some properties are only apparent in the environment of application. Which properties of nanoparticles are more critical will vary with the material system (metal, metal oxides) and with the application.
Surface functionalization is an important factor in determining many properties of nanoparticles. Species on the surface may be deliberately added to control particle behaviours such as aggregation and surface potential, or those picked from the surrounding environment. The characteristics of suspending media, such as pH, ionic strength, and temperature equally affect the behaviour of nanoparticles just like in a biological environment. With nanoparticle and medium as the system i.e. at the solid–liquid interface, the noticeable effects are particle aggregation, state of dispersion, stability/biodegradability, dissolution characteristics, hydration, effective surface charge (zeta potential) and valence of the surface layer. Thus the physicochemical property of an isolated nanoparticle may undergo changes when introduced in different media. The effective behaviour of the nanoparticle-media suspension will decide the further interaction at biological interfaces. The nanoparticle/medium can offer a surface for adsorption of ions, proteins, and natural organic materials; double layer formation; dissolution; or minimizing free surface energy by surface restructuring.24
In the following sections we consider a few examples of the how physicochemical properties of nanoparticles impact biological systems.
Surface charge
A nanoparticle suspended in water will have different interaction forces than suspended in a biological fluid. Consider an example of SiO2 nanoparticles which in contact with water may result in hydrated surfaces forming (Si–OH) groups which dissociate to result in negative surface charges. The repulsive electrostatic forces may keep the particles separate, however when suspended in a biological medium, which has high ionic strength, such electrostatic force may be shielded resulting in aggregation. The interaction of such nanoparticles with cells varies with the patchiness, non-rigid compliant membrane and heterogeneity of the cell surface. Surface charge of nanoparticles can also determine aqueous complexation or ligand enhanced dissolution, as observed for iron or zinc oxides.21,25Morphology
Shape, size, and aspect ratio are some of the characteristics which bring heterogeneity to the nanoparticle surface through which it interacts at the biological interface. Thus the interactions, such as cellular uptake and protein binding are strongly dependent on nanoparticle morphology. The apparent size (hydrodynamic radius) of nanoparticles is affected by the media parameters, such as pH, ionic strength. A few researchers have investigated a threshold radius for cellular entry of particles in nanometers and optimal particle size for cells to wrap a membrane around the particles during endocytosis/cell internalization.26–28 The threshold radius varies with particle shape (cylindrical and spherical) which is an indication to active contact points between nanoparticle and biological species vary with the aspect ratio.28 A few studies29,30 have shown, varying mechanisms (caveolae-mediated or clathrin-coated pits) for particle internalization depending on the size. The cell can condition the surface of nanoparticles, such as binding of proteins to the particle surface, which impacts the mechanism (endocytosis, particle phagocytosis, etc.) used for uptake of the nanoparticle. Particle shape is often critical for nanomaterials aimed for drug delivery design.Surface functionalization
The functional groups on the surface of nanoparticles affect particle interaction with the biological media and the interaction with cells. The surface charge, dynamic size, and biocompatibility of nanoparticles can be modified through this surface functionalization, which will affect the nanoparticle's behaviour based on the ligand size, ligand density, functional groups on surface, etc. Synthesis in different media – as discussed in a following section will determine the initial surface functionalization (which can be altered in various ways at later stages by processes such as ligand exchange).Functionalization will be impacted by particle morphology. Particles with high aspect ratio undergo longer wrapping times than spherical nanoparticles due to greater energy required for their engulfment, and therefore take longer time to internalize.31 Other than the aspect ratio/morphology of the particles, surface charge and/or presence of functional molecules also influence particle wrapping and therefore cellular internalization. Some studies32 have suggested high aspect ratio cationic PEG hydrogel particles undergo internalization rapidly. However it has also been shown that transferrin particle wrapping is slower in comparison to bare gold nanoparticles as compared to transferrin functionalized gold particles. The slower wrapping of transferrin coated gold nanoparticles may be attributed to increased particle size leading slower diffusion kinetics.31 Surface functionalization with PEG has also shown to increase circulation time and modify the bio-distribution of nanoparticles, by affecting the plasma protein absorption on PEG functionalized nanoparticles.
Synthesis dependent physicochemical properties of cerium oxide nanoparticles
Cerium compounds are known as antiemetic, bactericidal, bacteriostatic, antineoplastic agents and have been used as drug components and even marketed viz. dymal, ceolat, ceriform, introcid.33–37 Many technological applications of nanoceria, such as in solid oxide fuel cells,38 sensors,39 chemical mechanical planarization,40 catalyst,41 and UV-shielding,42 arise from the high oxygen mobility facilitated by the redox changes within the CeO2 lattice. Cerium exhibits dual valence state of +4 and +3 state and CeO2 are unique due to the ease of switching of the Ce valence state from one to other in favourable environment.43 This characteristic property of nanoceria can have diverse applications in biology as therapeutic agents, imaging, and drug delivery and we have extensively researched the implications in different biological systems.13,44–68 The most recent and widely studied therapeutic application of nanoceria stems from the catalytic reaction with reactive oxygen and nitrogen species, such as superoxide radical, hydrogen peroxide, hydroxyl radical, peroxynitrite, nitric oxide radicals, etc.69–71 Nanoceria have shown to possess superoxide dismutase mimetic and catalase mimetic activity.72–74A perfect cerium oxide, CeO2, (ceria) lattice has cubic fluorite structure (Fig. 1a) and in presence of oxygen vacancies it can exist as Ce2O3 (sesquioxide) with a hexagonal lattice. In a ceria lattice, the removal of an oxygen atom leads to localization of an electron in the 4f orbital of Ce. The absence of oxygen on the surface or within CeO2 lattice is accommodated by the switching of Ce4+ to Ce3+ states.18 The creation of oxygen vacancies and stabilization of the defect structure is more favourable in aqueous solution as the energy of formation of a vacancy is greatly compensated by the adsorption of water molecules on the CeO2 lattice. Molecular dynamics simulation can aid the experiments to quantify the physical and chemical properties of nanoceria.75 Oxygen modulations are critical to many biological processes at the cell level. The ability to extract, store and release oxygen by an external agent is therefore pivotal and nanoceria have this property of oxygen storage capacity.
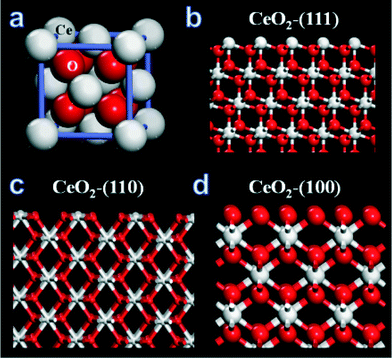 | ||
| Fig. 1 (a) Fluorite structure of cerium oxide (CeO2), (b) (111) plane of CeO2, (c) (110) plane CeO2 and (d) (100) plane of CeO2. | ||
The three commonly observed surfaces on nanoceria are (111), (110) and (100) as represented in Fig. 1b, c and d respectively. It has been shown through atomistic simulations that it is easier to extract oxygen from (100) and (110) surfaces compared to the (111) surface.76,77 If a CeO2 structure model defines high reactivity by the ease to release oxygen, then the (100) surface is found to be most reactive and (111) is the least reactive surface plane. Thus the composition of exposed planes on surface structure of ceria affects its chemical reactivity. As we have discussed in the earlier section, physico-chemical properties of nanomaterials control their behaviours at the biological interfaces.
Even apparently subtle changes in synthesis routes can have a dramatic influence on the properties of nanoparticles. Changes in the initial salt used to produce a form of oxidized iron results in significant changes in the rate and chemical reaction pathways for iron metal-core oxide-shell particles ultimately formed after several stages of processing.78 Synthesis temperature and sample processes also apparently alter particle behaviours of cerium oxide nanoparticles.12 (Detailed discussion in the section “Influence of environmental factors and aging”) The synthesis route has also been found to be particularly important for cerium oxide nanoparticles. The “same” nano-sized material can be prepared using a wide range of methods and medium with different synthesis parameters.13 The variety of synthesis routes is very helpful as physico-chemical properties can be tuned with size, shape etc. However, the differences in processing may also produce unintended or anticipated variations in particle behaviours.12
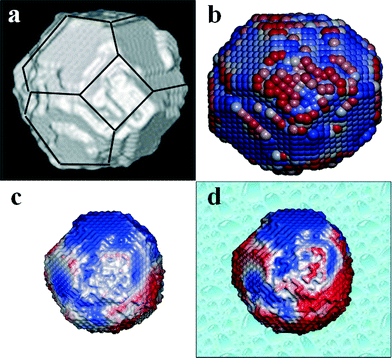
Structural and chemical quantification of the nanomaterials produced are central to reliable therapeutic applications. The structure of ceria nanoparticles depends upon synthetic protocol and atomistic modelling can generate lattice models by simulating the synthetic protocol. Fig. 2 shows the simulated crystallization of nanoceria (which can mimic experimentally produced nanoceria by flame pyrolysis) in vacuum from an amorphous structure to a polyhedral nanoparticle with different lattice planes emerging on the surface. Simulation can predict the relative surface area of surface lattice planes in a nanostructure from which it is easy to extract oxygen.79 Sayle et al. has used simulation to distinguish the surface reactivity of reduced and unreduced nanoceria, in vacuum and water environment. Another remarkable comparison was made for the superoxide radical scavenging ability between nanoceria in an aqueous environment with nanoceria which have been dried and rehydrated. The nanoceria in an aqueous environment, which was never dried, had higher activity than those dried and re-dispersed.79 Even though simulations may not be able to represent the experimental scenario completely, they were effective in showing the changes in surface reactivity of nanoceria with environmental variation.
 | ||
| Fig. 2 Atomistic simulation of ceria polyhedral nanoparticles from amorphous state. The yellow section within the structure shows the movement of crystallized lattice front. | ||
Physicochemical properties and redox activity
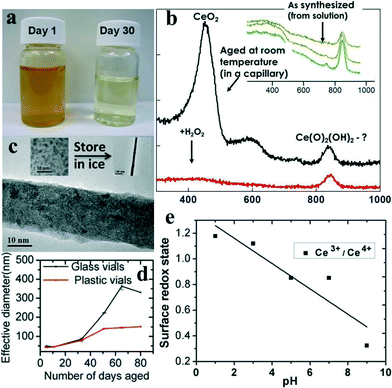
Fig. 3a shows a crystallized polyhedral CeO2 crystal from atomistic simulation with (111) and (100) surfaces exposed together with steps/edges. Fig. 3b shows only the surface oxygen atoms of the polyhedral nanoceria simulated lattice, colour coded to show the activity, the red oxygen atoms are easy to extract, blue oxygen atoms difficult to extract (red–white–blue gradient scale). Fig. 3(c and d) show the activity of oxygen in vacuum and aqueous environment respectively. In biological environments the fluid will influence the ease of extracting oxygen. In water it becomes easier to extract oxygen atoms because water molecules coordinate to surface cerium ions and prevent relaxation (oxygen thus ‘held’ less tightly). The nanoceria suspended in water are more reactive towards oxidative catalysis because of the ease of extracting oxygen. The simulations suggest that the reactivity of nanoceria surfaces and thus the activity at biological interface is dependent on environment of nanoceria as well as the history of environment which influences both surface functionalization as well as composition. In the following section we will briefly discuss the changes in nanoceria properties with different preparations which have an end effect on its biological application.It has been shown that the quenching of reactive oxygen species and reactive nitrogen species is a strong function of the redox state of nanoceria which varies with synthesis method and processing conditions.80 A general observation in nanoceria literature is that the Ce3+ state increases with decreasing particle size.81,82 We have reported that for simple water based synthesis of nanoceria without any surfactant or organic ligands, storage time and environment affects the redox state of nanoceria.11 This was an interesting observation for nanoceria because even without self-assembly, aggregation or, particle dissolution the valence state of nanoceria was non-linearly time-dependent and partly reversible. The nanoceria were synthesized by treating nitrate precursor with hydrogen peroxide. The change in the chemical state of nanoceria with time was apparent with visual inspection. One such visual comparison of these nanoceria samples is shown in Fig. 4a, where the dark yellow color of the sample represent predominant Ce in +4 state and light yellow color of the sample suggest Ce to be in +3 state. The change in color of the sample occurring due to the reduction of nanoceria was characterized in detail in earlier studies.11,16 Characterizing the nanoparticle suspension using transmission spectra collected from an ultraviolet-visible (UV-vis) spectrophotometer,11 it was found that Ce4+ concentration reached its peak concentration in a day after hydrogen peroxide treatment. The sample was dark yellow and with time the color changes to light yellow after a few weeks. The color change of the sample was confirmed with UV-vis spectra as reported in earlier study,11 indicating decrease in concentration of Ce4+ with simultaneous increase in Ce3+ with time. The results were also supported by another study16 utilizing X-ray photoelectron spectroscopy (XPS) to quantify the valence state of samples over four week time period. Transmission electron microscopy images were collected for the particles in solution to determine the size of nanoparticles during the study and it stayed unchanged.11 The finding was significant as it suggests two sets of nanoceria particles prepared using the same synthesis method but tested at different times after particle synthesis could produce variable results for ‘identical’ biological experiments because of differences in the Ce+3/Ce+4 ratio.
Preliminary laser Raman measurements (Fig. 4b) suggest that the smallest of these particles can transform in rather complex ways and that these transformations depend on particle size. Raman measurements made on small (~3 nm grain size) wet ceria particles, for which Ce4+ is observed by UV-Vis11 and XPS,16 were found to have a Raman signal characteristic of an oxy-hydroxide. In contrast, when UV-Vis11 and XPS16 indicate that Ce+3 dominates, the Raman spectra is consistent with the spectrum from ceria. Similar transformations are not observed for larger particles. These data show that small ceria particles – in this case having never been out of solution – change their structure and chemical state in a way not possible for larger ceria particles. In this case the Ce+3 appears to have the fluorite structure while the Ce4+ state for small grained particles is a type of superoxide. As this type of change is not seen for larger particles, a difference in biological effect depending upon particle size should not be a surprise.
Influence of environmental factors and aging
Differences in properties of nanoceria prepared using the same synthesis was also observed with concentration variation. Over a long period of time the low concentration (~5 mM nanoceria) may result in random agglomeration with storage condition, however a higher concentration (~30 mM nanoceria) undergoes self-assembly to form faceted fractal superoctahedral structures. Maintaining an acidic pH or refrigeration avoids agglomeration of the 3–5 nm sized nanoceria for both concentrations. However at sub-zero temperature, the low concentration nanoceria assembles into nanorods with time.83Fig. 4c shows the TEM image from one such sample of water-based nanoceria preparation stored at sub-zero temperature. The inset on the left side of Fig. 4c shows the TEM image of water based nanoceria synthesis and the inset on the right side of Fig. 4c shows a representative nanoceria nanorod of micron size. The morphological change in nanoceria from irregular nanoparticles to high aspect ratio nanorod is clearly observed with a difference in storage temperature of the sample. In the light of such a simple experiment involving no surfactant, polymers, or ionic stabilizers, it is clear that nanoceria chemistry is variable over time, concentration, processing and environment.With our extensive experience synthesizing nanoceria for biological and other catalytic applications, we have realized nanoceria need great attention and detail to control the properties. For the wet chemical synthesis of nanoceria discussed above, a surprising observation was made that the same synthesis protocol adapted in two different types of sample storage vials produced different particle properties.12Fig. 4d indicates the difference in hydrodynamic radius of same nanoceria sample preparation stored in different type of vials. The nanoceria aged differently in a sterile plastic polyethylene terephthalate than sterile glassware. Even though the nucleating nanoparticles were synthesized using the ‘same’ process for both cases, the sample in plastic vials started to dissolve within 8 week, whereas the sample in glass was stable for longer time.12 The pH of the nanoceria samples in glass and plastic vials were monitored regularly and size changes were consistent with the pH changes. Fig. 4e shows the changes in the redox state of Ce in a water-based nanoceria preparation with respect to pH variation. The redox state of the samples were determined by XPS characterization. The pH was varied with nitric acid (HNO3) and ammonium hydroxide (NH4OH). In another report on similar study, we have found that the chemical state (particle size and valence state) of nanoceria with same organic molecules (poly-ethylene glycol) varies with chain length and concentration.72 The chemical states of nanoceria were found to be different in acidic and basic environments with glucose and dextran conjugation in the aqueous suspension.
Other processing parameters such as impurities, temperature treatment, surface coatings, and lattice doping will influence the physic-chemical properties of nanoceria. High-temperature methods frequently result in nanoceria with particle size greater than 20 nm and this may affect the interaction kinetics at the biological interface. High temperature processing is also known to result in defect quenching and produce crystalline nanoceria with facets or edges, and this brings a differential property on the particle surface. The use of high temperature processes, such as hydrothermal or solvothermal methods, makes nanoparticles which are uniform in shape and size that show minimal changes with time and environment.
Taken together these results suggest that nanoparticle synthesis is both an art and a science. Therefore preparation of nanoceria for use in biological experiments should be well researched, giving particular attention to the history of chemical changes of the sample with storage, processing conditions, presence of surfactant or solvent molecules. Tests should be undertaken to assess the similarity or differences in material characteristics to be used in biological studies as close to the time of use as possible. It is equally important to have some information about the rates and types of change that particles may undergo during storage or preparation. Although the different synthesis routes are not discussed in detail in the following sections, consistent with the importance of noting the impacts of different synthesis routes, in each we call attention to the method used to synthesize the particles.
Effect of media or buffer on nanoceria
We have seen in the previous section that the synthesis route and processing of nanoceria influences their physico-chemical characteristics which play primary roles during interactions with biological systems. The second encounter of nanoceria at the biological interface is through the media. Interaction of nanoceria with biological molecules will usually modify the surface, so that the catalytic activity of nanoceria may become ineffective. In order to understand such interactions of nanoceria with buffers, we have pursued studies from which a few results are highlighted in the following paragraphs.Stability of suspension and agglomeration
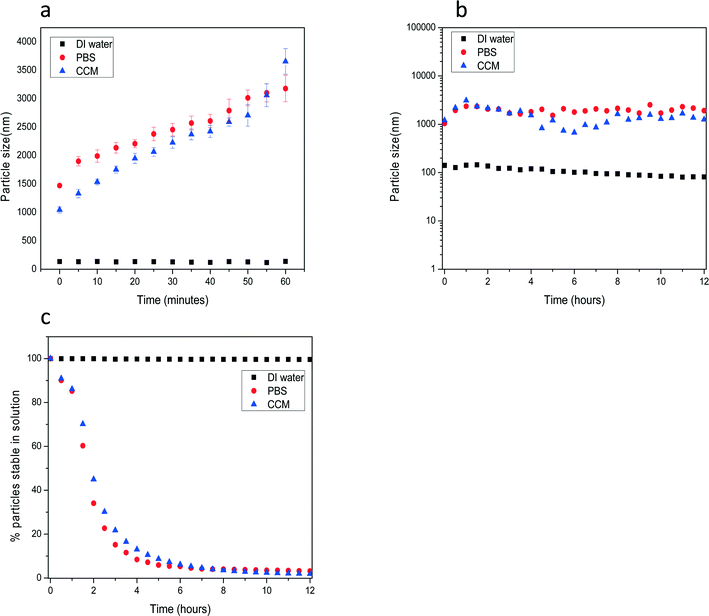
One important impact of dispersion and delivery media can be to alter particle aggregation or agglomeration. Nanoparticles that are initially suspended in solution may aggregate or dissolve in the relevant biological media based on particle properties, surface ligand and dispersion solution constituents. Sharma et al. describe the role that protein serum can have in limiting particle aggregation.84Fig. 5 shows the time dependent colloidal stability of unstabilized nanoceria after transfer into various dispersion solutions in the form of nanopowders. The nanoceria reported here were prepared by thermal hydrolysis precipitation methods. The crystallite size of the particles was ~10 nm as characterized by XRD and TEM measurements. For time dependent agglomeration studies dry powder particles were initially dispersed in DI water at a mass concentration of 50 mg ml−1 which was used as a stock solution for further dispersion in other media. Fig. 5(a) shows agglomeration kinetics and stability studies of nanoceria during the first hour after dispersion in cell culture media (CCM; RPMI 1640), DI water and phosphate buffered saline (PBS) at 100 μg ml−1. Even at the start of the measurements the size of particles (made up of many crystallites) in DI water were approximately 10 fold smaller than those in CCM and PBS. Furthermore, these non-stabilized particles showed a time dependent fast aggregation in PBS and CCM in comparison with the more stable particle size in DI water. The particle sizes at longer time points up to 12 h (Fig. 6b) showed no further hydrodynamic size increase in PBS and CCM. These results demonstrate that non-functionalized nanoceria in high ionic media such as PBS and RPMI aggregate to form larger particles within a few hours. As the particles grow by agglomeration to reach a size, larger particles drop out of solution (as shown below).We quantitatively evaluated the sedimentation stability of nanoceria in all three dispersion solutions as function of time, Fig. 5(c). The suspension stability index was calculated using UV-vis spectroscopy analysis to determine the % of nanoceria's absorbance intensity at λ = 300 nm at different time points (t = 0 to 12 h). The particles were stable in DI water without any significant decrease in absorbance intensity for the complete duration of measurements. In PBS and CCM more than 90% of particles dropped out of the solution within first 4 h. This correlated well to the particle agglomeration kinetics measurements where unstabilized particles formed large size agglomerates within the first few hours of transfer to biological media. Consistent with the work of Sharma et al. (ref. 84 above) on iron nanoparticles, preliminary studies for the nanoceria (not shown) indicated that when a large single protein (BSA) or more complex set of proteins (fetal bovine serum, FBS) were added to each of the above solutions, the agglomerate sizes in the CCM and PBS solutions decreased and the solution stability increased as the particles tended to remain in solution for the duration of the measurements.84
This study showed that in addition to the specific nature of the particles, the type of dispersion media, and media constituents including proteins can have a significant impact on particle agglomeration/aggregation. Understanding the biological impact and dose of particles will require knowledge of the aggregation or agglomeration state of the particles in the media of interest. Such effects are noted in several of the studies reported below.
Surface change
The role of media is important in modifying the surfaces to suit to certain biological applications, such as cellular targeting. The surface potentials of nanoceria influence the binding with specific proteins required for targeted delivery and cellular uptake. For any therapeutic application, during the transportation of nanoceria or cellular interaction, the local environment could influence the surface of NPs and affect protein binding. To explore such possibility, we used holo-transferrin (Tf) as the cellular targeting agent and studied its interaction with nanoceria in buffer of different pHs with single molecule force spectroscopy (SMFS).85 nanoceria with different surface charges were prepared by treating the nanoceria in deionized water with acidic (pH = 5) and basic (pH = 8 and 14) buffers. The nanoceria with pH buffers 5, 8 and 14 showed zeta potential (ZP) values of ~+36, +6, and ~−35 respectively and were used for single molecule force spectroscopy (SMFS) studies with Tf-conjugated atomic force microscopy (AFM) tip. The nanoceria were coated on silicon substrate for AFM based SMFS study.The force-extension profile of a Tf-coated AFM tip with nanoceria showed that adhesion between Tf and nanoceria decreases as the ZP of nanoceria changes from positive to negative values. Density functional computations were carried out to understand the interaction of Tf with nanoceria, and it was found that Tf forms strong hydrogen bonds with a protonated nanoceria surface. To test the adhesion of Tf coating on a protonated nanoceria surface (positive ZP), Tf coated nanoceria were incubated with human lung adenocarcinoma epithelial cells (A549) and human embryo lung fibroblast cells (WI-38). The ZP of nanoceria was observed to reduce by 20% after Tf. The presence of the coating was confirmed from Fourier Transform Infrared Spectroscopy (FTIR) and XPS. The A549 and W38 cells were incubated with varying concentrations (100 nM to 100 μM) of Tf coated and bare nanoceria. A549 cells exhibited preferential uptake of Tf coated nanoceria compared to bare nanoceria, which suggest that the uptake mechanism in cancer cells is by receptor-mediated endocytosis, which involves a two-step process. The first step is the attachment of Tf coated nanoceria to the Tf receptors on the cell membrane and in the second step, the complex is taken inside the cell and transferred to the endosomal compartment.
The uptake of bare nanoceria by cells was significantly lower than that for those coated with Tf and was suggested to occur through phagocytosis or pinocytosis process. W38 cells showed enhanced uptake of bare nanoceria in comparison to Tf:nanoceria and this was attributed to the steric hindrance of Tf by the negatively charged domains on cell membrane. The detailed study of nanoceria modified with Tf, showed that the interaction mechanism of nanoceria with cells can be tuned by changing nanoparticle surface charges in buffer for enhanced ligand coverage. The key to achieve preferential delivery to targeted cells is the interaction forces operating between the nanoceria and ligand (Tf here) and ligand conjugated nanoceria with cells. Thus for efficient targeting of nanoceria and physiological stability of the nanoceria, buffer treatment was found very beneficial in this study.
Surface modification can also shield the interaction between nanoparticles and biological system originating from other factors, such as morphology. Local particle shapes at the contact point of cells govern the cell interaction with nanoparticles and uptake process. Particles with local spherical shapes (measured by parameter Ω which is length-normalized curvature) that have a high length of curvature facilitates cell internalization, whereas lower length normalized curvature inhibits cell internalization.86,87 However, similar size nanoceria with different shapes (polygonal and spherical) synthesized using hexamethylenetetramine (HMT) showed no significant difference in cell viability.61 This indicates that the effect of surface modification with HMT can overcome the effect of different morphology in cell viability or cell interaction.
Catalytic/redox activity
We have also studied the superoxide dismutase (SOD) and catalase mimetic activity of nanoceria in biologically relevant media, buffers and ions.88 The SOD mimetic activity of nanoceria represents the extent to which it can convert superoxide (O2−) into oxygen and hydrogen peroxide while changing its redox state from Ce+3 to Ce+4. The catalase mimetic activity of nanoceria is a measure of extent to which it can decompose hydrogen peroxide to water and oxygen, while changing its redox state from Ce+4 to Ce+3. At first we incubated nanoceria synthesized by the wet chemical preparation in aqueous (pH = 7), acidic (pH = 3 and 5) and basic (pH = 9) media and followed the changes in absorption spectra collected by UV-vis. At higher pH (>9), the nanoceria showed aggregation and weren't investigated in that range. However other samples showed good stability in the acidic to basic range studied. No significant changes in redox state of Ce and superoxide scavenging properties were observed. We determined nanoceria stability in DMEM (with and without serum) and it formed a stable suspension. The sample was observed for 72 h of incubation and the SOD activity remained unaltered, which suggests any protein absorption from serum onto nanoceria does not affect its catalytic activity.Another common biological medium used in cell culture or animal models is phosphate buffer. We tested the nanoceria (100 μM) with phosphate buffer of different concentrations (50 μM–5 mM) and changes were observed in the UV-vis spectra for phosphate concentration higher than 50 μM. The peak corresponding to 250 nm (ascribed to Ce3+ in CeO2) was shifted to 275 nm (ascribed to Ce3+ in CePO4) for phosphate concentration higher than 50 μM. The SOD activity of the nanoceria, when suspended in phosphate buffer was also not observed, for 100 μM concentration of phosphate and higher, suggesting the affinity of phosphate anions on the surface of nanoceria cations. The most interesting observation was that, for the equimolar concentrations of nanoceria and phosphate buffer, the complete loss of SOD activity was accompanied with gain in the catalase activity. In another set of experiment (100 μM of nanoceria) the catalase activity increased in the phosphate buffer treated nanoceria in a dose dependent manner of phosphate buffer (10, 50, and 100 μM). Observing the effect of nanoceria surface modifications by phosphate anions, we studied nanoceria with other common anions such as carbonates and sulfates. However, we did not observe a change in UV-vis of carbonate and sulfate treated nanoceria with bare nanoceria in aqueous medium. The SOD activity of nanoceria in carbonate and sulfate anions was similar to those observed for untreated nanoceria. No catalase activity was observed for treated and untreated nanoceria. Thus phosphate was the only buffer which modified the nanoceria surface to seize the SOD activity and impart catalase activity. The study suggests that based on the phosphate present around cells and tissues, nanoceria can alter their SOD activity to catalase activity. Thus nanoceria which can scavenge superoxide radicals when brought to biological environment abundant with phosphate can only scavenge peroxides. We still have to study if shielding the nanoceria with anion or ligand followed by treatment with phosphate buffer can prevent the SOD activity of nanoceria, which is a gap in the existing literature. However, it is clear from our study of nanoceria in buffers that buffers play an important role in modifying the surfaces of nanoceria.
For in vivo studies with nanoceria in biological system, it would be interesting to observe the effect of blood on nanoceria. As of now we do not have experimental results on nanoceria in blood or blood mimicking fluid; however we need to understand the behaviour in buffer before looking into complex system like body fluid or blood. The current understanding on behaviour of nanoceria at different pH and in the common buffers used for dispersion or processing during in vitro studies can guide to move on to in vivo studies.
Fate of nanoparticles inside living systems/physicochemical properties and in vitro cell interaction
Understanding interactions at the nano–bio interface is one of key challenges to improving the design of bio-materials for medical uses, ranging from diagnostic to treatment. We have already highlighted the role that physiochemical properties of nanomaterials play in many particle behaviours and they are important in determining the nature of the interactions between nanomaterials and cells. The interaction of nanoparticles with a cell governs cellular response such as the pathway to internalization, the amount of internalization, intracellular localization and interference with cellular pathways. Physiochemical properties including size, shape, surface charge, surface modification or functional group present on the surface of the nanoparticles influence biological interaction. In addition, biological media contain different buffer ions, bio-molecules including proteins, amino acids etc.59 Due to high surface energy, nanoparticles trends to agglomerate or react with ions or absorb proteins present in the medium. Therefore, to understand the nanoparticle cell interactions, it is very important to investigate nanoparticle chemistry and physical properties in biologically relevant media.89 Not only the cell interaction and bio-availability, but bio-transformation or fates of the nanomaterials are topics to explore to understand the long term safety of nanomaterial. In this section we have summarized literature in which researchers have investigated the influence of physiochemical properties of nanoceria on cell interaction, internalization, localization and also chemical fate of nanoparticles after internalization.In vitro study
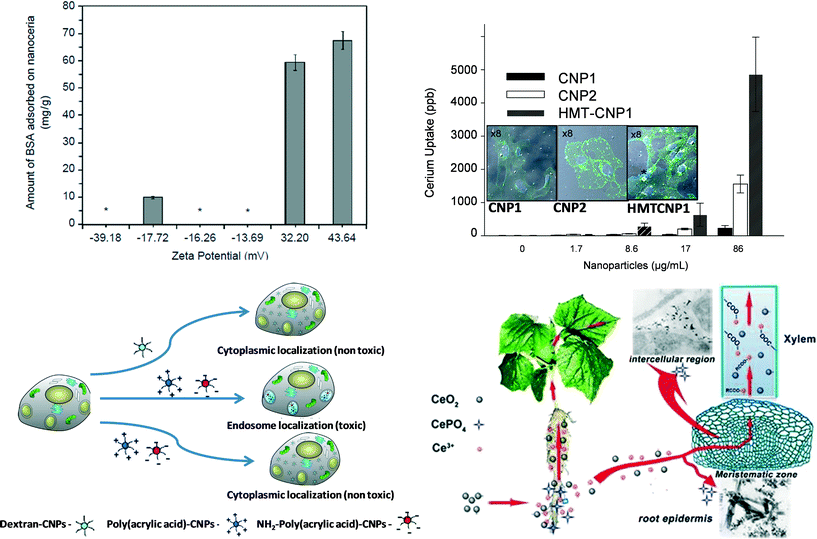
Bovine serum albumin (BSA) has been used to examine the effects of cell interaction due to protein adsorption on differently synthesized nanoceria (microemulsion and hydrothermal).89 Protein adsorption on the surface of the nanoparticles mainly depends on electrostatic interaction, hydrophobic interaction and specific chemical interactions between protein and the nanoparticle surface. Variations in protein adsorption were observed for nanoceria with different surface charge. Positively surface charged nanoceria adsorbed proteins, whereas negatively surface charged nanoceria did not significantly adsorb proteins (Fig. 6a). More positively charged protein adsorbed a higher amount of protein compared to lower positive charged proteins, this reflects importance of pH and electrolyte concentration of media for protein-nanoparticle interaction. On the other hand in case of nanoparticle cell internalization (A549 cell line; human alveolar basal epithelial cells), negative surface charged nanoparticles showed higher cellular uptake as compared to positive surface charged nanoceria for both microemulsion and hydrothermal synthesized nanoparticles. Similar to protein adsorption, cellular uptake also highly depends on the electrostatic interaction of nanoceria with the cell membrane. Other than surface charge of nanoceria, size was observed to play a role in cellular uptake. Cells efficiently took up smaller nanoceria (3–5 nm) prepared using a microemulsion method as compared to bigger nanoceria prepared using a hydrothermal method (8–10 nm).89Different shape of the nanoparticles could also interact differently with cells.54 3–5 nm nanoceria showed induction of tube formation at 10 μM concentration. However, bigger size (≥30 nm) and different shape nanoceria including cube, star or nanorod, did not show any induction of tube formation. Chemical property of nanoceria surface (Ce3+/Ce4+) tends to change with increasing size of the particles with larger particles often having more Ce4+ on the surface. Altered cellular responses were observed with different size and shape of the nanoceria. Specially, when considering nanorod, the aspect ratio of the nanorod played a major role in cell interaction. Nanorods with an aspect ratio 1–16 did not induce any cellular response. However, nanorods with an aspect ratio 22 and 31 induced pro-inflammatory (IL-1β) production and cytotoxicity. Higher aspect ratio nanorods ≥22 damaged lysosomes and therefore induced inflammatory response to the cells.90 Therefore, shape of the nanoparticles can be very important to the cellular response.
In addition to size, shape and surface change, surfactants and unwanted bio-molecules at the particle surface could influence the cellular interaction and the uptake of the nanoceria.61 In this study, different nanoceria were synthesized using wet-chemical method by using different reducing or oxidizing agents, such as hydrogen peroxide (nanoceria1), ammonium hydroxide (nanoceria2) or hexamethylenetetramine (HMT-nanoceria). Surface charges of these nanoceria in doubly distilled H2O were HMT-nanoceria (~34 mV) > nanoceria2 (~30 mV) > nanoceria1 (18 mV), however in cell culture media they showed a very similar distribution of charge (8–10 mV). Still, cellular uptake was found to differ for nanoceria1, nanoceria2 and HMT-nanoceria in human umbilical vein endothelial cells line. HMT-nanoceria found higher amount of cellular uptake, followed by nanoceria2 and minimum amount were observed in case of nanoceria1 (analyzed using ICP-MS) (Fig. 6b). Though HMT-nanoceria were found to have higher cell internalization, the size of HMT-nanoceria were bigger that nanoceria1 and nanoceria2. In this case different shapes of HMT-nanoceria did not show any difference in cell interaction (confirmed by cell survival study). Higher amounts of HMT adsorbed on the surface of nanoceria did not have any further influence on cell interaction. Another study showed surface functionalization with PEG increased nanoceria colloidal stability without affecting its catalytic activity. Moreover, induction of PEG spacer between antibodies to target plaque in Alzheimer model increased protection of neurone cells against β-amyloid challenge compared to the bare nanoparticle.91 From these results it can be concluded that presence of functional group/bio-molecule on the surface can play the most important role in cell-nanoparticles interaction or cellular uptake.
Following tissue or cell internalization, fate of nanoceria has been explored in just a few studies.92–95 It has been shown that nanoceria, when internalized in the squamous carcinoma cells, induced reactive oxygen species whereas they did not induce any oxidative stress in normal dermal fibroblasts. The increase in ROS generation is mainly due to the acidic environment of cancer cells as in acidic media nanoceria convert superoxide radical very effectively to hydrogen peroxide, but do not scavenge hydrogen peroxide, therefore accumulate hydrogen peroxide in cells.95 Similarly, a pH dependent induction of hydrogen peroxide generation has been observed by another study. Poly(acrylic acid) and aminated poly(acrylic acid) coated nanoceria, due to their intracellular localization in the acidic endosome of the A549 cell line, were observed to show oxidase like activity (Fig. 6c).94 Therefore, presence of nanoceria in subcellular compartment or micro-environment also can determine nanoceria reactivity.
In vivo study
Biological systems can sometimes transform the nature (structure and chemistry) of nanoceria. In a recent study92 soybean seeds were germinated to full maturity in presence of nanoceria. Next, X-ray fluorescence (μ-XRF) and micro-X-ray absorption near-edge structure were used to determine the form of nanoceria on soybean tissues. Signal from Ce was mainly observed in the epidermis and inside the nodule, and 88% of these signals were from cerium oxide. Further analysis of the spectra revealed that some of Ce was reduced to Ce3+. This result indicates that the nanoceria surface is susceptible to reduction in the biological environment. In another study, biotransformation of nanoceria was explored in the cucumber plant (Fig. 6d).93 Needle like clusters were observed in the intracellular space of cucumber plants root when plants were exposed to 2000 mg l−1 nanoceria for 21 days. The needle like structure is mainly identified as CePO4. Moreover, X-ray absorption fine structure (XANES) spectra analysis revealed that Ce was found in the root as CeO2 and CePO4 while in the shoots as CeO2 and cerium carboxylates. However, it is not very hard to conclude if nanoceria are absorbed in the intracellular space and then transformed either in phosphate or carboxylates. It has been hypothesized that nanoceria first adsorbed on the root and then partially dissolved on root surface by organic acids and reducing substances excreted by the roots. Then Ce3+ ion leached from the nanoceria reacts with phosphate or carboxylates, either at root surface or in the intracellular space/during translocation to the shoots.Current state and challenges of nanoceria at the nano–bio interface
The complexity of nanoparticle interaction with biological entities presents challenges for the development and engineering of nanoparticles before they can be put to application within the biological system. It is equally important to understand the fate, transport and transformation of nanoceria in the biological system of interest, some of these concerns are summarized in Table 1. Nanoceria have been shown to mimic the natural anti-oxidant in the body. This antioxidant type activity, such as: SOD and catalase, of nanoceria has been observed by several researchers both in vitro and in vivo. It is clear that nanoceria have the potential to be used as therapeutic agents against diseases/disorders associated with oxidative stress. In addition to the beneficial properties of nanoceria, some research shows the toxicity. It is our experience that the properties of nanoceria can be controlled or varied over a wide range and thereby tuned for a given application. Particularly at the bio-interface, seemingly negligible changes in the synthesis route or observed characteristics of a nanomaterial can result in widely different observations. Researchers working in this fascinating field need to realize the possibility that minor changes in nanomaterials may result in huge differences in biological impact.| Area | Observation/known | Future scope of study/comments |
|---|---|---|
| Synthesis and process route | (i) Amount and type of oxidizer or reducer, pH of the reaction mixture, temperature can influence nanoceria physicochemical properties and therefore have a huge impact on catalytic properties, interaction with environment and living system. | (i) Influence of type and purity of the precursors on physical and surface chemistry still needs to be explored in detail. It is well known that surface chemistry can be altered by surface contamination or by doping of other rare earth materials which will impact biological response. |
| (ii) Synthesis procedure and use of surfactant/stabilizer also can alter cell nanoceria interaction by changing surface charge, stability of nanoparticles suspension, directly interact to surface ligand/receptor present on cell membrane or guiding nanoparticles to different cellular compartments. | ||
| Impact of morphology | (i) Morphology, specifically cell nanoparticle interaction/biological responses in terms of toxicity or catalytic response (pro-angiogenesis induction) have been explored with nanoceria with different aspect ratio of nanorod, faceted nanoparticles, polygonal structure, star and irregular to spherical nanoparticles. | (i) The specific and different interactions of different morphology nanoceria with cells need to be understood. How does the morphology impact the cell? |
| (ii) Can different crystal planes of nanoparticles at cell-nanoparticles contact point influence interaction or biological response. | ||
| Variable chemical state | (i) With careful selection of precursor, size and controlling synthesis procedure, it is possible to tailor the surface oxidation state of nanoceria, however agglomeration, storage environment, container, pH also influence surface oxidation state. | (i) The nature and role of binding at specific sites (e.g. Ce3+) is not adequately understood and both experimental and computational approaches are needed to understand the roles of particle features and chemical binding for nanoceria catalytic activity. |
| (ii) Surface oxidation state of nanoceria controls its bio-catalytic properties towards scavenging reactive oxygen species and nitrogen species, and interaction with molecular oxygen. | ||
| Fate in biological systems | (i) Localization, clearance, and pharmacokinetics of nanoceria were explored and correlated with physicochemical properties of nanoceria after exposure to biological system such as cells, plants, animals. | (i) Long term safety and bio-transformation in animal models need additional systematic study to enrich understanding of nanoceria biological application. |
| (ii) Biotransformation of nanoceria is also known in plant system after encountering very high pH and ion rich environment. |
Challenges
The therapeutic beneficial effects of nanoceria are unique and open windows for further exploration. We have seen in the earlier discussion that nanoceria with different synthesis method are widely different and that the toxicity of one nanoceria preparation cannot be generalized to other nanoceria. The mixed beneficial and toxicity reports on nanoceria indicate that researchers need to be extremely careful in designing the nanoparticles and the biological study using those nanoparticles.The interaction of nanoparticles at various biointerfaces is dynamic and the nanoceria surfaces themselves are found to be dynamic. We have noticed that the same sample batch shows variation in the physico-chemical property with storage condition, container and aging. The variations in sample from batch to batch in well-developed and adapted methods have begun to unfold.96 Thus the necessity for detailed history and characterization of sample is important to avoid uncertainty in the synthesis methods. How one can standardize the current manufacturing practice for nanoparticle preparation methods is another major challenge.
For advancement of understanding of nanoceria it is important to study all forms which show promising results in biology. However to expedite the understanding in biological applications or impact on social life, those forms of nanoceria should be given attention, which have shown beneficial property or those nanoceria samples which are exposed to the environment through feasible route. Studying a batch of ceria in the laboratory which shows toxicity effects, may not be relevant to nanoceria found in environment.
What is needed to fill in the current gaps?
The current literature on nanoceria interaction with biological media is very motivating for further research in tailoring its properties towards biological applications. However, significant work is required to design nanoceria based drugs for therapeutic applications. In our view, the priority should be given to the following:1. Simulation and modelling – as discussed in this article, computer simulation is a valuable complementary tool that can help rationalise the behaviour of nanoparticles immersed in a particular environment. A future requirement for simulation is to couple atomistic with quantum chemical methods. In particular the microstructure of a nanoparticle (morphology and surfaces, dislocations, grain-boundaries, point defects, surface relaxation and strain) can dominate its reactivity. However, the number of atoms required to capture such microstructure is at present prohibitively large to be considered quantum chemically. Conversely, the chemical reactivity (bond breaking and bond formation) is best captured using quantum chemical methods. Coupling the two approaches will enable one to predict more accurately how the hierarchical structure of a nanomaterial, including its environment, can impact upon its reactivity.
2. In situ real-time measurements – the study of nanoceria in biological systems should be aimed for simultaneous characterization of nanoceria while the biological experiments are being carried out. Such in situ real-time measurements require high resolution and high sensitivity. Although a challenge, such measurements are increasingly possible. For example, a study of the adsorption of acetic acid nanoceria particles has been reported.97 Studies using scanning transmission X-ray microscopy (STXM) to probe the real time changes in chemical state of nanoceria in cells are currently being attempted. For nanoparticles research, sum-frequency-generation (SFG) spectroscopy can be used to probe biomolecules at nanoceria interfaces with molecular-level resolution in situ.
3. Particle classification – some immature and preliminary efforts are underway to classify or index nanoparticle therapeutics and subsequent toxicity. As of now, in the absence of a toxicity model for different types of nanoparticles or even toxicity rating for different nanoparticles within the same material system, the unique antioxidant like activity of certain nanoceria preparations cannot be neglected. This will involve keeping and reporting more detailed records of particle synthesis and processing history.
4. Focus or compare to particles with desired or known behaviours – toxicity studies are important to advance the therapeutic nanoparticles as it suggests what precautions should be taken. To meet the challenge of developing therapeutic nanoparticles, the studies on any nanoparticles which have shown good results should be studied at great detail and with great responsibility. The biological studies of a particular nanoceria preparation with beneficial effects cannot be generalized to any other nanoceria preparation in the light of discussion in this article. The exact mechanism of nanoceria behaviour within biological systems should be evaluated. It is also advised that the effect of any nanoparticles should be studied in conjunction with other control nanoparticles to distinguish the origin of nano–bio interactions. To determine the redox based anti-oxidant behaviour of nanoceria in biological system, a control nanoparticle can be any metal oxide, such as silicon oxide, zinc oxide, etc., of similar size, morphology, surface coating. A study was performed to compare the in vitro cytotoxicity of several nanoparticles on scale of nontoxic amorphous silica to toxic crocidolite asbestos.98 A oxidative stress study99 conducted on three different oxide nanoparticles (ZnO, TiO2, CeO2) demonstrates a range of biological responses which vary from cytotoxic to cytoprotective elucidated varying interaction of nanoparticles within biological system.
5. Enhanced collaboration and data sharing – apart from sharing through journal articles, more interactive and collaborative work is needed among the researchers working on the same nanoparticles with emphasis on those collaborations who have observed differing results from the same nanoparticle. For example there are several studies which suggest adverse response due to nanoceria100–103 in biological system. At the same time a large number of studies report beneficial effects104–107 of nanoceria in the biological system. Also, studies which have found ceria nanoparticles as benign have indicated that at lengths ≥200 nm and aspect ratios ≥22, CeO2 nanorods induced progressive pro-inflammatory effects and cytotoxicity.90 However, in the light of discussion of this article, it is apparent that nanoceria can reveal different biological response depending on its physico-chemical characteristics. Thus to dig deeper into exploring the engineered nanoceria for beneficial therapeutic property and to rule out nanoceria preparations which are deleterious in biological system, researchers with different observation on nanoceria should work together in collaboration. One such attempt was done through the SNO Special workshop on Nanoceria held at Santa Barbara in 2013.
6. Determine rates of change – we have studied the effect of aging of nanoceria in aqueous medium and found significant changes over time. The long term aging of nanoceria still needs to be studied for other reported biologically relevant nanoceria in literature.
7. Know source chemicals – one of the challenges is to obtain and use consistent precursor material from existing chemical suppliers for the synthesis of nanoceria. The responsibility of using consistent precursor materials lies with the vendor as well as the researcher. For e.g. if the precursor salt is hygroscopic, particular attention has to be given in using a batch over time and its storage and use condition.
8. Fate, transformation and transport – there is a need to understand both the uptake and removal and ultimate fate of nanoparticles as they move within a biological system (particle balance).
Acknowledgements
Dr. Seal and Dr. Self acknowledge NSF (NIRT: CBET-0708172, EECS 0901503, CBET-1261956) and NIH support (R01: AG031529-01). Dr Baer acknowledges NIEHS support under Center grant U19 ES019544. Drs. Seal and Self acknowledge travel support from for presenting at the nanoceria workshop at SNO meeting, Santa Barbara. We thank Drs. S.V.N.T. Kuchibhatla and C.F. Windisch Jr. for providing the preliminary Raman data in Fig. 4b. A portion of this work was conducted in EMSL, a national scientific user facility sponsored by DOE-BER and located at PNNL. This article is a product of a workshop on nanoceria held November 2, 2013 at Fess Parker's Doubletree Resort, Santa Barbara, California which was made possible by financial support from the Sustainable Nanotechnology Organization; NSF grant CBET-1343638 to UCSB; and the Tracy Farmer Institute for Sustainability and the Environment, Department of Pharmaceutical Sciences, Office of the Vice President for Research, and Associate Dean for Research of the College of Pharmacy, University of Kentucky.Notes and references
- T. Kim and T. Hyeon, Nanotechnology, 2014, 25 Search PubMed.
- N. Anton and T. F. Vandamme, Pharm. Res., 2014, 31, 20–34 CrossRef CAS PubMed.
- X. H. Tan and R. C. Jin, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 569–581 CrossRef CAS PubMed.
- N. A. Kotov, J. O. Winter, I. P. Clements, E. Jan, B. P. Timko, S. Campidelli, S. Pathak, A. Mazzatenta, C. M. Lieber, M. Prato, R. V. Bellamkonda, G. A. Silva, N. W. S. Kam, F. Patolsky and L. Ballerini, Adv. Mater., 2009, 21, 3970–4004 CrossRef CAS.
- I. Willner and B. Willner, Coord. Chem. Rev., 2003, 245, 139–151 CrossRef CAS.
- P. A. Schulte, C. L. Geraci, V. Murashov, E. D. Kuempel, R. D. Zumwalde, V. Castranova, M. D. Hoover, L. Hodson and K. F. Martinez, J. Nanopart. Res., 2013, 16 Search PubMed.
- C. E. H. Beaudrie, T. Satterfield, M. Kandlikar and B. H. Harthorn, PLoS One, 2013, 8 Search PubMed.
- D. R. Hristozov, S. Gottardo, A. Critto and A. Marcomini, Nanotoxicology, 2012, 6, 880–898 CrossRef CAS PubMed.
- S. Seal and B. Karn, Safety Sci., 2014, 63, 217–225 CrossRef PubMed.
- W. J. Stark, Angew. Chem., Int. Ed., 2011, 50, 1242–1258 CrossRef CAS PubMed.
- S. V. Kuchibhatla, A. S. Karakoti, D. R. Baer, S. Samudrala, M. H. Engelhard, J. E. Amonette, S. Thevuthasan and S. Seal, J. Phys. Chem. C, Nanomater. Interfaces, 2012, 116, 14108–14114 CrossRef CAS PubMed.
- A. S. Karakoti, P. Munusamy, K. Hostetler, V. Kodali, S. Kuchibhatla, G. Orr, J. G. Pounds, J. G. Teeguarden, B. D. Thrall and D. R. Baer, Surf. Interface Anal., 2012, 44, 882–889 CrossRef CAS PubMed.
- T. Sakthivel, S. Das, A. Kumar, D. L. Reid, A. Gupta, D. C. Sayle and S. Seal, ChemPlusChem, 2013, 78, 1446–1455 CrossRef CAS.
- A. B. Stefaniak, V. A. Hackley, G. Roebben, K. Ehara, S. Hankin, M. T. Postek, I. Lynch, W.-E. Fu, T. P. J. Linsinger and A. F. Thünemann, Nanotoxicology, 2012, 1–13, DOI:10.3109/17435390.2012.739664.
- D. R. Beverhof and R. M. David, Anal. Bioanal. Chem., 2010, 396, 953–961 CrossRef PubMed.
- D. R. Baer, M. H. Engelhard, G. E. Johnson, J. Laskin, K. Mueller, P. Munusamy, S. Thevuthasan, H. Wang, N. Washton, A. Elder, B. L. Baisch, A. Karakoti, S. V. N. T. Kuchibhatla and D. W. Moon, J. Vac. Sci. Technol., A, 2013, 31, 050820 Search PubMed.
- R. M. Crist, J. H. Grossman, A. K. Patri, S. T. Stern, M. A. Dobrovolskaia, P. P. Adiseshaiah, J. D. Clogston and S. E. McNeil, Integr. Biol., 2013, 5, 66–73 RSC.
- A. Kumar, R. Devanathan, V. Shutthanandan, S. Kuchibhata, A. S. Karakoti, Y. Yong, S. Thevuthasan and S. Seal, J. Phys. Chem. C, 2012, 116, 361–366 CAS.
- M. Pickup, S. Novitskiy and H. L. Moses, Nat. Rev. Cancer, 2013, 13, 788–799 CrossRef CAS PubMed.
- Z. Wang, N. Li, J. Zhao, J. C. White, P. Qu and B. Xing, Chem. Res. Toxicol., 2012, 25, 1512–1521 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS PubMed.
- B. Pelaz, S. Jaber, D. J. de Aberasturi, V. Wulf, T. Aida, J. M. de la Fuente, J. Feldmann, H. E. Gaub, L. Josephson, C. R. Kagan, N. A. Kotov, L. M. Liz-Marzán, H. Mattoussi, P. Mulvaney, C. B. Murray, A. L. Rogach, P. S. Weiss, I. Willner and W. J. Parak, ACS Nano, 2012, 6, 8468–8483 CrossRef CAS PubMed.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- Surface Complexation at the Iron Oxide/Water Interface. Experimental Investigations and Theoretical Developments, http://hdl.handle.net/2077/15697.
- P. Decuzzi and M. Ferrari, Biomaterials, 2007, 28, 2915–2922 CrossRef CAS PubMed.
- H. Gao, W. Shi and L. B. Freund, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9469–9474 CrossRef CAS PubMed.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS PubMed.
- W.-L. L. Suen and Y. Chau, J. Pharm. Pharmacol., 2013, 66, 564–573 CrossRef PubMed.
- J. Rejman, V. Oberle, I. S. Zuhorn and D. Hoekstra, Biochem. J., 2004, 377, 159–169 CrossRef CAS PubMed.
- B. D. Chithrani and W. C. W. Chan, Nano Lett., 2007, 7, 1542–1550 CrossRef CAS PubMed.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613–11618 CrossRef CAS PubMed.
- M. A. Jakupec, P. Unfried and B. K. Keppler, Rev. Physiol., Biochem. Pharmacol., 2005, 153, 101–111 CAS.
- J. P. Garner and P. S. Heppell, Burns, 2005, 31, 539–547 CrossRef CAS PubMed.
- A. B. Lansdown, S. R. Myers, J. A. Clarke and P. O'Sullivan, J. Wound Care, 2003, 12, 113–118 CrossRef CAS.
- W. W. Monafo, S. N. Tandon, V. H. Ayvazian, J. Tuchschmidt, A. M. Skinner and F. Deitz, Surgery, 1976, 80, 465–473 CAS.
- C. L. Fox Jr., W. W. Monafo Jr., V. H. Ayvazian, A. M. Skinner, S. Modak, J. Stanford and C. Condict, Surg., Gynecol. Obstet., 1977, 144, 668–672 CAS.
- S. C. DeCaluwe, M. E. Grass, C. Zhang, F. E. Gabaly, H. Bluhm, Z. Liu, G. S. Jackson, A. H. McDaniel, K. F. McCarty, R. L. Farrow, M. A. Linne, Z. Hussain and B. W. Eichhorn, J. Phys. Chem. C, 2010, 114, 19853–19861 CAS.
- L. Liao, H. X. Mai, Q. Yuan, H. B. Lu, J. C. Li, C. Liu, C. H. Yan, Z. X. Shen and T. Yu, J. Phys. Chem. C, 2008, 112, 9061–9065 CAS.
- J. C. Yang, D. W. Oh, G. W. Lee, C. L. Song and T. Kim, Wear, 2010, 268, 505–510 CrossRef CAS PubMed.
- D. Knapp and T. Ziegler, J. Phys. Chem. C, 2008, 112, 17311–17318 CAS.
- L. Truffault, B. Winton, B. Choquenet, C. Andreazza, C. Simmonard, T. Devers, K. Konstantinov, C. Couteau and L. J. M. Coiffard, Mater. Lett., 2012, 68, 357–360 CrossRef CAS PubMed.
- A. Kumar, S. Babu, A. S. Karakoti, A. Schulte and S. Seal, Langmuir, 2009, 25, 10998–11007 CrossRef CAS PubMed.
- M. Das, S. Patil, N. Bhargava, J. F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS PubMed.
- J. J. Hickman, M. Das, S. Patil, N. Bhargava, J. F. Kang, L. Riedel and S. Seal, Tissue Eng., 2007, 13, 873–874 Search PubMed.
- J. Chen, S. Patil, S. Seal and J. F. McGinnis, in Recent Advances in Retinal Degeneration, ed. R. E. Anderson, M. M. LaVail and J. G. Hollyfield, Springer-Verlag Berlin, Berlin, 2008, vol. 613, pp. 53–59 Search PubMed.
- J. Colon, L. Herrera, S. Patil, S. Seal, W. Jenkins, P. Kupelian and C. Baker, Int. J. Radiat. Oncol., Biol., Phys., 2008, 72, S700–S701 CrossRef PubMed.
- S. M. Hirst, A. D. Peairs, R. Gogal, S. Seal and C. M. Reilly, FASEB J., 2008, 22 Search PubMed.
- J. Colon, N. Hsieh, A. Ferguson, P. Kupelian, S. Seal, D. W. Jenkins and C. H. Baker, Nanomedicine, 2010, 6, 698–705 CrossRef CAS PubMed.
- J. M. Dowding, S. Lubitz, A. Karakoti, A. Kim, S. Seal, M. Ellisman, G. Perkins, E. Bossy-Wetzel and W. Self, Free Radical Biol. Med., 2010, 49, S181–S181 CrossRef PubMed.
- X. H. Zhou, L. L. Wong, A. S. Karakoti, S. Seal and J. F. McGinnis, PLoS One, 2011, 6 Search PubMed.
- X. Cai, S. A. Sezate, S. Seal and J. F. McGinnis, Biomaterials, 2012, 33, 8771–8781 CrossRef CAS PubMed.
- S. Das, S. Singh, S. Chigurupati, M. R. Mughal, A. Kumar, C. R. Patra, S. Oommen, N. E. Vlahakis, D. Mukhopadhyay, W. T. Self, M. P. Mattson and S. Seal, Wound Repair Regen., 2012, 20, A20–A20 Search PubMed.
- S. Das, S. Singh, J. M. Dowding, S. Oommen, A. Kumar, T. X. T. Sayle, S. Saraf, C. R. Patra, N. E. Vlahakis, D. C. Sayle, W. T. Self and S. Seal, Biomaterials, 2012, 33, 7746–7755 CrossRef CAS PubMed.
- R. A. Madero-Visbal, B. E. Alvarado, J. F. Colon, C. H. Baker, M. S. Wason, B. Isley, S. Seal, C. M. Lee, S. Das and R. Manon, Nanomedicine, 2012, 8, 1223–1231 CrossRef CAS PubMed.
- V. Shah, S. Shah, H. Shah, F. J. Rispoli, K. T. McDonnell, S. Workeneh, A. Karakoti, A. Kumar and S. Seal, PLoS One, 2012, 7 Search PubMed.
- Z. Vujaskovic, P. Xu, B. Maidment, Z. Rabbani, I. L. Jackson, A. Zodda, C. Hadley, A. Burkhalter, S. Das and S. Seal, Int. J. Radiat. Oncol., Biol., Phys., 2012, 84, S683–S683 CrossRef PubMed.
- L. Alili, M. Sack, C. von Montfort, S. Giri, S. Das, K. S. Carroll, K. Zanger, S. Seal and P. Brenneisen, Antioxid. Redox Signaling, 2013, 19, 765–778 CrossRef CAS PubMed.
- K. Chaudhury, K. N. Babu, A. K. Singh, S. Das, A. Kumar and S. Seal, Nanomedicine, 2013, 9, 439–448 CrossRef CAS PubMed.
- S. Chigurupati, M. R. Mughal, E. Okun, S. Das, A. Kumar, M. McCaffery, S. Seal and M. P. Mattson, Biomaterials, 2013, 34, 2194–2201 CrossRef CAS PubMed.
- J. M. Dowding, S. Das, A. Kumar, T. Dosani, R. McCormack, A. Gupta, T. X. T. Sayle, D. C. Sayle, L. von Kalm, S. Seal and W. T. Self, ACS Nano, 2013, 7, 4855–4868 CrossRef CAS PubMed.
- S. Giri, A. Karakoti, R. P. Graham, J. L. Maguire, C. M. Reilly, S. Seal, R. Rattan and V. Shridhar, PLoS One, 2013, 8 Search PubMed.
- S. M. Hirst, A. Karakoti, S. Singh, W. Self, R. Tyler, S. Seal and C. M. Reilly, Environ. Toxicol., 2013, 28, 107–118 CrossRef CAS PubMed.
- K. E. Klump, X. Cai, R. Towner, S. Seal, M. Dyer and J. McGinnis, FASEB J., 2013, 27 Search PubMed.
- S. V. Kyosseva, L. J. Chen, S. Seal and J. F. McGinnis, Exp. Eye Res., 2013, 116, 63–74 CrossRef CAS PubMed.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. H. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- L. L. Wong, S. M. Hirst, Q. N. Pye, C. M. Reilly, S. Seal and J. F. McGinnis, PLoS One, 2013, 8 Search PubMed.
- X. Cai, S. Seal and J. F. McGinnis, Biomaterials, 2014, 35, 249–258 CrossRef CAS PubMed.
- A. Karakoti, S. Singh, J. M. Dowding, S. Seal and W. T. Self, Chem. Soc. Rev., 2010, 39, 4422–4432 RSC.
- Y. Xue, Y. Zhai, K. Zhou, L. Wang, H. Tan, Q. Luan and X. Yao, Chem. – Eur. J., 2012, 18, 11115–11122 CrossRef CAS PubMed.
- J. M. Dowding, T. Dosani, A. Kumar, S. Seal and W. T. Self, Chem. Commun., 2012, 48, 4896–4898 RSC.
- A. S. Karakoti, S. Singh, A. Kumar, M. Malinska, S. Kuchibhatla, K. Wozniak, W. T. Self and S. Seal, J. Am. Chem. Soc., 2009, 131, 14144–14145 CrossRef CAS PubMed.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- W. T. Self and S. Seal, Google Pat., 2009 Search PubMed.
- D. C. Sayle, S. A. Maicaneanu and G. W. Watson, J. Am. Chem. Soc., 2002, 124, 11429–11439 CrossRef CAS PubMed.
- T. X. T. Sayle, S. C. Parker and C. R. A. Catlow, Surf. Sci., 1994, 316, 329–336 CrossRef CAS.
- J. C. Conesa, Surf. Sci., 1995, 339, 337–352 CrossRef CAS.
- K. Moore, B. Forsberg, D. R. Baer, W. A. Arnold and R. L. Penn, J. Environ. Eng., 2011, 137, 889–896 CrossRef CAS.
- T. X. T. Sayle, M. Molinari, S. Das, U. M. Bhatta, G. Mobus, S. C. Parker, S. Seal and D. C. Sayle, Nanoscale, 2013, 5, 6063–6073 RSC.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. S. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- S. Deshpande, S. Patil, S. Kuchibhatla and S. Seal, Appl. Phys. Lett., 2005, 87 Search PubMed.
- L. Wu, H. J. Wiesmann, A. R. Moodenbaugh, R. F. Klie, Y. Zhu, D. O. Welch and M. Suenaga, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 125415 CrossRef.
- A. S. Karakoti, S. Kuchibhatla, D. R. Baer, S. Thevuthasan, D. C. Sayle and S. Seal, Small, 2008, 4, 1210–1216 CrossRef CAS PubMed.
- G. Sharma, V. Kodali, M. Gaffrey, W. Wang, K. R. Minard, N. J. Karin, J. G. Teeguarden and B. D. Thrall, Nanotoxicology, 2013, 8, 663–675 CrossRef PubMed.
- A. Vincent, S. Babu, E. Heckert, J. Dowding, S. M. Hirst, T. M. Inerbaev, W. T. Self, C. M. Reilly, A. E. Masunov, T. S. Rahman and S. Seal, ACS Nano, 2009, 3, 1203–1211 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4930–4934 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Pharm. Res., 2009, 26, 244–249 CrossRef CAS PubMed.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753 CrossRef CAS PubMed.
- S. Patil, A. Sandberg, E. Heckert, W. Self and S. Seal, Biomaterials, 2007, 28, 4600–4607 CrossRef CAS PubMed.
- Z. Ji, X. Wang, H. Zhang, S. Lin, H. Meng, B. Sun, S. George, T. Xia, A. E. Nel and J. I. Zink, ACS Nano, 2012, 6, 5366–5380 CrossRef CAS PubMed.
- A. Cimini, B. D'Angelo, S. Das, R. Gentile, E. Benedetti, V. Singh, A. M. Monaco, S. Santucci and S. Seal, Acta Biomater., 2012, 8, 2056–2067 CrossRef CAS PubMed.
- J. A. Hernandez-Viezcas, H. Castillo-Michel, J. C. Andrews, M. Cotte, C. Rico, J. R. Peralta-Videa, Y. Ge, J. H. Priester, P. A. Holden and J. L. Gardea-Torresdey, ACS Nano, 2013, 7, 1415–1423 CrossRef CAS PubMed.
- P. Zhang, Y. Ma, Z. Zhang, X. He, J. Zhang, Z. Guo, R. Tai, Y. Zhao and Z. Chai, ACS Nano, 2012, 6, 9943–9950 CrossRef CAS PubMed.
- A. Asati, S. Santra, C. Kaittanis and J. M. Perez, ACS Nano, 2010, 4, 5321–5331 CrossRef CAS PubMed.
- L. Alili, M. Sack, A. S. Karakoti, S. Teuber, K. Puschmann, S. M. Hirst, C. M. Reilly, K. Zanger, W. Stahl and S. Das, Biomaterials, 2011, 32, 2918–2929 CrossRef CAS PubMed.
- R. Franca, X. F. Zhang, T. Veres, L. Yahia and E. Sacher, J. Colloid Interface Sci., 2013, 389, 292–297 CrossRef CAS PubMed.
- Z. Lu, A. Karakoti, L. Velarde, W. Wang, P. Yang, S. Thevuthasan and H.-F. Wang, J. Phys. Chem. C, 2013, 117, 24329–24338 CAS.
- T. J. Brunner, P. Wick, P. Manser, P. Spohn, R. N. Grass, L. K. Limbach, A. Bruinink and W. J. Stark, Environ. Sci. Technol., 2006, 40, 4374–4381 CrossRef CAS.
- T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS PubMed.
- J. Y. Ma, R. R. Mercer, M. Barger, D. Schwegler-Berry, J. Scabilloni, J. K. Ma and V. Castranova, Toxicol. Appl. Pharmacol., 2012, 262, 255–264 CrossRef CAS PubMed.
- A. Srinivas, P. J. Rao, G. Selvam, P. B. Murthy and P. N. Reddy, Toxicol. Lett., 2011, 205, 105–115 CrossRef CAS PubMed.
- E.-J. Park, J. Choi, Y.-K. Park and K. Park, Toxicology, 2008, 245, 90–100 CrossRef CAS PubMed.
- W. Lin, Y.-W. Huang, X.-D. Zhou and Y. Ma, Int. J. Toxicol., 2006, 25, 451–457 CrossRef CAS PubMed.
- S. Chen, Y. Hou, G. Cheng, C. Zhang, S. Wang and J. Zhang, Biol. Trace Elem. Res., 2013, 154, 156–166 CrossRef CAS PubMed.
- M. Das, S. Patil, N. Bhargava, J.-F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS PubMed.
- A. Clark, A. Zhu, K. Sun and H. Petty, J. Nanopart. Res., 2011, 13, 5547–5555 CrossRef CAS PubMed.
- J. Niu, A. Azfer, L. M. Rogers, X. Wang and P. E. Kolattukudy, Cardiovasc. Res., 2007, 73, 549–559 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |