Enhanced adsorption of carbon nanocomposites exhausted with 2,4-dichlorophenoxyacetic acid after regeneration by thermal oxidation and microwave irradiation†
Anthony B.
Dichiara
,
Jordan
Benton-Smith
and
Reginald E.
Rogers
*
Department of Chemical and Biomedical Engineering, Rochester Institute of Technology (R. I. T.), Rochester, NY 14623, USA. E-mail: rerche@rit.edu; Tel: +1 585 475 4157
First published on 7th February 2014
Abstract
Graphene nanoplatelet–single-walled carbon nanotube hybrid papers exhausted with 2,4-dichlorophenoxyacetic acid were recovered by a combination of thermal oxidation and microwave irradiation. The recycling efficiency investigated after multiple saturation/regeneration cycles based upon changes in optical absorption spectra significantly exceeded the adsorption capacity of the original nanocomposites.
Nano impactThe available supplies of freshwater are decreasing due to extended droughts, population growth, and increasing groundwater and environmental pollution. Advances in nanoscale science and engineering are providing new opportunities to develop more performant, cost-effective and sustainable materials that will help the world facing its upcoming challenges to meet rising demands of drinking water. The use of graphene nanoplatelet–single-walled carbon nanotube papers as new generation adsorbents for organic compounds offer not only fast adsorption rates, improved stability and large capacity, but exhibit very high regeneration efficiency potentially providing a sustainable solution for future purification systems. |
With the rapid growth of the world population, water is fast becoming the planet's most precious natural resource. While industrial activity keeps rising, the need to reuse water and reduce effluent pollution has led to the development of various methods to remove contaminants from aquatic environments.1 To this aim, advanced techniques such as nanofiltration, ultrafiltration and reverse osmosis can be used.2 However, their high cost limits their application at a global scale.3 One of the most commonly employed methods consists of using sorbents of high binding affinity and capacity to selectively remove residues of undesirable chemicals from the aqueous phase.4 Due to their large surface area, high hydrophobicity and strong interactions with organic compounds, carbon nanomaterials have shown great promise in environmental remediation.5 Both graphene, a two-dimensional material composed of a single sheet of hexagonally packed carbon atoms, and carbon nanotubes (CNTs), defined as graphene sheets rolled into seamless cylinders, have demonstrated higher adsorption capacities than activated carbon (AC), the most widely used material for water purification.6–8 Recently, opportunities have been found for enhanced adsorption using a nanocomposite based on the hybridization of single-walled CNTs (SWCNTs) with graphene nanoplatelets (GnPs).9 This material exhibits higher stability and performance than either component alone. Nevertheless, the large-scale applicability of adsorbents for removal of pollutants from water depends not only on the adsorption capacity, but also on the regeneration properties and reusability.10
The rising concern for pollutants in the environment, along with more restrictive environmental regulations and the increased price of adsorbents in recent years, has motivated the development of regeneration systems to allow a wider application of adsorption processes and ensure their economic feasibility. As adsorption is a widely applicable non-destructive technology, various methods have been reported to recover and reuse saturated adsorbents with organic contaminants, such as thermal treatment,11 surfactant based desorption,12 chemical oxidation,13 electrochemical oxidation,14 ultrasonic desorption,15 bioregeneration16 and microwave irradiation.17 However, these techniques are often time and/or energy consuming and usually induce significant deterioration of the adsorbent's structure, reducing its adsorption capacity. Hence, the recycling efficiency decreases as the number of regeneration cycles increases.18,19 Along these lines, we report the regeneration of SWCNT–GnP hybrid papers saturated with 2,4-dichlorophenoxyacetic acid (2,4-D), one of the most extensively applied herbicides for the control of broad-leaved weeds in fields,20 to such an extent that the original adsorption capacity is significantly exceeded even after reuse for several cycles.
The composite sorbents were prepared by a vacuum filtration process and purified through a combination of non-oxidative acid soaking and thermal oxidation in air under similar conditions to those previously reported.9 The advantage of using paper-like materials is that they can be retrieved from solution easily, without magnetic or centrifugation separation. Before each regeneration cycle, duplicate sections of the as-prepared hybrid papers weighing 0.7 mg were exhausted at six different initial concentrations (20.0, 30.0, 40.0, 50.0, 60.0 and 100.0 μg mL−1), which had a pH of 7 (±1), and constantly agitated on an orbital shaker (Chang Bioscience, KJ-201BD) at 120 rpm for 24 hours. Adsorption of 2,4-D molecules onto the surface of carbon nanostructures is attributed to overlapping benzene rings forming noncovalent π–π bonds.21,22 Equilibrium was achieved at the time beyond which there are no appreciable changes in the mass of 2,4-D adsorbed onto the carbon nanomaterials as measured by optical absorption spectroscopy (Perkin Elmer Lambda 950 UV/Vis/NIR). The adsorption capacities, q, were computed through mass balance on 2,4-D in the bulk solution using optical spectroscopy at the maximum adsorption peak (λ = 283 nm) of 2,4-D, as previously reported.6 Then, the adsorbent papers were retrieved from the solutions and dried at 110 °C for 24 hours before being regenerated by thermal oxidation at 560 °C in a muffle furnace (Thermo-Scientific, Thermolyne 1300) for 10 min followed by microwave irradiation in air at 1200 W for 15 min. The regeneration of pristine hybrids was also performed for comparison purposes and was referred to as “initially treated”. All regenerated papers were dried at 110 °C for 24 hours before re-adsorption. The saturation/regeneration process was carried out for four cycles. It is noteworthy that the hybrid papers exhibited high structural stability as no significant deterioration and negligible mass losses were observed after several saturation/regeneration cycles.
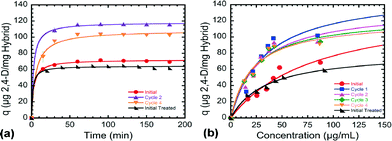
The time-dependent adsorption of a 50 μg mL−1 solution of 2,4-D, which has a pH of 7 (±1), onto hybrid papers after different regeneration cycles at 20 °C over a 3 hour time period is depicted in Fig. 1a. It can be seen that the evolution of the amount of 2,4-D adsorbed on the nanocomposites follows a pseudo-second order kinetic model.23 The adsorption rate was faster at the early stages and progressively slowed with time before eventually approaching equilibrium and reaching saturation. This is associated with the large number of vacant sites available for adsorption at the initial stage compared to the later stages and the repulsive forces existing between 2,4-D molecules on the adsorbent surface and those in solution at the later stages. Fig. 1a also shows that the aforementioned regeneration process significantly improved the adsorption of 2,4-D on the recovered nanocomposites. After four cycles reuse, the adsorption rate of the hybrids remained much higher than that of the native materials, while the adsorption of 2,4-D slightly decreased in the case of the “initially treated” samples. Fig. 1b exhibits the isotherms of 2,4-D adsorbed onto the surface of both original and regenerated hybrid papers. The trend in the data, with a progressive increase at lower concentrations, is consistent with reported isotherms for 2,4-D adsorption on AC.24 The Langmuir isotherm was used to fit the data and the adsorption capacity was calculated after reuse for multiple cycles. The monolayer adsorption capacity significantly increased after the first regeneration cycle, but slightly decreased for the “initially treated” materials. The adsorption capacity of the nanocomposites reached 0.124 mg of 2,4-D per mg of hybrids after the first regeneration cycle, which is 50% higher than that of the initial hybrids (i.e. 0.068 mg of 2,4-D per mg of hybrids). After subsequent saturation/regeneration cycles, it can be seen that the removal of 2,4-D was slightly reduced compared to that after the first cycle, which is consistent with the kinetics data presented in Fig. 1a. For instance, the adsorption capacities obtained at cycles 2, 3 and 4 were 0.114, 0.109 and 0.106 mg of 2,4-D per mg of hybrids, respectively. Regardless, the uptake of 2,4-D after each regeneration cycle always exceeded that of the original adsorbents.
Fig. 2a exhibits the recycling efficiency (RE) of the nanocomposites as a function of 2,4-D initial concentration after each regeneration cycle. RE corresponds to the ratio between the adsorption capacity of a given cycle and that of the original nanocomposites. While the RE gradually decreases after subsequent cycles (except at 20 μg mL−1), with this effect being more pronounced as the number of cycles increases, RE always reaches values above 100%. In order to evaluate the desorption yield in the different cycles, the evolution of the step stripping efficiency (SSE), defined as the ratio between the adsorption capacity of a given cycle and that of the previous one, is plotted in Fig. 2b. SSE drops down dramatically after the first cycle, while it remains relatively stable after subsequent cycles. On the basis of these results, it can be inferred that the aforementioned recycling process contributes to a very high regeneration efficiency and an increase in the life span of carbonaceous adsorbents. Even after several regeneration cycles, RE always remains above 100%, while the adsorption capacities of 2,4-D reported herein are significantly higher than those in other reports on 2,4-D adsorbed onto different types of AC.24–26
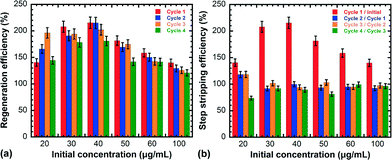 | ||
| Fig. 2 Evolution of the regeneration efficiency (RE) (a) and the step stripping efficiency (SSE) (b) as a function of 2,4-D initial concentration after each regeneration cycle. | ||
Improvement in adsorption of the regenerated materials suggests that the recycling process induces structural modifications (i.e. surface area, pore size, surface chemistry, etc.) which are beneficial for further retention of 2,4-D molecules.27 Any change in the intrinsic structure of the surface should indeed influence the chemical properties of the carbon nanocomposites, which will eventually affect the adsorbent/adsorbate interface. Enhanced RE was previously reported for bulk carbonaceous adsorbents like AC28 and graphite29 and was attributed to the activation of coke deposits formed due to thermal cracking of the adsorbate, thereby counteracting the site plugging effect. However, these studies reported slight improvements in RE after each regeneration cycle, unlike the data presented herein. This suggests that another mechanism accounting for the abnormally high RE may take place. The regeneration process was found to enhance the basicity of the recovered nanocomposite surface, as revealed by Boehm titration (Fig. S1†). This agrees with the prediction that thermal treatment and microwave irradiation would produce AC with basic properties.30 The increase of basic properties after regeneration might be caused by the formation of pyrone or chromene-like structures on the adsorbent surface.31,32 The addition of these surface basic groups influences the adsorption performance in two different ways. Due to 2,4-D's acidic nature, it can contribute to improve adsorption through the donor–acceptor complex mechanism.33 Nevertheless, adsorption of 2,4-D onto the surface of carbon nanomaterials is mainly attributed to π–π stacking,21 whereas it is affected by hydrophobicity and electrostatic effects only to a limited extent.22,34 Therefore, the addition of surface basic groups would mostly result in a larger density of π electrons, which leads to stronger dispersion forces in the π–π interactions.35 Hence, the stronger dispersive interactions between the π electrons on the basic surface of the regenerated adsorbent and the free electrons of the 2,4-D molecule present in the aromatic rings and multiple bonds promote adsorption.36 Moreover, it is worth noting that no improvement in adsorption was observed in the case of the “initially treated” samples (Fig. 1), indicating that recycling-induced structural modification does not occur in the absence of adsorbates. Fig. 2 shows the influence of the adsorbate concentration on RE and SSE. It can be seen that RE increases accordingly with the initial concentration and reaches the maximum for each cycle at 40 μg mL−1 before slightly decreasing upon increasing the initial concentration further. Therefore, the decomposition of 2,4-D molecules during regeneration may induce the aforementioned changes on the adsorbent structure. However, the positive effect of surface basicity might be balanced by the reduction of the adsorbent surface area and pore size due to the formation of these basic groups as well as some other possible textural changes (Fig. S2†). The slight decrease in RE observed after subsequent regeneration cycles may thus be due to the deposition of decomposition residues onto the adsorbent surface, decreasing the number of sites available for further adsorption.19 As a result, structural changes induced in the carbon nanocomposites after each regeneration cycle might be attributed to the competition between basic group functionalization, increasing adsorption through a larger density of π electrons, and the side plugging effect, reducing the number of sites available for adsorption. For instance, at low concentration (20 μg mL−1), the amount of 2,4-D byproducts blocking the adsorption sites is reduced, which may explain why RE keeps increasing after a few cycles, as observed in Fig. 2.
In summary, we have presented the reactivation of SWCNT–GnP nanocomposite papers saturated with 2,4-D by thermal oxidation and microwave irradiation such that the original adsorption capacity is significantly exceeded and the maximum RE reaches values above 200%. The adsorbents regenerated even after several cycles always performed better than the native ones, which is significantly higher than other reports on 2,4-D adsorbed onto different types of both fresh and recycled AC. A larger adsorption capacity together with improved stability and enhanced recovery/recycle ability make carbon nanocomposite papers high-potential adsorbents for future aqueous purification systems.
Acknowledgements
We thank J.C. Wilson and T.J. Sherwood for their support. We also acknowledge funding support from the Kate Gleason gift. JBS thanks the Kate Gleason College of Engineering Honors Program for undergraduate student support.Notes and references
- N. Bolong, A. F. Ismail, M. R. Salim and T. Matsuura, Desalination, 2009, 239, 229 CrossRef CAS PubMed.
- M. G. Buonomenna, RSC Adv., 2013, 3, 5694 RSC.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946 CAS.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 8075 CAS.
- M. M. Titirici, R. J. White, C. Falco and M. Sevilla, Energy Environ. Sci., 2012, 5, 6796 Search PubMed.
- X. Ren, C. Chen, M. Nagatsu and X. Wang, Chem. Eng. J., 2011, 170, 395 CrossRef CAS PubMed.
- R. E. Rogers, T. I. Bardsley, S. J. Weinstein and B. J. Landi, Chem. Eng. J., 2011, 173, 486 CrossRef CAS PubMed.
- K. C. Kemp, H. Seema, M. Saleh, N. H. Le, K. Mahesh, V. Chandra and K. S. Kim, Nanoscale, 2013, 5, 3149 RSC.
- A. B. Dichiara, T. J. Sherwood and R. E. Rogers, J. Mater. Chem. A, 2013, 1, 14480 CAS.
- H. Marsh and F. Rodriguez-Renoso, The economics of activated carbon, Roskill Information Services Ltd., Clapham Road, SW9 OJA, London, 1998, vol. 17 Search PubMed.
- R. Berenguer, J. P. Marco-Lozar, C. Quijada, D. Cazorla-Amoros and E. Morallon, Energy Fuels, 2010, 24, 3366 CrossRef CAS.
- J. S. Gutsch and H. Sixta, Ind. Eng. Chem. Res., 2012, 51, 8624 CrossRef.
- A. Hutson, S. Ko and S. G. Huling, Chemosphere, 2012, 89, 1218 CrossRef CAS PubMed.
- C. O. Ania and F. Beguin, Environ. Sci. Technol., 2008, 42, 4500 CrossRef CAS.
- O. Hamdaoui, E. Naffrechoux, J. Suptil and C. Fachinger, Chem. Eng. J., 2005, 106, 153 CrossRef CAS PubMed.
- C. Coelho, A. S. Oliveira, M. F. R. Pereira and O. C. Nunes, J. Hazard. Mater., 2006, 138, 343 CrossRef CAS PubMed.
- F. K. Yuen and B. H. Hameed, Adv. Colloid Interface Sci., 2009, 149, 19 CrossRef CAS PubMed.
- X. Quan, X. Liu, L. Bo, S. Chen, Y. Zhao and X. Cui, Water Res., 2004, 38, 4484 CrossRef CAS PubMed.
- J. Wang, X. Peng, Z. Luan and C. Zhao, J. Hazard. Mater., 2010, 178, 1125 CrossRef CAS PubMed.
- Pesticides industry sales and usage report, EPA-733-R-04-001, United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances (7503C), Washington DC, 2004.
- B. Pan and B. Xing, Environ. Sci. Technol., 2008, 42, 9005 CrossRef CAS.
- P. E. Diaz-Flores, R. Leyva-Ramos, J. R. Rangel-Mendez, M. M. Ortiz, R. M. Guerrero-Coronado and J. Mendoza-Barron, J. Environ. Eng. Manage., 2006, 16, 249 CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- J. M. Salman, V. O. Njoku and B. H. Hameed, Chem. Eng. J., 2011, 174, 33 CrossRef CAS PubMed.
- Z. Aksu and E. Kabasakal, Sep. Purif. Technol., 2004, 35, 223 CrossRef CAS.
- B. H. Hameed and J. M. Salman, J. Hazard. Mater., 2009, 163, 121 CrossRef CAS PubMed.
- C. O. Ania, J. B. Parra, J. A. Menendez and J. J. Pis, Water Res., 2007, 41, 3299 CrossRef CAS PubMed.
- C. O. Ania, J. B. Parra, J. A. Menendez and J. J. Pis, Microporous Mesoporous Mater., 2005, 85, 7 CrossRef CAS PubMed.
- J. M. Skowronski and P. Krawczyk, Chem. Eng. J., 2009, 152, 464 CrossRef CAS PubMed.
- J. M. Valente Nabais, P. J. M. Carrott, M. M. L. Ribeiro Carrott and J. A. Menendez, Carbon, 2004, 42, 1315 CrossRef PubMed.
- U. Zielke, K. J. Huttinger and W. P. Hoffman, Carbon, 1996, 34, 983 CrossRef CAS.
- H. P. Boehm, Carbon, 1994, 32, 759 CrossRef CAS.
- K. Laszlo, P. Podkoscienly and A. Dabrowski, Appl. Surf. Sci., 2006, 252, 5752 CrossRef CAS PubMed.
- W. Chen, L. Duan and D. Zhu, Environ. Sci. Technol., 2007, 41, 8295 CrossRef CAS.
- A. H. Norzilah, A. Fakhru'l-Razi, T. S. Y. Choong and A. Luqman Chuah, J. Nanomater., 2011, 495676, 18 Search PubMed.
- P. C. C. Faria, J. J. M. Orfao and M. F. R. Pereira, Water Res., 2004, 38, 2043 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental procedure and Boehm titration. See DOI: 10.1039/c3en00093a |
| This journal is © The Royal Society of Chemistry 2014 |