Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater
Nadia
von Moos
a,
Paul
Bowen
b and
Vera I.
Slaveykova
*a
aEnvironmental Biogeochemistry and Ecotoxicology, Institute F.-A. Forel, Earth and Environmental Sciences, University of Geneva, 10, route de Suisse, CH-1290 Versoix, Switzerland. E-mail: vera.slaveykova@unige.ch; Fax: +41 22 379 03 29; Tel: +41 22 379 03 35
bPowder Technology Laboratory, Institute of Materials, School of Engineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
First published on 19th March 2014
Abstract
Over the past few years, engineered nanomaterials (ENMs) have penetrated nearly every sector of modern life and their broad-scale use is steadily and rapidly increasing. The (expected) elevated levels of ENMs in the environment raise concerns with regard to their potential environmental impact, but environmental risk assessment of released ENMs lags behind invention and today's global consumption volumes. Although considerable progress has been achieved in understanding particle behavior in complex systems and numerous studies have investigated the environmental hazards of ENMs in recent years, the link between these two aspects is less developed. This review provides an overview of what is known about ENMs in freshwater systems and explores the applicability of the bioavailability concept known from aquatic trace metal toxicology. The concept of bioavailability may provide a useful framework to link the “chemical and physical speciation” of ENMs with their possible biological effects but likely requires some ENM specific adaptations. However, there are still considerable knowledge gaps with respect to ENM “speciation” in natural aquatic systems and it remains unclear if it is realistic (by analogy to free metal ions) to search for a specific ENM form that could be used as a measure of biological reactivity. Major knowledge gaps concern the effects of agglomeration on bioavailability, cellular internalization routes, intracellular compartmentalization as well as dissolved organic matter–protein competition on the surface of internalized ENPs.
Nano impactThis review provides a thorough overview of what is currently known about environmental transformations of nanomaterials as well as their interactions with and their toxicity towards bacteria and microalgae, two principal model microorganisms of aquatic risk assessment. In this way, we hope to provide a comprehensive synopsis that can serve as a practical reference study to help guide future research efforts in nanomaterial hazard and risk assessment. |
Introduction
Biological availability (bioavailability) is defined as “the extent to which a contaminant in a source is free for uptake by an organism and to which it can cause an effect at the site of action”1,2 (Box 1). It is considered a key concept in aquatic ecotoxicology and emerged from the recognition that not all metal forms present in natural waters are available to aquatic biota – it was, for example, found that total or dissolved metal concentrations are usually not good predictors of the acute toxicity of metals to aquatic biota.3 Indeed, a basic premise in aquatic ecotoxicology is that toxicants must first be accumulated above normally regulated levels by the organism in which an adverse effect is elicited. In other words, biota only respond to metals which become associated either in or on them. This association between metals and organisms is fundamental to the bioavailability concept and understanding this process is critical to the evaluation of potential adverse effects. The bioavailability concept has been adopted in the regulatory context for trace metals4–8 and enables the quantitative link between chemical speciation and biological effects, as well as the integration of the modifying role of basic water quality parameters, such as water hardness ions, pH, dissolved organic matter and salinity.Conversely, though there is an ever growing understanding of the physical and chemical transformations and toxic effects of engineered nanomaterials (ENMs, definition in Box 1), a general conceptual framework linking ENM behavior and effects as well as the influence of environmental parameters thereon is still lacking. As for trace metals, the bioavailability of ENMs could provide a conceptual framework for the integration of their environmental transformations, the influence of modifying factors and possible toxic effects and thus provide a means to understand and predict their potential (eco-)toxicity.9–13 However, it is currently unclear to what extent this concept could apply to inorganic ENMs and what specific adaptations are likely required.
This review provides an overview of the main processes underlying the availability of inorganic ENMs to unicellular microorganisms, explores how physical and chemical transformations affect their toxicity towards bacteria and microalgae, two very common model aquatic microorganisms (AMOs) of (eco-)toxicology, and examines the applicability of the bioavailability concept in understanding ENM toxicity. The main topics covered are (i) direct interactions between inorganic metal and metal oxide ENMs and AMOs, adsorption to biosurfaces, penetration of cellular barriers and intracellular fate, (ii) environmental transformations of ENPs that affect physical and chemical “speciation” of ENMs in contact with biota and their bioavailability, such as agglomeration, dissolution, binding to dissolved organic matter, sulfidation and oxidation.
1. Bioavailability of engineered nanomaterials
In order to induce an effect in AMOs, ENMs suspended in the water column must first translate from the bulk medium to the immediate vicinity of a microorganism via diffusion (mass transport, Fig. 1). ENMs that agglomerate and reach a critical threshold size high enough for gravitational forces to outweigh buoyancy forces22 will settle and thus decrease the particle number that can encounter planktonic algal or bacterial cells. During mass transport, ENMs are not necessarily inert and undergo physical and chemical transformations.23–27 Thus, ENMs will interact with AMOs in their environmentally transformed forms rather than in their original, as-manufactured or as-released forms.21,28,29 ENMs interact with biological surfaces via adsorption and desorption processes either as released ions, single nanoparticles, agglomerates, or complexes (Fig. 1). At the nano–bio interface, target AMOs can modify interactions by their surface properties (e.g. by the presence of exopolymeric substances, surface molecules etc.) and by the release of small proteins and other exudates.30 Dynamics at this interface stem from patchiness of the distribution and spatial localization of surface charge or embedded biomolecules (e.g. proteins, lipids, and glycosylated structures), which undergo constant flux due to normal cellular metabolism (e.g. protein turnover, ion transport, secretion) and reactions in response to environmental fluxes.13 The bioavailability of ENMs is thus ultimately shaped by the sum of the above processes (Fig. 1) occurring successively or simultaneously31,32 in the inherently complex, heterogeneous and highly dynamic natural aquatic systems, which contribute yet another degree of complexity by their high spatial and temporal variability.18,21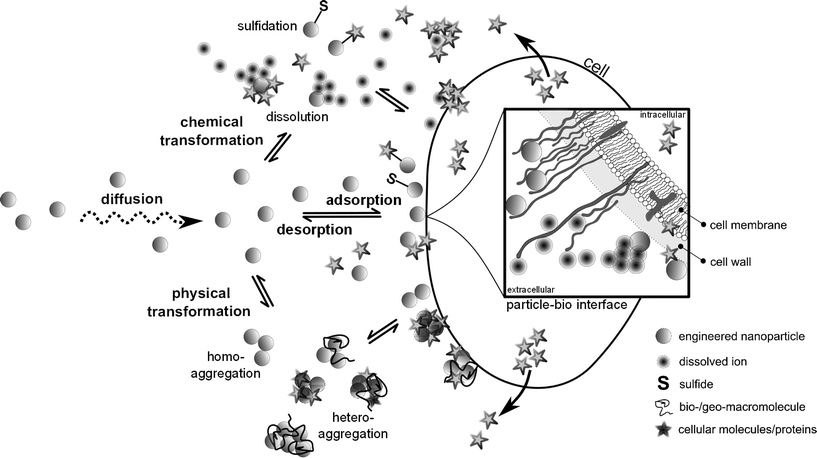 | ||
| Fig. 1 Processes at the medium–bio-interface underlying the bioavailability of engineered nanomaterials to aquatic microorganisms. Engineered nanomaterials in the environment undergo chemical (e.g. dissolution, sulfidation, redox surface reactions, (de-)protonation, ligand exchange, photodegradation, complexation etc.) and physical (homo- and heteroaggregation) transformations as a function of their material properties and the abiotic factors of the ambient medium. They interact with biological surfaces by adsorption and desorption, either as released ions, transformed single nanoparticles, agglomerates or complexes. Cells can modify these interactions by their specific cell surface properties (e.g. by the presence of extracellular polymeric substances etc.) or by the release of small molecules/proteins. Following adsorption, engineered nanomaterials may, depending on their material properties and the biological target organism, actively or passively be internalized by cells and induce biological responses, which however can also be independent of direct interactions and internalization. All processes are highly dynamic. Adapted from ref. 33 and 13, not to scale. | ||
The quantitative understanding of ENM bioavailability requires insights into their behavior during transport from the ambient medium to the AMO interface and of the processes underlying adsorption, internalization as well as intracellular fate, which will be discussed in the next sections. Although these processes depend on the ENMs' physical and chemical properties such as size, shape, surface properties, energy bandgap etc., the emphasis of this review lies on the environmental factors affecting the above processes, since the importance of ENMs' material characteristics has already been thoroughly reviewed.16,17,34 For more detailed information concerning these processes in wastewater systems, the authors direct readers to recent reviews by Eduok et al.,35 Liu et al.36 or Brar et al.37
2. ENM uptake by planktonic bacteria and microalgae
ENMs undergo environmental transformations that yield a complex mix of different ENM forms, comparable to the speciation of trace metals in the aquatic environment, which include homo- and hetero-agglomerates, free/complexed dissolved and partially dissolved ENMs. This section is devoted to the uptake of (undissolved) ENMs, which is defined as a process involving adsorption and internalization, as well as to the intracellular fate of internalized ENMs. The uptake of dissolved, inorganic species (ions) is well known from research on trace metals.32.1 Adsorption
Adsorption and direct contact of ENMs with AMOs are a prerequisite for toxicity38–42 and the penetration of biological membranes. Adsorption of ENMs onto surfaces is governed by the combined effects of the nature and physicochemical properties of the ENMs and the ambient media.43 Adsorption and internalization also strongly depend on the biological substrate, i.e. the cell type, cell membrane, differentiation stage and the pathways of cellular processing.13 The forces involved in adsorption are van der Waals forces, hydrophobic forces, electrostatic attraction and specific chemical interactions, including hydrogen bonding and receptor–ligand interactions.44 Microorganisms in general,45 particularly bacteria and microalgae, normally exhibit a net negative surface charge at freshwater pH values due to the dissociation of carboxylic, phosphate and other acidic functional groups of the membrane.38,46,47 Electrostatic attraction is therefore likely to occur between AMOs and ENMs with a positive zeta potential, be it inherent or the result of previous modifying interaction processes at the particle surface.38,47 Indeed, the importance of electrostatic attractions has, for example, been evidenced by interactions between positively charged nano-CeO2 (at neutral pH) and outer membranes of Escherichia coli.48 However, the simple electrostatic approach sometimes fails to explain direct interactions between ENPs and AMOs. This was, for instance, the case for nano-TiO2 (−25.72 ± 0.5 mV) and the bacterial freshwater isolate Bacillus licheniformis (−28.47 ± 0.62 mV) whose negative zeta potentials in lake water seemed to preclude nano–bio interactions based on the electric charge but whose mechanism of toxicity under dark conditions was predominantly found to be due to the attachment of nano-TiO2 to the bacterial cell membrane.49 Electrostatic interactions were also deemed to be of secondary importance when no difference in the adhesion of more (carboxyl-PEG-QDs) or less (amine-PEG-QDs) negatively charged CdSe/ZnS quantum dots (QDs) onto the negatively charged bacterium Cupriavidus metallidurans CH34 was observed.29 Negatively charged nano-Ag interacted with membranes of negatively charged E. coli cells50 and un-capped nano-TiO2 induced toxicity in the negatively charged bacteria, regardless of their own negative surface charge at physiological pH.38 Hence, the possibility of other mechanisms than electrostatic forces governing the adhesion of ENPs to cell surfaces has been suggested.51 Nonetheless, electrostatic attraction cannot generally be ruled out.Adsorption of ENMs onto unicellular freshwater microalgae (Table 1) has been shown for nano-Au and the microalga Scenedesmus subspicatus52 as well as for SiO2 and CeO2 and the microalga Pseudokirchneriella subcapitata.39,53,54 According to Aruoja et al.,55 the same microalga, P. subcapitata, adsorbed 2.3 times its own weight of nano-TiO2 and the adsorption kinetics depended on pH, with the adsorption maximum at pH 5.5.56 Similarly, nano-TiO2 was shown to adsorb onto the cell walls of the microalga Chlamydomonas reinhardtii57 and so did nano-Ag58 and CdSe/ZnS QDs.59 Furthermore, negatively charged carboxyl CdSe/ZnS QDs were adsorbed onto cells of the wall-less strain but not onto wild-type C. reinhardtii cells.60
| ENP (size) | Organism | Location | Uptake mechanism | Analytical technique | Ref. |
|---|---|---|---|---|---|
| Ag (1–35 nm) | Ochromonas danica | Inside cells | Suggested: increased membrane permeability | TEM and STEM | 61 |
| Ag (29 nm) | Chlamydomonas reinhardtii | Inside cells | NA | HR-ICP-MS | 58 |
| Au (10 nm, amine-coated) | Scenedesmus subspicatus | In the cell wall | NA | TEM | 52 |
| FITC-mannose generation 0 (G0) glycodendrimer-coated Au (2 nm) | Wt and wall-less Chlamydomonas reinhardtii | On the cell wall of wt and inside wt and wall-less strains | NA | Fluorescence confocal microscopy and flow cytometry | 62 |
| CdTe/CdS quantum dots (core 3–4 nm, 5.7 ± 0.4 nm, 4.3 ± 0.4 nm, 4.2 ± 1.2 nm) | Chlamydomonas reinhardtii | Cd inside cells | NA | Graphite furnace atomic absorption spectrometry and ICP-MS | 63 |
| Carboxyl CdTe/ZnS quantum dots | Wall-less Chlamydomonas reinhardtii | Association with cell | NA | ICP-MS and flow cytometry | 60 |
| CuO, bare and polymer-coated (30–40 nm/148 nm, 65.4 nm) | Chlamydomonas reinhardtii | In cytoplasm | NA | TEM and ICP-AES | 64 |
| CuO (30–40 nm) | Chlamydomonas reinhardtii | Inside cells | Suggested: endocytosis, carrier proteins or ion channels | TEM | 65 |
| TiO2 (21 nm) | Chlamydomonas reinhardtii | In the cell wall, plasma membrane and cytoplasm | NA | SEM, TEM | 57 |
The adsorption of ENPs onto bacterial cells (Table 2) has, for instance, been shown for nano-CeO2 and the bacteria E. coli and Synechocystis,48,66 nano-Ag and laboratory-grown P. putida biofilm bacteria,67 CeO2 and the cyanobacterium Anabaena CPB4337,54 Fe0 and the cyanobacterium Microcystis aeruginosa,68 TiO2 and B. licheniformis,49 TiO2 and Al2O3 and C. metallidurans and E. coli,69 as well as for Al2O3, SiO2, TiO2, ZnO and the three bacteria B. subtilis, E. coli and Pseudomonas fluorescens.51 The same was observed for nano-Ag and the bacteria E. coli, V. cholerae, P. aeruginosa and S. typhus, which also entered the bacterial cells.47 Based on the observation that internalized nano-Ag were approximately the same size as those adsorbed onto the bacterial membrane, it was suggested that uptake may occur via changes in membrane permeability induced by interactions with the ENPs.47 As mentioned earlier, it is therefore very likely that interaction and adsorption are preconditions for the internalization of undissolved ENPs into bacterial cells. This presumption is supported by the hypothesis that adsorption may induce cell wall pitting50 or membrane damage, e.g. perforation,70,71 and thereby increase membrane permeability.44
| ENP (size) | Organism | Location | Uptake mechanism | Analytical technique | Ref. |
|---|---|---|---|---|---|
| Ag (12 nm) | Escherichia coli | In the membrane structure and inside cells | Suggested: increased membrane permeability | SEM, TEM and EDAX | 50 |
| Ag (16 nm) | Escherichia coli | On the cell membrane and inside cells | Suggested: increased membrane permeability | High angle annular dark field STEM and TEM | 47 |
| Ag (10–15 nm) | Escherichia coli | On the cell wall and inside the cell | NA | TEM | 70 |
| Ag (30–50 nm) | Pseudomonas putida biofilms | On and inside cells | NA | TEM, graphite furnace atomic absorption and ICP-MS | 67 |
| Ag (104–118 nm) | Escherichia coli and Bacillus subtilis | Inside cells | NA | High resolution microscopy, TEM, TEM-EDS | 72 |
| CuO (<5 nm) | Cyanobacterium Microcystis aeruginosa | Inside cells | Cell wall pores, endocytosis | FAAS, HRTEM, SEM, TEM and EDS | 73 |
| TiO2, Al2O3 (12–707 and 13, respectively) | Cupriavidus metallidurans and Escherichia coli | In the periplasmic compartment | NA | TEM and STEM | 69 |
| TiO2 (192 nm) | Cyanobacterium Anabaena variabilis | Inside cells | NA | HRTEM and Raman imaging | 71 |
| TiO2 (20–100 nm) | Bacillus licheniformis | Outer and inner sides of the plasma membrane and inside cells | Increased membrane permeability | SEM, TEM, FTIR and ICP-OES | 49 |
| Carboxyl-CdSe/ZnS core/shell QDs (12.9 nm) | Cupriavidus metallidurans | In periplasmic space | Loss of membrane integrity | TEM, EDS, AFlFFF and ICP-MS | 74 |
| ZnO (ca. 7, 260 and 800) | Escherichia coli and Staphylococcus aureus | On/in membranes and in the cytoplasm | NA | TEM, HRTEM, EDS | 75 |
| ZnO (30 nm), TiO2 (50 nm) | Escherichia coli | On the cell wall and inside cells | NA | TEM, SEM | 76 |
| ZnO (30 nm), TiO2 (50 nm) | Salmonella typhimurium | Smaller ENPs inside cells, larger ones on the membrane | NA | TEM and flow cytometry | 77 |
Overall, the adsorption of ENPs onto biosurfaces has been shown for various planktonic bacteria and microalgae, although the underlying mechanisms and modifying factors are not yet well understood. Electrostatic attraction is the most obvious explanatory factor for surface adsorption but does not explain all direct interactions. Other factors may come into play. Molecular dynamic simulations have shown that the adsorption of polymers and dispersants is sensitive to the affinity of the surface for water.78 This has recently been illustrated for the adsorption of polyacrylic acid (PAA) and polyaspartic acid (p-ASP) on calcite surfaces78 and the attachment of small peptides onto an amorphous TiO2 surface.79 The different conformations of PAA and p-ASP lead to different disruptions of the ordered water layer at the calcite crystal surface and thus contributed to different adsorption free energies, which resulted in a more rapid adsorption and longer residence times for p-ASP at the calcite surface.78 Also, the adsorption geometry of peptides was found to be directly influenced by local density changes in the water structure on the Ti or Si surface, which resulted in subtle differences in the adsorption configurations of different amino acid side chains (flat vs. upright). Calculations of adsorption free energy profiles revealed a correlation between the adhesion forces and the nanoscale features of the water structure at the solid/liquid interface. The authors concluded that electrostatic interactions are the major driving force behind approaching surfaces of opposite charge density but are of secondary importance for adhesion forces.79
Furthermore, the kinetics of the adsorption process is also largely unknown. The kinetics of particle adhesion (and internalization) with respect to the diffusion flux from the bulk solution to the cell surface can indicate the limiting factor of the bioavailable fraction (and thus of the observed effects). In the case where mass transfer is slower than the adsorption process, bioavailability will primarily be determined by the diffusion flux, which depends on the concentration of the contaminant. In the inverse case, bioavailability will largely depend on cell properties governing adsorption (i.e. ligand binding etc.), in which case surface properties of ENPs (such as coatings, adsorbed DOM layer etc.) and cells (such as cell wall structure, extracellular polymeric substances etc.) will likely play an important modifying role. For example, it has been suggested that the reduced cytotoxicity (and thus bioavailability) of polyelectrolyte/NOM-coated nano-ZVI towards E. coli cells may be explained by DOM preventing direct physical contact with cells.80 In bacteria, it has variously been shown that extracellular polymeric substances (EPS) can exert a protective function by decreasing ENP bioavailability by fending off ENP adsorption and toxicity. This was, for example, the case for Synechocystis cyanobacteria, which were able to prevent the adsorption of CeO2 ENPs onto their outer membrane by trapping the ENPs with extracellular polysaccharides, much in contrast to E. coli that does not excrete EPS.81Synechocystis thereby protected itself from direct toxic effects mediated by ROS, which require a close contact between ENPs and cell membranes, a finding also supported by a more recent study on Synechocystis and TiO2 ENPs.82 However, the extracellular polysaccharide layer of Synechocystis did not provide sufficient protection from indirect toxicity mediated by released toxic ions originating from CeO2 ENPs.81 The protective function of EPS has also been demonstrated for the bacterium Pseudomonas chlororaphis exposed to nano-CuO.83 Furthermore, engineered E. coli and S. meliloti overproduced EPS in reaction to nano-Ag exposure and thereby protected themselves from toxicity by trapping ENPs with EPS outside the cell.84
However, to our knowledge, no studies have directly addressed the kinetics of adsorption onto AMOs with respect to bioavailability (and toxicity) so far. Further progress is probably limited by the available techniques, since most direct evidence comes from imaging techniques, which require important sample preparation and are not well suited for kinetic studies in liquid media.
2.2 Internalization
The internalization of ENPs by bacteria and microalgae has variously been described (Tables 1 and 2), disproving the common hypothesis that ENMs cannot penetrate the protective, relatively thick cell walls of bacteria and microalgae12,71 without prior damage. For example, nano-TiO2 localized in the cell wall and cytoplasm of the microalga C. reinhardtii57 and inside cells of the nitrogen-fixing, prokaryotic blue-green alga Anabaena variabilis.71 Gold ENPs penetrated both the wild-type and the cell wall-deficient mutant C. reinhardtii strains62 and adsorbed onto the cellulosic layer of the cell walls of the microalga S. subspicatus but did not enter its cytoplasm.52 Bare and polymer-coated nano-CuO also penetrated cells of the microalga C. reinhardtii, and the higher toxicity of the polymer-coated nano-CuO was attributed to their greater cell-penetrating capacity.64 Another study on C. reinhardtii suggested, based on the measured intracellular Ag, that the number of bioavailable nano-Ag was limited compared to cationic Ag.58 However, nano-Ag was readily internalized and accumulated by the mixotrophic freshwater alga Ochromonas danica.61 Furthermore, Ag ENPs penetrated Gram-negative E. coli cells47,72 and the Gram-positive B. subtilis.72 ZnO and TiO2 ENPs were observed inside E. coli.76 Similarly, CuO ENPs were internalized by the cyanobacterium M. aeruginosa and uptake was enhanced by the presence of SRFA.73 The internalization of ZnO was observed in both the Gram-negative bacterium E. coli as well as the Gram-positive bacterium Staphylococcus aureus and was found to be size-dependent, i.e. smaller particles were internalized to a greater extent than larger ones.75 Maybe less surprisingly (considering their smaller size range), CdTe/CdS QDs were also taken up by C. reinhardtii cells, but the observed bioaccumulation, in contrast, was greatest for the largest (orange) QDs.63 However, above observations should be considered cautiously as the techniques for the investigation of ENM uptake have several limitations. While visual techniques provide qualitative information on subcellular localization, techniques based on mass spectrometry provide quantitative data on uptake but cannot differentiate between ionic and particulate forms. Flow cytometry can also furnish quantitative information on uptake but cannot necessarily distinguish between uptake and surface-associated ENMs. Although there is evidence for the internalization of ENMs in AMOs such as algae and bacteria, the underlying mechanisms are neither understood nor are the uptake rates known.Nonetheless, the tough, more or less tensile and rigid, ca. 20 nm thick59 cell walls of bacteria and microalgae form efficient first-tier barriers that screen the few nanometer thick cell membranes enwrapping the cytoplasm from the environment.44 Cell walls are thus an important modulating factor of nano–bio interactions as demonstrated by a study on CdSe/ZnS QDs and the microalga C. reinhardtii, in which an association of QDs with the wall-less mutant strain was observed but not with the walled wild type.60 Similarly, the cell wall-deficient marine microalga D. tertiolecta was more sensitive to nano-Ag induced ROS and lipid peroxidation than the freshwater microalga C. vulgaris.85 Notwithstanding the generally suggested protective function of cell walls, it has also been shown that algal (C. reinhardtii) and bacterial (Vibrio fischeri) cells were more sensitive to nano-Au than two mammalian cell lines.86 Cell walls contain pores with diameters ranging 5–20 nm.87,88 These pores are potential entry ports for small ENMs, which may then become accessible for internalization across the lipid bilayer. The permeability of cell walls changes throughout cell cycling especially during the delicate phase of cell division in the course of which cell walls are newly synthesized.73,89–91 Also, there is the possibility that interactions of cells with ENMs may even induce the formation of larger new pores permeable to bigger ENMs.89
Once the cell wall has been penetrated, the cellular internalization of single particles or aggregates occurs through direct ingestion or across epithelial boundaries,92i.e. the semi-permeable phospholipid bilayer or plasma membrane. Stable primary ENPs can theoretically passively diffuse through hydrophobic lipid bilayers, given they are nonpolar, i.e. uncharged and not too large. However, this has, to the best of our knowledge, not yet been shown to occur in bacteria and microalgae.
Theoretically, ENPs may possibly also appropriate membrane fusion as a non-invasive entry route, commonly observed to occur with biomacromolecules or alternatively, if cationic, may also be capable of creating transient membrane holes,93 both of which remain to be shown. An uptake of ENPs via transport proteins for ions is deemed rather unlikely because they are much larger than ions (ranging from 30 pm to ≥200 pm) and therefore most likely do not fit the respective binding sites,43 unless of course, if they dissolve.
Endocytic pathways via membrane-bound vesicles that emerge from inward folded plasma membrane buds, which are then pinched off, have been suggested to be a possible internalization route for ENMs into the cytoplasm. However, prokaryotes do not have endocytic mechanisms for the transport of “bulk” particles across their membranes. Much in contrast to eukaryotes that have evolved highly developed mechanisms for the active internalization of bulk materials via endocytosis92 (Fig. 2). Endocytosis has, in some cases, been suggested as the main ENM uptake route into bacteria and microalgae (see Tables 1 and 2), but it remains to be shown whether endocytosis is the predominant uptake pathway of ENMs into AMOs. In most cases, the actual uptake routes are unknown.
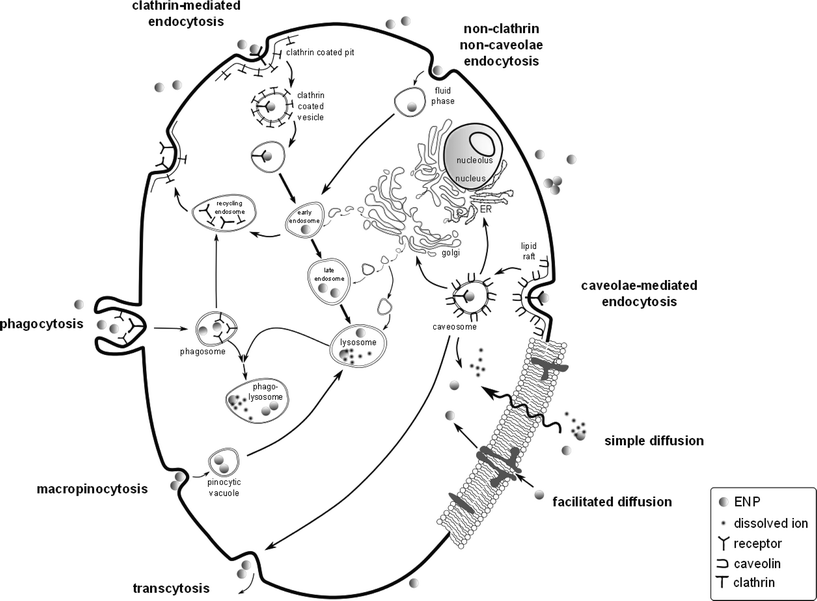 | ||
| Fig. 2 Active and passive cellular uptake pathways for ENMs in eukaryotic cells. Passive uptake occurs via diffusion and facilitated diffusion via transport proteins, i.e. gated channel proteins and carrier proteins. Active uptake pathways involve transmembrane carrier proteins and endocytic pathways including receptor-mediated phagocytosis, clathrin-mediated endocytosis (120 nm, via clathrin-coated pits) and caveolae-mediated endocytosis (60 nm, via lipid rafts), non-specific endocytosis by macropinocytosis and non-clathrin, non-caveolae endocytosis (90 nm, fluid phase). All pathways except caveolae-mediated endocytosis and diffusion merge with the lysosomal degradation system comprising numerous vesicle maturation steps within the cell. A lysosome typically ranges from 0.2 to 0.5 μm in diameter.98 Phagocytosis is mediated by specific membrane-receptors that are activated upon contact with a ligand to produce phagosomes (>250 nm). During their maturation process, phagosomes transform into late phagosomes, which fuse with lysosomes to form phagolysosomes. During macropinocytosis, internalization occurs via an unspecific invagination resulting in pinocytic vesicles (<150 nm), which eventually merge with lysosomes. Clathrin-mediated endocytosis and non-clathrin, non-caveolae-mediated endocytosis produce endosomes which evolve into early and late endosomes that mature into acidic lysosomes (pH = 5). Caveolae-mediated endocytosis produces caveosomes that either transfer their contents into the Golgi apparatus, endoplasmatic reticulum (ER) or into the cytosol or may also undergo transcytosis. Adapted from ref. 11, 92, 99 and 100, not to scale. | ||
Ample evidence from mammalian cells suggests that endocytosis is size-dependent.94 It has been put forth that the optimal sizes for endocytosis are diameters around 50 nm,95 which corresponds well to theoretical model calculations yielding optimal radii between 27 and 30 nm for spherical particles.94 Thus, the 1–100 nm scale can be considered the most critical size dimensions for biological interfaces,96 especially since it is assumed that the two main endocytic entry routes for ENMs into eukaryotic cells are the clathrin- and caveolae-mediated endocytic pathways.92 Simulation results have furthermore suggested that cationic ENPs are more readily internalized than anionic ENPs, which must first overcome an energy barrier to reach the net negatively charged surface of the lipid bilayer.97 However, this hypothesis remains to be corroborated by conclusive experimental data for AMOs.
Uptake of dissolved ENMs occurs by the same internalization routes as for trace metals, which principally depends on the metal form (i.e. charge, valency, size etc.) and the biological substrate (i.e. availability of pathways). These include active and passive transport mechanisms via (non-)specific transporter (carrier) proteins and channels, co-transport, endocytosis or simple diffusion.1,3
Hence, ENPs of different chemical composition and various sizes (Tables 1 and 2) are capable of penetrating the cell walls and plasma membranes of Gram-negative and Gram-positive bacteria as well as microalgae. However, it seems that in at least some cases membrane damage occurs during the process and it remains to be shown if this is size or particle related. Furthermore, excretion of ENMs can theoretically occur by exocytosis,43 but we have found no experimental accounts of this mechanism for microalgae and bacteria.
2.3 Intracellular fate
Understanding the intracellular fate of ENMs is critical to the quantitative link of internalized ENMs to their toxicity, since biological effects are a complex function of a contaminant's cellular concentrations, intracellular distribution and speciation. ENMs internalized by AMOs reach the cytoplasm where they potentially interact with cellular organelles. However, data concerning their intracellular distribution and localization in cells and organisms of ecotoxicological relevance are sparse, apart from a handful of isolated accounts. For example, nano-CuO in C. reinhardtii cells formed large agglomerates which mainly localized in the cytoplasm and were likely enclosed by vacuoles.64 Nano-TiO2 accumulated inside its cell wall and plasma membrane.57 Similarly, gold ENPs localized in the cellulosic layer of the trilaminar membrane of S. subspicatus microalgal cells.52 Nano-Ag was found in irregularly shaped vacuoles of the microalga O. danica and it remained unclear whether Ag ENPs exerted toxicity directly by their presence or indirectly by the release of Ag+.61 Similar observations have been made in bacteria models. For instance, nano-Ag was found in the membrane and inside E. coli.47,50,70 TiO2 and Al2O3 ENPs adsorbed onto the surfaces of C. metallidurans and E. coli and accumulated in the periplasmic compartments of both species.69 Similarly, CdSe/ZnS functionalized QDs adsorbed onto the bacterium C. metallidurans and accumulated in its periplasmic space.74 In the Gram-positive bacterium B. licheniformis, nano-TiO2 accumulated on the outer and inner sides of the plasma membrane and in the cytoplasm.49 ZnO ENPs were detected in the membranes and cytoplasm of E. coli and S. aureus.75 Another study described the uptake of ZnO and TiO2 ENPs into E. coli cells and observed a uniform distribution of smaller ENPs in the cell and the adherence of agglomerated particles onto the cell wall77 (Table 2).Intracellular localization is largely determined by the chemistry of the biological fluids92 and the dynamic biopolymer (e.g. protein) corona on the particle surface.13 Unfried et al.99 emphasize the importance of clarifying the relation between uptake and subsequent cellular distribution patterns in future research. ENMs in physiological fluids such as cytoplasm or interstitial fluids are immediately coated by biomolecules and proteins that form a dynamically changing biopolymer corona of partial (to, rather unlikely, full) coverage on a particle's surface that confers a unique biological identity.101,102 The currently prevailing hypothesis proposes a highly dynamic corona consisting of a strongly associated hard phase and a more loosely bound soft phase. Polymer-coated ENPs may be more resistant to the formation of a protein corona, especially since coatings often are specifically designed to increase their biocompatibility. For ENPs which have previously acquired a dissolved organic matter (DOM) coating in the environment, the formation of a protein corona will be dominated by the competitive exchange between proteins, DOM and other present coatings for the core surface. However, we are not aware of any studies investigating these interactions.
What we can derive so far is that protein adsorption kinetics (association and dissociation) is governed by competitive binding mechanisms and furthermore by time, particle size (i.e. surface curvature), initial material surface properties, previous surface interactions and by the proteins present in the surrounding media and their respective equilibrium constants.13,103,104 Protein concentrations and equilibrium binding constants determine the composition of a corona at any given time101 and particle–protein complexes can last microseconds to days.13 But it is still unknown how the epitopes of proteins embedded in the corona are affected in their ability to perform their usual biological functions.103,105 It is however recognized that the protein corona strongly influences all interactions at the particle–bio interface as well as the biological fate and effect of ENMs and that biological responses may either reflect the adsorbed protein corona, the material itself or both.101 Yet, the ramifications for nanoecotoxicology have, to our knowledge, hardly been investigated and research in this area has almost entirely focussed on mammalian model cells.
While the bioavailability (and toxicity) of trace metals is thought to be directly linked to their ability of crossing membranes and is commonly predicted by internal metal concentrations or uptake rates,106 this relation does not necessarily apply to ENMs in the same way. Firstly, specific uptake rates and bioconcentration factors of ENPs are currently not available for AMOs. Secondly, the current body of nanotoxicological evidence suggests that the bioavailability (and toxicity) of ENMs is possibly independent of internalization and thus more complex. It has, for instance, been shown that ENPs can indirectly trigger adverse biological responses without direct contact or uptake (via ions and ROS) and it has also been shown that internalized ENPs do not cause harm per se.41 Thus, the actual toxicity mediating the ENM form has so far not yet been explicitly identified. Whether ENP toxicity is ion or particle mediated is still an avidly debated question in nanoecotoxicology and the literature contains copious contradictory findings, which are treated in the section on dissolution.
In the following sections, toxicity will thus be considered as a preliminary proxy for the bioavailability of ENPs to AMOs. Sections three and four are devoted to the effects of agglomeration and dissolution on ENP bioavailability as well as to the modifying environmental factors.
3. ENM agglomeration and bioavailability
ENP agglomeration behavior can largely be explained by fundamental colloid science and there is increasing knowledge of the physical transformations of ENPs in freshwater and how these are affected by water quality parameters.107 However, there is little knowledge of how agglomeration influences ENP bioavailability (and toxicity); in fact, very few studies actually address the direct relation between ENM agglomeration state and bioavailability to AMOs. Since the role of undissolved, particulate ENMs is still uncertain with respect to ENM toxicity, the role of agglomeration can only be resolved once the bioavailable ENM form responsible for the observed toxic effects has clearly been identified and quantified (i.e. particles vs. dissolved fractions etc.).Generally, agglomeration is expected to diminish ENM fluxes towards biointerfaces (by increasing hydrodynamic size and decreasing their diffusivity) as well as the number concentration and available surface area and interfacial free energy for adsorption and reaction.31,53,108,109 But even this basic premise is still controversial since very few existing studies on AMOs report contradicting findings. The above assumption was, for example, confirmed for the crustacean Daphnia magna (immobilization) exposed to nano-Ag in OECD medium109 but was refuted by a recent study investigating the effect of agglomeration of nano-CeO2 on growth of the microalga Pseudokirchneriella subcapitata, which was independent of agglomeration. Similar findings were reported for tungsten carbide, albeit for gill cells of the rainbow trout Oncorhynchus mykiss, in which rapid agglomeration in minimal exposure media did not prevent uptake and subsequent toxicity.110
In addition to particle-specific characteristics, medium pH, ionic strength and DOM concentrations cause changes in the particle surface charge of metal oxides,22,27 thus affecting interparticle interaction forces and agglomeration behavior.111,112 Hence, medium properties likely affect ENM bioavailability. For example, Guzman et al.22 pointed out that various materials have pHIEP values far from the pH of natural systems, e.g. uranium oxides (pHIEP ∼ 5), iron oxides (pHIEP ∼ 8), zinc sulfides (pHIEP ∼ 2) and aluminum oxides (pHIEP ∼ 9), which, in light of the basic premise discussed above, favor stable suspensions and high fluxes towards biointerfaces. At any given pH, the aggregation of nano-TiO2 suspensions increased with increasing ionic strength.112 This was also observed for nano-Ag.113 The influence of the nature of electrolytes present in the aqueous suspensions under investigation was nicely illustrated in a study comparing the effects of increasing Na+ and Ca2+ concentrations by French et al.114 who observed the agglomeration of nano-TiO2 in the presence of the divalent cation Ca2+ within 5 min, which is significantly faster than the 15 min agglomeration time observed in the presence of monovalent Na+ at the same ionic strength (0.0128 M) and pH (4.8). The above non-exhaustive examples illustrate that water hardness (e.g. Ca2+) and increased water salinity (e.g. high ionic strength) could favor ENM agglomeration. By the same token, water hardness and other major ionic species are thus expected to mitigate ENM bioavailability and thus protect planktonic bacteria and microalgae from ENM-induced stress, but further experimental evidence is necessary to support such as a general consideration.
The effects of DOM on ENM suspensions are complex and can be difficult to predict.31 Depending on DOM composition, pH and ionic strength conditions, DOM can either have a stabilizing effect on suspensions,24,25,113 favor agglomeration via bridging or even cause de-agglomeration of a given ENM.10,23,25,31,53,89,112,115–117 The stabilizing effect of DOM is thought to be due to the combined effects of increased steric hindrance118 and increased electrostatic inter-particle repulsion due to increased particle surface charges resulting from the adsorption of the negatively charged DOM.24 For example, the nano-ZnO agglomerates formed at pHIEP (pH = 9.3) decreased in size as concentrations of Suwannee River humic acid (SRHA) were increased from 0.1 to 0.5 mg L−1, which is explained by the formation of an adsorbed layer of negatively charged SRHA causing an increase in surface potentials and the resulting electric double layer repulsive energy and steric repulsion.119 Stabilizing effects of DOM have, for example, been also shown for nano-TiO2 (<25 nm) in lake water,49 15–30 nm sized nano-TiO2, nano-ZnO and nano-CeO2 suspensions under many different natural conditions120 as well as for TiO2 ENPs (5 nm) in the presence of SRFA.26 Similarly, bacterial extracellular proteins were found to stabilize nano-Ag.121 In yet another study, DOM was even found to counteract the agglomeration-inducing effect of increasing ionic strength towards nano-Ag suspensions, producing more stable suspensions.122 Chowdhury et al.123 found that both the bacterium E. coli and SRHA significantly stabilized nano-TiO2 suspensions but that the stabilizing effect of DOM was greater. They also observed less nano-TiO2 deposition in the combined presence of DOM and E. coli compared to each of the compounds alone, implying highly complex interactions. DOM-induced destabilization has also been variously observed. For example, nano-TiO2 suspensions were destabilized by the two organic acids, oxalic and adipic acids.108 DOM-induced de-agglomeration has also been described for iron oxide ENPs at pH 7. It was found that high concentrations of SRHA – as opposed to low SRHA concentrations or none – induced the de-agglomeration of the iron oxide ENPs over time.124 In the same way, SRHA caused a partial disaggregation of nano-Ag agglomerates by coating individual ENPs with a thin film, which stabilized the particles by charge and steric effects yielding nicely dispersed, stable suspensions of primary Ag ENPs.67 Another study investigating the effects of environmental concentrations of SRHA (0.02 to 0.5 mg L−1) on nano-ZnO suspensions concluded that the SRHA surface coatings on the ZnO ENPs induced the disaggregation of ZnO aggregates and a reduction in their size with increasing SRHA concentrations.119 Environmental concentrations of HAs and alginates were found to not only stabilize TiO2 suspensions (even at high concentrations) but also cause the disaggregation and dispersion of already formed TiO2 aggregates.115 Domingos et al.112 observed stable dispersions of TiO2 ENPs under environmentally relevant conditions (SRFA, pH, I) and inferred that stable TiO2 dispersions may occur more frequently in natural aquatic environments than predicted. A similar conclusion was also drawn by Cumberland and Lead113 and Khan et al.121 from their observations on the behavior of nano-Ag under environmentally relevant conditions, which implied longer residence times and thus higher bioavailability than initially expected. A recent study investigating different divalent cations (Ca2+ or Mg2+) and pH values with DOM concentrations of European streams demonstrated that the agglomeration behavior of nano-Au was, to a large extent, determined by DOM.107 Therefore, depending on ENP properties and environmental conditions, certain ENMs form well-dispersed, stable suspensions in the water column. Others will form large particle clusters/agglomerates, which at a critical threshold size, sediment and decrease the particle concentration in the water column, at least temporarily.125,126 According to the above premise, the former case thus favors high mobility125 and fluxes towards microorganisms, while in the latter case, sedimentation leads to a decrease of the number of suspended ENMs available for interaction with planktonic AMOs.
Another important assumption, especially with respect to ENPs whose main bioavailable forms are ionic (see next section), is that agglomeration can minimize dissolution by kinetically interfering with the diffusion process.127 This has, for example, been confirmed for the dissolution rate of nano-Ag, which tended to be slower for agglomerated ENPs.128,129
Apart from these single accounts, the question of how agglomeration relates to the bioavailability of ENMs to AMOs has, to the best of our knowledge, not yet been systematically addressed. The scarcity of articles may possibly also be due to a publication bias in favour of positive (toxic) effects. Bridging the gap between ENP behavior and effects as well as the identification of the critical bioavailable ENM forms thus remains a major research priority.
4. ENM dissolution and bioavailability
On the one hand, dissolution leads to reduced environmental persistence of ENMs and their long-term bioavailability.31 On the other hand, the released toxic ions are immediately and easily bioavailable to AMOs and may trigger acute toxic effects.130 Thus, dissolution decisively influences ENM bioavailability (and toxicity)60,89,131 to algae and bacteria, which are thus exposed to complex mixtures of ENMs, partially dissolved ENMs, free/complexed dissolved species and ions adsorbed to ENM surfaces.132Ion-mediated toxicity of ENPs has been demonstrated by numerous studies, most prominently for nano-ZnO, nano-CuO and nano-Ag.131 A recent literature review on the ecotoxicity of ZnO ENPs has, for example, concluded that ionic Zn was the main mediator of detrimental effects towards bacteria, algae and plants, aquatic and terrestrial invertebrates and vertebrates.12 Examples of Zn2+ as mediators of nano-ZnO toxicity towards AMOs include the freshwater microalga P. subcapitata,55,111 the bacteria E. coli, B. subtilis, V. fischeri and P. putida (measured as inhibition of bacterial luminescence (INH), minimal inhibitory concentration (MIC), mortality, growth inhibition, or cell viability).133–135 Likewise, the solubilized bioavailable fraction of nano-CuO ENPs was linked to the growth inhibition of microalga P. subcapitata.55 There is also a wealth of articles describing Ag+ ion-mediated antimicrobial effects of nano-Ag.47,72,136–143 Recently, Xiu et al.144 decidedly ruled out direct particle-specific biological effects by showing no toxicity of nano-Ag towards the bacterium E. coli under anaerobic conditions, precluding the oxidation of Ag and thus the release of Ag+. The hypothesis of ion-mediated ENM toxicity is further supported by findings showing how organic molecules can decrease the bioavailability of the released ions and thereby mitigate acute, short-term toxicity. For example, the complexing agent tannic acid significantly decreased the toxicity of nano-ZnO towards the bacterium P. putida by decreasing the bioavailable fraction of dissolved Zn2+ and was more efficient in doing so than humic, fulvic and alginic acids.134 On the other hand, the effects of nano-Ag towards the planktonic bacterium P. fluorescens were independent of the presence of standard SRHA.67 Furthermore, the toxicity of ZnO ENPs towards E. coli, mediated by dissolved Zn2+, was mitigated by the presence of cations (Ca2+, Mg2+) and increasing pH, HPO4− and DOM.145 Thus, in the above cases where the main bioavailable fraction responsible for ENM toxicity is unequivocally found to be mediated by dissolved species, the conventional bioavailability models described at the end of this section can be directly applied.
However, despite the numerous studies supporting the role of dissolution in toxicity, the current body of evidence suggests that the toxicity of soluble ENMs is more complex and cannot generally be simply reduced to dissolution, i.e. that the major bioavailable forms are not ionic but may be particulate or a combination of different forms (both particulate and ionic). As such, the growth inhibition of CuO, NiO, ZnO, and Sb2O3 ENPs towards the three model bacterial species E. coli, B. subtilis and S. aureus was found to be principally due to particle rather than to ion “species”146 and the MIC of ZnO ENPs towards the bacterium S. meliloti was higher than that of ionic Zn.147 Also, it was concluded that dissolved Ce4+ was not responsible for the observed growth inhibition of CeO2 ENPs towards the freshwater microalga P. subcapitata.148 A specific ENP intrinsic effect has also been shown for Ag ENPs towards the bacterium P. fluorescens67 and towards activated sludge communities composed of various bacteria.149 Results obtained by Burchardt et al.150 suggest a shared effect of Ag ENPs and released Ag+ towards the diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp., which has also been observed for Ag ENPs and E. coli (via ROS, measured by a recombinant luminescent bacterium)151 and Ag ENPs and the microalga C. reinhardtii (measured as photosynthetic yield).152 Similarly, Dimkpa et al.83 concluded that released Cu ions only partially account for the observed effects of CuO ENPs towards the soil bacterium Pseudomonas chlororaphis O6 and neither did released Zn2+ ions fully explain the Chlorella sp. growth inhibition by ZnO ENPs.153 Other results suggest that the observed effects of the metal oxide ENPs Al2O3, SiO2 and ZnO towards the bacteria B. subtilis, E. coli and P. fluorescens were not only due to released ions but also due to their tendency to attach to the cell walls of the exposed organisms.51 Stable colloidal suspensions of Ag ENPs were more toxic towards P. fluorescens biofilms than purely ionic Ag and highly aggregated Ag ENP dispersions, but Ag+ significantly contributed to the reduction of viability.154 Hence, there are a considerable number of cases in which ionic species do not seem to be the primary bioavailable ENM form responsible for toxic effects. In these cases, a clearer picture of ENM toxicity mechanisms is necessary to enable appropriate modifications to the conventional bioavailability models.
Dissolution of ENMs, especially under natural conditions, remains difficult to predict, as discussed elsewhere.132 The most relevant medium properties include temperature, pressure, pH, metal solubility, ionic species and the presence of complexing agents (e.g. biological metal ion chelators, DOM),17,34,131 which affect both ENP solubility and the chemical speciation in the medium.
Overall, a thorough understanding of dissolution processes and the chemical speciation of released metal ions is required for the proper description of ENM bioavailability and ion-mediated toxicity. In this regard, the existing general models applied to trace metals, which incorporate chemical speciation (such as the free ion activity model155), the competition between metal species and biotic ligands (i.e., biotic ligand model (BLM)),8 biodynamics156 or more general dynamics linking chemo- and biodynamic models130 may be useful starting points for the development of similar models for ENPs, especially for those cases in which ionic species are not the sole mediators of biological effects, for which particles or a mix of the two likely play crucial roles. We have seen that the interactions (and thus the resulting effects) of both dissolved ions and ENPs with biota are affected by different environmental factors including pH, major cations and anions, trace elements, ligands of different origins, dissolved organic matter and natural colloids. In general, hardness cations such as Ca2+ and Mg2+ are known to play a protective role with respect to biota by competing with toxic metal ions for the binding sites on biological membranes. Therefore, major cations could be expected to mitigate the toxicity of the ENPs by competing with released Ag+, Cu2+ and Zn2+ ions for the uptake sites on algal and bacterial surfaces33 and thereby modifying ENM surface properties and charge, favoring aggregation of the ENPs and decreasing their persistence and the probability of contact with biota. pH affects ENP dissolution, surface charge, aggregation and thus ENP reactivity. DOM could promote the dissolution of ENMs, but on the other hand DOM decreases the bioavailability of the dissolved ions by complexation. Further studies, particularly for low, environmentally relevant concentrations of ENMs, are necessary to better understand which conditions favor the action of which specific ENM species (i.e. dissolved ions or particles).
5. ENM surface transformations and bioavailability
In addition to dissolution and aggregation processes, ENMs can bind different natural and anthropogenic substances in the ambient medium or undergo other chemical transformations such as sulfidation and oxidation. Surface coatings of ENMs can play crucial roles in nano–bio interactions. Engineered or natural (DOM, proteins, lipids etc.) surface coatings transform ENP surface properties and thus play a very important role in the bioavailability of ENMs.157 For example, it has been shown that citrate-functionalized nano-Ag exhibited more ready dissolution and higher toxicity towards the nitrifying bacterium Nitrosomonas europaea than the less well stabilized gum arabic and polyvinylpyrrolidone-stabilized nano-Ag suspensions.158 Surface functionalization also strongly influenced the toxicity of three differently organo-coated nano-Ag ENPs towards the bacterium E. coli159 and increased the bactericidal properties of glucosamine-functionalized nano-Cu by UV irradiation.160 A comparison of the toxicities of bare and polymer-coated nano-CuO towards the microalga C. reinhardtii showed that coated nano-CuO was more toxic than uncoated nano-CuO whose cell-penetrating capacity was lower.64 Another study demonstrated how G0 polyamidoamine dendrimer-coated nano-Au induced very low toxicity in two mammalian cells (N2A and Vero) but caused adverse effects on the bacterium V. fischeri and the microalga C. reinhardtii, which was explained by the different cellular structures and by the different modification of the ENP surface properties in the respective cell culture media.86 Hence, exposure media play a crucial role and acquired surface coatings of ENPs in the environment (e.g. protein/DOM corona) can decisively influence internalization processes.44 This was nicely illustrated by findings showing enhanced cellular uptake and subsequent toxicity of nano-CuO in the prokaryotic microalga M. aeruginosa in the presence of SRFA.73ENM transformation by sulfidation was first detected for nano-Ag161 but is especially relevant for class B soft metal cations (e.g. Ag, Zn and Cu) due to their high affinities for electron-dense S.31 Sulfidation has been shown to decrease the dissolution of nano-Ag and effectively reduce its bactericidal properties.143,162–164 Other findings in environmentally realistic exposure scenarios have recently observed adverse effects of nano-Ag towards microbes in spite of prior transformation in biosolids through oxidation and sulfidation.165 The oxidation of nano-Ag produced Ag(NH3)+ in solution and led to an increase in dissolution rates and ion-mediated toxicity towards the ammonia-oxidizing bacterium Nitrosomonas europaea.166
ENPs in the environment are also known to adsorb anthropogenic substances in aquatic systems, such as surfactants,167 trace metals or persistent organic pollutants,168 which could considerably modify their bioavailability. This is supported by findings showing an increased adsorption of Cd(II) on HA-coated TiO2 ENPs as compared to bare, uncoated TiO2 ENPs57 and increased phenanthrene sorption by HA-coated TiO2 and ZnO ENPs,169 which may lead to either a decrease or an increase in the pollutants' bioavailabilities. This is evidenced by a study showing decreased bioavailabilities of Cu and Pb in solution to the algal strains Chlorella kessleri and C. reinhardtii in the presence of carboxyl-CdSe/ZnS QDs by a factor of 2.5 and 2, respectively.60 In fact, ENMs such as nano-Fe, nano-TiO2 or silica monoliths are also being applied in the targeted decontamination of wastewater from various organic environmental contaminants via adsorption.37,170 On the other hand, the same QDs increased the bioavailability of Cu and Pb to the wall-less C. reinhardtii strain by a factor of 4 and 3.5, respectively.60 In the case of increased bioavailability, ENPs become carriers that facilitate the internalization of previously unavailable pollutants by the so-called “Trojan Horse” mechanism. These kinds of mixed effects have received very little attention so far and remain to be investigated systematically. What is more, for nano-Ag, it was found that laundry surfactants (anionic sodium dodecylbenzenesulfonate (LAS), cationic dodecyl trimethylammonium chloride (DTAC), and nonionic Berol 266) modified ENP surface and significantly influenced speciation and stability.167 Depending on charge and concentration, surfactants either reduced or enhanced the degree of particle agglomeration. These interactions affected the mobility of Ag ENPs, which, depending on the reversibility of the surface modifications and exposure settings, can positively or negatively affect ENP bioavailability.89 Apart from this rather singular account, we virtually know nothing about mixed effects of this type, which potentially complicate the prediction of ENM behavior and bioavailability in natural aquatic systems. Their possible impact on ENP bioavailability remains to be examined.
6. Conclusions and outlook
Though an ever more accurate understanding of particle behavior in complex systems has been attained and a wealth of ecotoxicological information has been gathered on the environmental hazards of ENMs in recent years, the link between the two aspects is comparably less developed. There are still considerable knowledge gaps with respect to ENM bioavailability in the environment. Most importantly, ENM “speciation” in natural aquatic systems is not well understood or quantified. The major bioavailable fractions responsible for toxic effects have not been concludingly identified either. Considerable knowledge gaps remain with respect to the effects of agglomeration on bioavailability as well as on the exact uptake routes, intracellular compartmentalization as well as DOM–protein competition on the surface of internalized ENPs. Hence, the concept of bioavailability may provide a useful framework to link “chemical and physical speciation” of ENMs with their possible biological effects but require some ENM specific adaptations. In the environment, ENMs are present as complex mixtures of different chemical and physical forms including particles, particles complexed with DOM, sulfidized/oxidized forms, agglomerated and (partially) dissolved forms etc. For a given ENM, laboratory studies revealed that the relative proportion of these forms depends on the environmental parameters such as water hardness ions, pH and DOM.In the case where particles as such are the major bioavailable fraction mediating toxicity, water hardness cations are expected to reduce the bioavailability of ENMs by favoring aggregation and thus protecting algae and bacteria from ENM stress. In the case where solubilized ENM forms are the main bioavailable form, hardness cations will reduce their bioavailability by competing with the released ions for sensitive sites on biological membranes. Similarly, ambient pH values are anticipated to affect aggregation, dissolution and interactions with bio-interfaces. Circumneutral pH values typically found in surface waters will favor the agglomeration of the ENMs with close pHIEP. However, it has already been shown that the aggregation behavior of ENPs in surface waters is largely determined by DOM. Indeed, DOM is anticipated to play a key but dual role in ENM bioavailability to algae and bacteria. On the one hand, DOM is expected to affect chemical and physical “speciation”, e.g. by complexing ENPs and favoring the formation of stable suspensions, which would favor their contact with biointerfaces. On the other hand, DOM also binds a solubilized metal, which will decrease the bioavailability and uptake of dissolved ENM species. Therefore, for the same ENM, different bioavailabilities must be expected under different environmental conditions.
It is currently unclear if it is realistic (by analogy to free metal ions) to search for a specific form that could be used as a measure of the biological reactivity of the medium. Accordingly, the development of analytical tools and models that enable the characterization of ENM “speciation” or the measurement of specific dissolved and/or nanoparticulate forms would be highly useful. Also, it is still unclear if specific properties can universally be considered important for the uptake and toxicity of inorganic ENMs. In the case of soluble ENMs, it seems important to quantify and distinguish the contribution of soluble and particulate fractions to toxicity, as well as to define the “limiting” condition corresponding to the prevailing contribution of one or the other form.
To verify if basic ecotoxicological paradigms are applicable to ENM toxicity, it is crucial to improve our current understanding of possible particle-specific uptake routes in planktonic bacteria and microalgae, the kinetics of the uptake and excretion processes, evaluation of the importance of internalization with respect to adsorption to surfaces, as well as the intracellular fate of ENMs in algae and bacteria. Furthermore, it is still unsure whether endocytic pathways are common in microorganisms such as planktonic bacteria and microalgae or rather are an exception. It is also still unclear if cellular uptake is a precondition for biological effects and if such a general principle, which is proven for conventional micropollutants, is also valid for ENMs. We have seen that biological effects can, but not necessarily, be triggered by ENMs directly reacting with sensitive sites on the biological membrane and/or by the penetration of biological barriers (internalization), i.e. cell walls or epithelial boundaries and membranes. However, there are processes and indirect mechanisms of toxicity for which direct contact of ENMs with biota is not necessarily required. These include (i) the dissolution of ions, (ii) the production of ROS (mediated by UV, discontinuous crystal planes or material defects) and (iii) redox cycling and catalytic chemistry (e.g. Fenton and Quinone reactions), which can lead to oxidative damage.41,171,172 In the case in which these processes dominate, the applicability of the bioavailability concept to ENMs may be limited due to the complexity of these interactions. The Trojan Horse mechanism is another important possible mechanism of indirect ENM toxicity, which is not considered in the bioavailability framework. Despite these limitations, the integration of key modifying parameters is critical to develop a deeper understanding of the bioavailability of ENMs and to establish general principles that allow us to link environmental transformations of ENMs with their potential effects. Furthermore, a well-established bioavailability concept would allow the development of descriptive and predictive models capable of taking into account specificities of different environmental systems and enable extrapolations of laboratory results to the natural environment. Important advances in understanding environmental hazards of ENMs to different organisms and their interactions at nano–biointerfaces were mainly achieved by bioassays in well-controlled media with relatively high ENP concentrations. However, in the environment, ENPs are subjected to complex and highly dynamic physical, chemical and biological transformations, which will alter their interactions with biota and toxicity.31,162 A first attempt has already been done to predict the speciation and prevailing forms of Ag based on thermodynamic principles in various environmental scenarios relevant for natural and constructed environments (wastewater treatment plants).162
Box 1. Definitions
| Nanomaterials |
The European Commission has recently recommended the following definition of nanomaterials:14
“‘Nanomaterial’ means a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1 and 50%.” There is currently no universally accepted definition which is equally practical and unambiguous to industry, consumers, legislators and scientists alike. There are good scientific grounds to argue that the 100 nm limit is scientifically somewhat imprecise, if not arbitrary.15–17 Some argue that the nano-specific, non-bulk properties only emerge below a critical size of about 20–30 nm or below.17,18 Kreyling, Semmler-Behnke and Chaudhry15 provided a good overview of the different definitions currently in use. Furthermore, it is also important to clearly distinguish between engineered and naturally occurring nanoparticles,19 which are ubiquitous in all water bodies and crucially influence water chemistry.20 For the purpose of this review, we will adhere to the above definition and designate manufactured nanomaterials and nanoparticles “engineered” nanomaterials (ENMs) and nanoparticles (ENPs), respectively. |
| Bioavailability | According to the International Union of Pure and Applied Chemistry, biological availability is defined as “the extent of absorption of a substance by a living organism compared to a standard system”.2 |
| Agglomeration and aggregation | The term agglomeration is used for loosely adhering clusters of particles without any chemical bonds. Aggregates are clusters of particles irreversibly held together by chemical bonds21 (the two terms are, however, commonly used ambiguously and interchangeably). |
Acknowledgements
Many thanks to the anonymous reviewers who have helped improve this article. This work was financed by the Swiss National Science Foundation in the framework of the Swiss National Research Program 64 on the Opportunities and Risk of Nanomaterials.References
- M. Newman, Fundamentals of ecotoxicology, CRC Press, Boca Raton, FL, USA, 3rd edn, 2010 Search PubMed.
- M. Nordberg, J. H. Duffus and D. M. Templeton, Explanatory dictionary of key terms in toxicology: Part II, Pure Appl. Chem., 2010, 82, 679–751 CrossRef CAS.
- A. Tessier and D. R. Turner, Metal speciation and bioavailability in aquatic systems, Wiley, Chichester, UK, 1995, vol. 3 Search PubMed.
- EPA, U.S. Environmental Protection Agency Office of Water, Washington, DC, 2007.
- W. Ahlf, W. Drost and S. Heise, Incorporation of metal bioavailability into regulatory frameworks—metal exposure in water and sediment, J. Soils Sediments, 2009, 9, 411–419 CrossRef CAS PubMed.
- A. Fairbrother, R. Wenstel, K. Sappington and W. Wood, Framework for metals risk assessment, Ecotoxicol. Environ. Saf., 2007, 68, 145–227 CrossRef CAS PubMed.
- P. G. Campbell, P. M. Chapman and B. A. Hale, Risk assessment of metals in the environment, Environ. Sci. Technol., 2006, 22, 102–131 CAS.
- P. R. Paquin, et al., The biotic ligand model: a historical overview, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 3–35 CrossRef.
- A. J. Bone, et al., Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles: Part 2–Toxicity and Ag speciation, Environ. Sci. Technol., 2012, 46, 6925–6933 CrossRef CAS PubMed.
- A. Elsaesser and C. V. Howard, Toxicology of nanoparticles, Adv. Drug Delivery Rev., 2012, 64, 129–37 CrossRef CAS PubMed.
- A. Kunzmann, et al., Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation, Biochim. Biophys. Acta, Gen. Subj., 2011, 1810, 361–373 CrossRef CAS PubMed.
- H. Ma, P. L. Williams and S. A. Diamond, Ecotoxicity of manufactured ZnO nanoparticles – A review, Environ. Pollut., 2013, 172, 76–85 CrossRef CAS PubMed.
- A. E. Nel, et al., Understanding biophysicochemical interactions at the nano–bio interface, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- The European Commission, Definition of the Term “Nanomaterial”, 2011, http://www.ec.europa.eu/.
- W. G. Kreyling, M. Semmler-Behnke and Q. Chaudhry, A complementary definition of nanomaterial, Nano Today, 2010, 5, 165–168 CrossRef PubMed.
- M. Auffan, J. Y. Bottero, C. Chaneac and J. Rose, Inorganic manufactured nanoparticles: how their physicochemical properties influence their biological effects in aqueous environments, Nanomedicine, 2010, 5, 999–1007 CrossRef CAS PubMed.
- M. Auffan, et al., Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS PubMed.
- S. J. Klaine, et al., Nanomaterials in the environment: Behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- M. F. Hochella and A. S. Madden, Earth's nano-compartment for toxic metals, Elements, 2005, 1, 199–203 CrossRef CAS.
- A. Hartland, J. Lead, V. Slaveykova, D. O'Carroll and E. Valsami-Jones, The environmental significance of natural nanoparticles, Nature Education Knowledge, 2013, 4, 7 Search PubMed.
- S. J. Klaine, et al., Paradigms to assess the environmental impact of manufactured nanomaterials, Environ. Toxicol. Chem., 2012, 31, 3–14 CrossRef CAS PubMed.
- K. A. D. Guzman, M. P. Finnegan and J. F. Banfield, Influence of surface potential on aggregation and transport of titania nanoparticles, Environ. Sci. Technol., 2006, 40, 7688–7693 CrossRef.
- A. B. A. Boxall, K. Tiede and M. Q. Chaudhry, Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health?, Nanomedicine, 2007, 2, 919 CrossRef CAS PubMed.
- J. T. K. Quik, et al., Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water, Chemosphere, 2010, 81, 711–715 CrossRef CAS PubMed.
- K. Tiede, M. Hassellov, E. Breitbarth, Q. Chaudhry and A. B. A. Boxall, Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles, J. Chromatogr. A, 2009, 1216, 503–509 CrossRef CAS PubMed.
- R. F. Domingos, et al., Characterizing manufactured nanoparticles in the environment: multimethod determination of particle sizes, Environ. Sci. Technol., 2009, 43, 7277–7284 CrossRef CAS.
- V. Stone, et al., Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physico-chemical characterisation, Sci. Total Environ., 2010, 408, 1745–1754 CrossRef CAS PubMed.
- B. Nowack, et al., Potential scenarios for nanomaterial release and subsequent alteration in the environment, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS PubMed.
- V. I. Slaveykova and K. Startchev, Effect of natural organic matter and green microalga on carboxyl-polyethylene glycol coated CdSe/ZnS quantum dots stability and transformations under freshwater conditions, Environ. Pollut., 2009, 157, 3445–3450 CrossRef CAS PubMed.
- J. M. Unrine, B. P. Colman, A. J. Bone, A. P. Gondikas and C. W. Matson, Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles. Part 1. Aggregation and dissolution, Environ. Sci. Technol., 2012, 46, 6915–6924 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- G. V. Lowry, et al., Environmental occurrences, behavior, fate, and ecological effects of nanomaterials: an introduction to the special series, J. Environ. Qual., 2010, 39, 1867–1874 CrossRef CAS.
- V. I. Slaveykova and K. J. Wilkinson, Predicting the bioavailability of metals and metal complexes: Critical review of the biotic ligand model, Environ. Chem., 2005, 2, 9–24 CrossRef CAS.
- M. Auffan, J. Rose, M. R. Wiesner and J. Y. Bottero, Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro, Environ. Pollut., 2009, 157, 1127–1133 CrossRef CAS PubMed.
- S. Eduok, et al., Evaluation of engineered nanoparticle toxic effect on wastewater microorganisms: Current status and challenges, Ecotoxicol. Environ. Saf., 2013, 95, 1–9 CrossRef CAS PubMed.
- Y. Liu, M. Tourbin, S. Lachaize and P. Guiraud, Nanoparticles in wastewaters: Hazards, fate and remediation, Powder Technol., 2014, 255, 149–156 CrossRef CAS PubMed.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Engineered nanoparticles in wastewater and wastewater sludge – Evidence and impacts, Waste Manage., 2010, 30, 504–520 CrossRef CAS PubMed.
- A. L. Neal, What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles?, Ecotoxicology, 2008, 17, 362–371 CrossRef CAS PubMed.
- K. Van Hoecke, et al., Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests, Environ. Sci. Technol., 2009, 43, 4537–4546 CrossRef CAS.
- N. B. Hartmann, et al., Algal testing of titanium dioxide nanoparticles--testing considerations, inhibitory effects and modification of cadmium bioavailability, Toxicology, 2010, 269, 190–197 CrossRef CAS PubMed.
- N. von Moos and V. Slaveykova, Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae - state of the art and knowledge gaps, Nanotoxicology, 2013, 8, 605–630 CrossRef PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Fate and risks of nanomaterials in aquatic and terrestrial environments, Acc. Chem. Res., 2013, 46, 854–862 CrossRef CAS PubMed.
- R. D. Handy, R. Owen and E. Valsami-Jones, The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs, Ecotoxicology, 2008, 17, 315–325 CrossRef CAS PubMed.
- S. Ma and D. Lin, The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: adsorption and internalization, Environ. Sci.: Processes Impacts, 2013, 14, 145–160 Search PubMed.
- X. K. Hu, S. Cook, P. Wang and H. M. Hwang, In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles, Sci. Total Environ., 2009, 407, 3070–3072 CrossRef CAS PubMed.
- J. Gregory, Particles in water: properties and processes, CRC Press, Taylor & Franci Group, Boca Raton, FL, 2006, pp. 33487–2742 Search PubMed.
- J. R. Morones, et al., The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- A. Thill, et al., Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS.
- S. Dalai, S. Pakrashi, R. S. S. Kumar, N. Chandrasekaran and A. Mukherjee, A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations, Toxicol. Res., 2012, 1, 116–130 RSC.
- I. Sondi and B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- W. Jiang, H. Mashayekhi and B. S. Xing, Bacterial toxicity comparison between nano- and micro-scaled oxide particles, Environ. Pollut., 2009, 157, 1619–1625 CrossRef CAS PubMed.
- S. Renault, et al., Impacts of gold nanoparticle exposure on two freshwater species: a phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea), Gold Bull., 2008, 41, 116–126 CrossRef CAS.
- K. Van Hoecke, K. A. C. De Schamphelaere, P. Van der Meeren, S. Lucas and C. R. Janssen, Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: Importance of surface area, Environ. Toxicol. Chem., 2008, 27, 1948–1957 CrossRef CAS.
- I. Rodea-Palomares, et al., Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms, Toxicol. Sci., 2011, 119, 135–145 CrossRef CAS PubMed.
- V. Aruoja, H. C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata, Sci. Total Environ., 2009, 407, 1461–1468 CrossRef CAS PubMed.
- C. Huang, D. Cha and S. Ismat, Progress report: short-term chronic toxicity of photocatalytic nanoparticles to bacteria, algae, and zooplankton, University of Delaware, 2005 Search PubMed.
- L. Z. Chen, et al. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii, Ecotoxicol. Environ. Saf., 2012, 84, 155–162 CrossRef CAS PubMed.
- F. Piccapietra, C. G. Allué, L. Sigg and R. Behra, Intracellular silver accumulation in Chlamydomonas reinhardtii upon exposure to carbonate coated silver nanoparticles and silver nitrate, Environ. Sci. Technol., 2012, 46, 7390–7397 CrossRef CAS PubMed.
- S. Lin, P. Bhattacharya, N. C. Rajapakse, D. E. Brune and P. C. Ke, Effects of quantum dots adsorption on algal photosynthesis, J. Phys. Chem. C, 2009, 113, 10962–10966 CAS.
- I. A. M. Worms, J. Boltzman, M. Garcia and V. I. Slaveykova, Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae, Environ. Pollut., 2012, 167, 27–33 CrossRef CAS PubMed.
- A.-J. Miao, et al., Intracellular Uptake: A Possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica, PLoS One, 2010, 5, e15196 CAS.
- F. Perreault, N. Bogdan, M. Morin, J. Claverie and R. Popovic, Interaction of gold nanoglycodendrimers with algal cells (Chlamydomonas reinhardtii) and their effect on physiological processes, Nanotoxicology, 2012, 6, 109–120 CrossRef CAS PubMed.
- R. F. Domingos, D. F. Simon, C. Hauser and K. J. Wilkinson, Bioaccumulation and effects of CdTe/CdS quantum dots on Chlamydomonas reinhardtii - Nanoparticles or the free ions?, Environ. Sci. Technol., 2011, 45, 7664–7669 CrossRef CAS PubMed.
- F. Perreault, A. Oukarroum, S. P. Melegari, W. G. Matias and R. Popovic, Polymer coating of copper oxide nanoparticles increases nanoparticles uptake and toxicity in the green alga Chlamydomonas reinhardtii, Chemosphere, 2012, 87, 1388–1394 CrossRef CAS PubMed.
- S. P. Melegari, F. Perreault, R. H. R. Costa, R. Popovic and W. G. Matias, Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii, Aquat. Toxicol., 2013, 142–143, 431–440 CrossRef CAS PubMed.
- L. K. Limbach, et al., Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency, Environ. Sci. Technol., 2008, 42, 5828–5833 CrossRef CAS.
- J. Fabrega, S. R. Fawcett, J. C. Renshaw and J. R. Lead, Silver nanoparticle impact on bacterial growth: Effect of pH, concentration, and organic matter, Environ. Sci. Technol., 2009, 43, 7285–7290 CrossRef CAS.
- B. Marsalek, et al., Multimodal action and selective toxicity of zerovalent iron nanoparticles against cyanobacteria, Environ. Sci. Technol., 2012, 46, 2316–2323 CrossRef CAS PubMed.
- A. Simon-Deckers, et al., Size-, composition- and shape-dependent toxicological impact of metal oxide Nanoparticles and carbon nanotubes toward bacteria, Environ. Sci. Technol., 2009, 43, 8423–8429 CrossRef CAS PubMed.
- S. Shrivastava, et al., Characterization of enhanced antibacterial effects of novel silver nanoparticles, Nanotechnology, 2007, 18, 225103 CrossRef.
- C. Cherchi, T. Chernenko, M. Diem and A. Z. Gu, Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization, Environ. Toxicol. Chem., 2011, 30, 861–869 CrossRef CAS PubMed.
- S. W. Kim, Y. W. Baek and Y. J. An, Assay-dependent effect of silver nanoparticles to Escherichia coli and Bacillus subtilis, Appl. Microbiol. Biotechnol., 2011, 92, 1045–1052 CrossRef CAS PubMed.
- Z. Y. Wang, J. Li, J. Zhao and B. S. Xing, Toxicity and Internalization of CuO Nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter, Environ. Sci. Technol., 2011, 45, 6032–6040 CrossRef CAS PubMed.
- V. I. Slaveykova, J. P. Pinheiro, M. Floriani and M. Garcia, Interactions of core–shell quantum dots with metal resistant bacterium Cupriavidus metallidurans: Consequences for Cu and Pb removal, J. Hazard. Mater., 2013, 261, 123–129 CrossRef CAS PubMed.
- G. Applerot, et al., Enhanced antibacterial activity of nanocrystalline ZnO Due to increased ROS-mediated cell injury, Adv. Funct. Mater., 2009, 19, 842–852 CrossRef CAS.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli, Free Radical Biol. Med., 2011, 51, 1872–1881 CrossRef CAS PubMed.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells, Chemosphere, 2011, 83, 1124–1132 CrossRef CAS PubMed.
- U. Aschauer, D. Spagnoli, P. Bowen and S. C. Parker, Growth modification of seeded calcite using carboxylic acids: Atomistic simulations, J. Colloid Interface Sci., 2010, 346, 226–231 CrossRef CAS PubMed.
- J. Schneider and L. Colombi Ciacchi, Specific material recognition by small peptides mediated by the interfacial solvent structure, J. Am. Chem. Soc., 2012, 134, 2407–2413 CrossRef CAS PubMed.
- Z. Q. Li, K. Greden, P. J. J. Alvarez, K. B. Gregory and G. V. Lowry, Adsorbed polymer and NOM limits adhesion and toxicity of nano scale zerovalent iron to E. coli, Environ. Sci. Technol., 2010, 44, 3462–3467 CrossRef CAS PubMed.
- O. Zeyons, et al., Direct and indirect CeO2 nanoparticles toxicity for Escherichia coli and Synechocystis, Nanotoxicology, 2009, 3, 284–295 CrossRef CAS.
- M. Planchon, et al., Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters, J. Colloid Interface Sci., 2013, 405, 35–43 CrossRef CAS PubMed.
- C. O. Dimkpa, A. Calder, D. W. Britt, J. E. McLean and A. J. Anderson, Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions, Environ. Pollut., 2011, 159, 1749–1756 CrossRef CAS PubMed.
- N. Joshi, B. T. Ngwenya and C. E. French, Enhanced resistance to nanoparticle toxicity is conferred by overproduction of extracellular polymeric substances, J. Hazard. Mater., 2012, 241–242, 363–370 CrossRef CAS PubMed.
- A. Oukarroum, S. Bras, F. Perreault and R. Popovic, Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta, Ecotoxicol. Environ. Saf., 2012, 78, 80–85 CrossRef CAS PubMed.
- F. Perreault, et al., Toxicity of pamam-coated gold nanoparticles in different unicellular models, Environ. Toxicol., 2012, 29, 328–36 CrossRef PubMed.
- I. Bhatt and B. N. Tripathi, Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment, Chemosphere, 2011, 82, 308–317 CrossRef CAS PubMed.
- W. L. Zemke-White, K. D. Clements and P. J. Harris, Acid lysis of macroalgae by marine herbivorous fishes: effects of acid pH on cell wall porosity, J. Exp. Mar. Biol. Ecol., 2000, 245, 57–68 CrossRef CAS.
- E. Navarro, et al., Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- M. Ovečka, et al., Endocytosis and vesicle trafficking during tip growth of root hairs, Protoplasma, 2005, 226, 39–54 CrossRef PubMed.
- J. G. Wessels, Wall growth, protein excretion and morphogenesis in fungi, New Phytol., 1993, 123, 397–413 CrossRef CAS.
- M. N. Moore, Do nanoparticles present ecotoxicological risks for the health of the aquatic environment?, Environ. Int., 2006, 32, 967–976 CrossRef CAS PubMed.
- A. Verma, et al., Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles, Nat. Mater., 2008, 7, 588–595 CrossRef CAS PubMed.
- H. Gao, W. Shi and L. B. Freund, Mechanics of receptor-mediated endocytosis, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9469–9474 CrossRef CAS PubMed.
- F. Osaki, T. Kanamori, S. Sando, T. Sera and Y. Aoyama, A Quantum dot conjugated sugar ball and Its cellular uptake. On the size effects of endocytosis in the subviral region, J. Am. Chem. Soc., 2004, 126, 6520–6521 CrossRef CAS PubMed.
- A. M. Alkilany and C. J. Murphy, Toxicity and cellular uptake of gold nanoparticles: what we have learned so far?, J. Nanopart. Res., 2010, 12, 2313–2333 CrossRef CAS PubMed.
- J. Q. Lin, H. W. Zhang, Z. Chen and Y. G. Zheng, Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship, ACS Nano, 2010, 4, 5421–5429 CrossRef CAS PubMed.
- B. Alberts, et al., Essential cell biology, Garland Science, New York, USA, 2nd edn, 2004, vol. 1 Search PubMed.
- K. Unfried, et al., Cellular responses to nanoparticles: Target structures and mechanisms, Nanotoxicology, 2007, 1, 52–71 CrossRef CAS.
- V. Mailänder and K. Landfester, Interaction of Nanoparticles with Cells, Biomacromolecules, 2009, 2379–2400 CrossRef PubMed.
- I. Lynch and K. A. Dawson, Protein-nanoparticle interactions, Nano Today, 2008, 3, 40–47 CrossRef CAS.
- C. Röcker, M. Potzl, F. Zhang, W. J. Parak and G. U. Nienhaus, A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles, Nat. Nanotechnol., 2009, 4, 577–580 CrossRef PubMed.
- M. Lundqvist, et al., Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- T. Cedervall, et al., Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2050–2055 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. Puntes, Time Evolution of the Nanoparticle Protein Corona, ACS Nano, 2010, 4, 3623–3632 CrossRef CAS PubMed.
- K. J. Wilkinson and J. Buffle, in Physicochemical Kinetics and Transport at Biointerfaces, John Wiley & Sons, Ltd., 2004, pp. 445–533 Search PubMed.
- J. Liu, F. von der Kammer, B. Zhang, S. Legros and T. Hofmann, Combining spatially resolved hydrochemical data with in-vitro nanoparticle stability testing: Assessing environmental behavior of functionalized gold nanoparticles on a continental scale, Environ. Int., 2013, 59, 53–62 CrossRef CAS PubMed.
- J. M. Pettibone, D. M. Cwiertny, M. Scherer and V. H. Grassian, Adsorption of organic acids on TiO2 nanoparticles: Effects of pH, nanoparticle size, and nanoparticle aggregation, Langmuir, 2008, 24, 6659–6667 CrossRef CAS PubMed.
- I. Römer, et al., Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests, J. Chromatogr. A, 2011, 1218, 4226–33 CrossRef PubMed.
- D. Kühnel, et al., Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line, Aquat. Toxicol., 2009, 93, 91–99 CrossRef PubMed.
- N. M. Franklin, et al., Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility, Environ. Sci. Technol., 2007, 41, 8484–8490 CrossRef CAS.
- R. F. Domingos, N. Tufenkji and K. J. Wilkinson, Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid, Environ. Sci. Technol., 2009, 43, 1282–1286 CrossRef CAS.
- S. A. Cumberland and J. R. Lead, Particle size distributions of silver nanoparticles at environmentally relevant conditions, J. Chromatogr. A, 2009, 1216, 9099–9105 CrossRef CAS PubMed.
- R. A. French, et al., Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of Titanium Dioxide Nanoparticles, Environ. Sci. Technol., 2009, 43, 1354–1359 CrossRef CAS.
- F. Loosli, P. Le Coustumer and S. Stoll, TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability, Water Res., 2013, 47, 6052–63 CrossRef CAS PubMed.
- M. Farré, K. Gajda-Schrantz, L. Kantiani and D. Barcelo, Ecotoxicity and analysis of nanomaterials in the aquatic environment, Anal. Bioanal. Chem., 2009, 393, 81–95 CrossRef PubMed.
- V. K. Sharma, Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment-A Review, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 1485–1495 CrossRef CAS PubMed.
- P. Bowen, H. Hofmann, M. Staiger, R. Steiger, P.-A. Brugger and K. Peternell, Colloidal processing of nanoceramic powders for porous ceramic film applications, Key Eng. Mater., 2002, 206–213, 1977–1980 CrossRef CAS.
- F. Mohd Omar, H. Abdul Aziz and S. Stoll, Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid, Sci. Total Environ., 2014, 468–469, 195–201 CrossRef CAS PubMed.
- A. A. Keller, et al., Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- S. S. Khan, P. Srivatsan, N. Vaishnavi, A. Mukherjee and N. Chandrasekaran, Interaction of silver nanoparticles (SNPs) with bacterial extracellular proteins (ECPs) and its adsorption isotherms and kinetics, J. Hazard. Mater., 2011, 192, 299–306 CAS.
- M. Delay, T. Dolt, A. Woellhaf, R. Sembritzki and F. H. Frimmel, Interactions and stability of silver nanoparticles in the aqueous phase: Influence of natural organic matter (NOM) and ionic strength, J. Chromatogr. A, 2011, 1218, 4206–4212 CrossRef CAS PubMed.
- I. Chowdhury, D. M. Cwiertny and S. L. Walker, Combined factors influencing the aggregation and deposition of nano-TiO2 in the presence of humic acid and bacteria, Environ. Sci. Technol., 2012, 46, 6968–6976 CrossRef CAS PubMed.
- M. Baalousha, Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter, Sci. Total Environ., 2009, 407, 2093–2101 CrossRef CAS PubMed.
- D. Lin, X. Tian, F. Wu and B. Xing, Fate and transport of engineered nanomaterials in the environment, J. Environ. Qual., 2010, 39, 1896–1908 CrossRef.
- L. K. Limbach, et al., Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations, Environ. Sci. Technol., 2005, 39, 9370–9376 CrossRef CAS.
- P. Borm, et al., Research strategies for safety evaluation of nanomaterials, Part V: Role of dissolution in biological fate and effects of nanoscale particles, Toxicol. Sci., 2006, 90, 23–32 CrossRef CAS PubMed.
- A. P. Gondikas, et al., Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation, Environ. Sci. Technol., 2012, 46, 7037–7045 CrossRef CAS PubMed.
- D. He, J. J. Dorantes-Aranda and T. D. Waite, Silver nanoparticle—algae interactions: oxidative dissolution, reactive oxygen species generation and synergistic toxic effects, Environ. Sci. Technol., 2012, 46, 8731–8738 CrossRef CAS PubMed.
- J. Buffle, K. J. Wilkinson and H. P. van Leeuwen, Chemodynamics and bioavailability in natural waters, Environ. Sci. Technol., 2009, 43, 7170–7174 CrossRef CAS.
- O. Bondarenko, et al., Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review, Arch. Toxicol., 2013, 87, 1181–1200 CrossRef CAS PubMed.
- S. K. Misra, A. Dybowska, D. Berhanu, S. N. Luoma and E. Valsami-Jones, The complexity of nanoparticle dissolution and its importance in nanotoxicological studies, Sci. Total Environ., 2012, 438, 225–232 CrossRef CAS PubMed.
- M. Li, L. Zhu and D. Lin, Toxicity of ZnO Nanoparticles to Escherichia coli: Mechanism and the influence of medium components, Environ. Sci. Technol., 2011, 45, 1977–1983 CrossRef CAS PubMed.
- M. H. Li, et al., Stability, bioavailability, and bacterial toxicity of ZnO and Iron-Doped ZnO nanoparticles in aquatic media, Environ. Sci. Technol., 2011, 45, 755–761 CrossRef CAS PubMed.
- M. Heinlaan, A. Ivask, I. Blinova, H. C. Dubourguier and A. Kahru, Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus, Chemosphere, 2008, 71, 1308–1316 CrossRef CAS PubMed.
- H. J. Jo, J. W. Choi, S. H. Lee and S. W. Hong, Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods, J. Hazard. Mater., 2012, 227, 301–308 CrossRef PubMed.
- R. Kumar and H. Münstedt, Silver ion release from antimicrobial polyamide/silver composites, Biomaterials, 2005, 26, 2081–2088 CrossRef CAS PubMed.
- O. Choi, et al., The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth, Water Res., 2008, 42, 3066–3074 CrossRef CAS PubMed.
- J. Dobias and R. Bernier-Latmani, Silver release from silver nanoparticles in natural waters, Environ. Sci. Technol., 2013, 47, 4140–4146 CrossRef CAS PubMed.
- J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway and J. R. Lead, Silver nanoparticles: Behaviour and effects in the aquatic environment, Environ. Int., 2011, 37, 517–531 CrossRef CAS PubMed.
- J. S. Kim, et al., Antimicrobial effects of silver nanoparticles, Nanomedicine: Nanotechnology, Biology and Medicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- C. N. Lok, et al., Silver nanoparticles: partial oxidation and antibacterial activities, JBIC, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef CAS PubMed.
- B. C. Reinsch, et al., Sulfidation of silver nanoparticles decreases Escherichia coli growth inhibition, Environ. Sci. Technol., 2012, 46, 6992–7000 CrossRef CAS PubMed.
- Z.-M. Xiu, Q.-B. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Negligible particle-specific antibacterial activity of silver nanoparticles, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- M. Li, D. Lin and L. Zhu, Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli, Environ. Pollut., 2013, 173, 97–102 CrossRef CAS PubMed.
- Y. W. Baek and Y. J. An, Microbial toxicity of metal oxide nanoparticles CuO, NiO, ZnO, and Sb2O3 to Escherichia coli, Bacillus subtilis, and Streptococcus aureus, Sci. Total Environ., 2011, 409, 1603–1608 CrossRef CAS PubMed.
- S. Bandyopadhyay, J. R. Peralta-Videa, G. Plascencia-Villa, M. José-Yacamán and J. L. Gardea-Torresdey, Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: Use of advanced microscopic and spectroscopic techniques, J. Hazard. Mater., 2012, 241–242, 379–386 CrossRef CAS PubMed.
- N. J. Rogers, et al., Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater, Environ. Chem., 2010, 7, 50–60 CrossRef CAS.
- Y. Yang, et al., Pyrosequencing reveals higher impact of silver nanoparticles than Ag+ on the microbial community structure of activated sludge, Water Res., 2014, 48, 317–325 CrossRef CAS PubMed.
- A. D. Burchardt, et al., Effects of silver nanoparticles in diatom Thalassiosira pseudonana and Cyanobacterium Synechococcus sp., Environ. Sci. Technol., 2012, 46, 11336–11344 CrossRef CAS PubMed.
- A. Ivask, O. Bondarenko, N. Jepihhina and A. Kahru, Profiling of the reactive oxygen species-related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: differentiating the impact of particles and solubilised metals, Anal. Bioanal. Chem., 2010, 398, 701–716 CrossRef CAS PubMed.
- E. Navarro, et al., Toxicity of silver nanoparticles to Chlamydomonas reinhardtii, Environ. Sci. Technol., 2008, 42, 8959–8964 CrossRef CAS.
- J. Ji, Z. Long and D. Lin, Toxicity of oxide nanoparticles to the green algae Chlorella sp, Chem. Eng. J., 2011, 170, 525–530 CrossRef CAS PubMed.
- S. M. Wirth, G. V. Lowry and R. D. Tilton, Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved silver, Environ. Sci. Technol., 2012, 46, 12687–12696 CrossRef CAS PubMed.
- M. A. Anderson, F. M. M. Morel and R. R. L. Guillard, Growth limitation of a coastal diatom by low zinc ion activity, Nature, 1978, 276, 70–71 CrossRef CAS.
- S. N. Luoma and P. S. Rainbow, Why is metal bioaccumulation so variable? Biodynamics as a unifying concept, Environ. Sci. Technol., 2005, 39, 1921–1931 CrossRef CAS.
- M. A. Kiser, D. A. Ladner, K. D. Hristovski and P. K. Westerhoff, Nanomaterial transformation and association with fresh and freeze-dried wastewater activated sludge: Implications for testing protocol and environmental fate, Environ. Sci. Technol., 2012, 46, 7046–7053 CrossRef CAS PubMed.
- C. L. Arnaout and C. K. Gunsch, Impacts of silver nanoparticle coating on the nitrification potential of Nitrosomonas europaea, Environ. Sci. Technol., 2012, 46, 5387–5395 CrossRef CAS PubMed.
- T. Silva, et al., Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity, Sci. Total Environ., 2014, 468–469, 968–976 CrossRef CAS PubMed.
- M. Veerapandian, S. Sadhasivam, J. Choi and K. Yun, Glucosamine functionalized copper nanoparticles: Preparation, characterization and enhancement of anti-bacterial activity by ultraviolet irradiation, Chem. Eng. J., 2012, 209, 558–567 CrossRef CAS PubMed.
- B. Kim, C.-S. Park, M. Murayama and M. F. Hochella, Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products, Environ. Sci. Technol., 2010, 44, 7509–7514 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Environmental transformations of silver nanoparticles: Impact on stability and toxicity, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- C. Levard, et al., Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate, Environ. Sci. Technol., 2011, 45, 5260–5266 CrossRef CAS PubMed.
- O. Choi, et al., Role of sulfide and ligand strength in controlling nanosilver toxicity, Water Res., 2009, 43, 1879–1886 CrossRef CAS PubMed.
- B. P. Colman, et al., Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario, PLoS One, 2013, 8, e57189 CAS.
- C. Kostigen Mumper, A.-K. Ostermeyer, L. Semprini and T. S. Radniecki, Influence of ammonia on silver nanoparticle dissolution and toxicity to Nitrosomonas europaea, Chemosphere, 2013, 93, 2493–2498 CrossRef CAS PubMed.
- S. Skoglund, et al., Effect of laundry surfactants on surface charge and colloidal stability of silver nanoparticles (Ag NPs), Langmuir, 2013, 29, 8882–8891 CrossRef CAS PubMed.
- J. Hammes, J. A. Gallego-Urrea and M. Hassellöv, Geographically distributed classification of surface water chemical parameters influencing fate and behavior of nanoparticles and colloid facilitated contaminant transport, Water Res., 2013, 47, 5350–5361 CrossRef CAS PubMed.
- K. Yang and B. Xing, Sorption of phenanthrene by humic acid-coated nanosized TiO2 and ZnO, Environ. Sci. Technol., 2009, 43, 1845–1851 CrossRef CAS.
- D. Rodrigues, T. A. P. Rocha-Santos, A. C. Freitas, A. M. P. Gomes and A. C. Duarte, Strategies based on silica monoliths for removing pollutants from wastewater effluents: A review, Sci. Total Environ., 2013, 461–462, 126–138 CrossRef CAS PubMed.
- A. S. Karakoti, L. L. Hench and S. Seal, The potential toxicity of nanomaterials - The role of surfaces, JOM, 2006, 58, 77–82 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Madler and N. Li, Toxic potential of materials at the nanolevel, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



