A minor lipid component of soy lecithin causes growth of triangular prismatic gold nanoparticles
Benjamin R.
Ayres†
a and
Scott M.
Reed
*b
aDepartment of Chemistry, Portland State University, Portland, Oregon 97201, USA
bDepartment of Chemistry, University of Colorado Denver, Denver, CO 80204, USA. E-mail: scott.reed@ucdenver.edu
First published on 28th November 2013
Abstract
Reduction of bromoauric acid by ascorbic acid in the presence of crude soybean lecithin produced a mixture of spherical and triangular prismatic nanoparticles while higher purity phosphatidylcholine (PC) produced only spherical nanoparticles. The triangular prismatic nanoparticles made with lecithin had an average edge length of 90 nm and a localized surface plasmon resonance between 700 and 1050 nm, depending on the synthetic conditions used. Although crude soy lecithin is composed of 75% PC and phosphatidylethanolamine, additional lipids and other small molecules are present. Preparatory gel electrophoresis was used to separate the different nanoparticle shapes and phosphatidic acid (PA) was identified as bound to the triangular prismatic nanoparticles by mass spectrometry of the gel-purified nanoprisms. Furthermore, PA proved essential to the asymmetric growth. When bromoauric acid was reduced by ascorbic acid using a mixture of pure PA and pure PC, triangular prismatic gold nanoparticles also resulted, confirming the role of PA and providing a second route to these near infrared active nanoparticles. Nanoparticles prepared in soy lecithin or PA–PC mixtures exhibited extended stability with no aggregation after months of storage, in contrast to nanoparticles prepared in pure PC. These nanoparticles were prepared without the use of the alkyl ammonium salts that have limited in vivo applications with other asymmetric gold nanoparticles. This is a versatile synthetic method providing stable, shape-controlled gold nanoparticles using a mild reducing agent and soy lecithin as an environmentally benign ligand source.
Nano impactPlant-derived lipids have the potential to function as versatile, environmentally benign ligands for nanoparticle synthesis, although the complex chemistry of the lipids must be understood in order to use them effectively. This work adds to the growing list of nanoparticle syntheses performed using renewable, plant-derived ligands in place of fossil fuel derived chemicals. This synthetic route provides control over the shape and the optical properties of the gold nanoparticles, making the resultant materials useful for biomedical, catalytic, and photonic applications. One critical impact of this work comes from the identification of a specific molecular component in soy lecithin that is responsible for causing asymmetric growth. Through this molecular level understanding it becomes possible to optimize the nanoparticle synthesis both from the perspective of the desired application of the nanoparticles and from the perspective of the environmental impact of their synthesis. |
Introduction
Anisotropic transition metal nanocrystals have important applications in optics,1 electronics,2 and catalysis.3 Shape plays a significant role in governing the optical and electronic properties of these nanoscale materials.4,5 Anisotropic nanoparticles have been prepared by a variety of strategies including electrochemical,6 microemulsion,7 phase-transfer,8 and seed-mediated9 syntheses. Ligand choice is one parameter that affects both shape and stability in nanomaterials.10 Anisotropic gold nanoparticles (GNPs) with a localized surface plasmon resonance (LSPR) in the near infrared (NIR) are of significant interest as these materials have been used as sensors,11 have increased catalytic activity,12 and can be used for in vivo imaging and phototherapeutic treatment of tumors.13 The ability to tune the dimensions of GNPs by controlling the reaction parameters is critical to these applications.It has been found that seed-mediated synthesis using alkyl ammonium salts such as cetyltrimethylammonium bromide (CTAB) works well to control the shape of GNPs.14,15 CTAB is used to form gold nanorods that have an LSPR near 530 nm and a larger LSPR in the NIR due to the transverse and the longitudinal electron oscillations, respectively. By switching to a three-step CTAB growth solution with hydroxide, the nanorod method instead provides gold nanoprisms.16 CTAB binds to the growing GNP surface, directing growth in one or two dimensions.17,18 However, a large excess of the ligand is required to stabilize the GNPs and therefore removal of CTAB is required prior to in vivo use. CTAB is not biocompatible19 and therefore, if GNPs are to be used in medical applications, CTAB has to be removed by a process such as ligand exchange which often leads to instability.13,20 Silver nitrate and benzyldimethylhexadecylammonium chloride are common additives used to tune gold nanorod aspect ratio21 and it has also been shown that single crystalline seeds are needed for gold nanorod growth.22
One approach for controlling biocompatibility is the use of green synthetic methods such as selecting benign reagents from renewable sources.23–26 A common method of preparing biocompatible nanoparticles is to use biogenic extracts as has been done with lemongrass,27 tea leaves,24 and pears.28 This approach is particularly promising for nanomaterials being developed for biomedical applications. Lipids are a major component of the plant extracts and lipids are optimal biocompatible ligands for surface functionalization of GNPs.13,29 Phospholipids have been studied extensively and are known to form stabilized liposomes that are used in drug delivery.30 PC is the major component of cell membranes and therefore PC can be obtained from inexpensive, readily available, renewable feedstocks such as soy lecithin.
Here, we demonstrate a seed-mediated, green synthetic route that produces asymmetric nanoparticles with an LSPR in the NIR using soy lecithin and ascorbic acid. We also report a facile separation of these different GNP shapes using preparative gel electrophoresis. The electronic and structural properties of these nanoparticles were investigated by UV-visible spectroscopy (UV-Vis) and transmission electron microscopy (TEM). It was found that GNPs synthesized with a less pure form of soy extract (PC30) showed a NIR LSPR and were stable for months. When a 95% pure form of PC (PC95) was used, stability was reduced, the nanoparticles aggregated over time, and no LSPR was observed in the NIR. However, it was found that PC is not the shape-directing component of lecithin. Using gel electrophoresis for GNP separation and analysis of the extracted lipid by LC-MS, it was found that PA was bound to the triangular prismatic nanoparticles. When growth solutions of PC95 were spiked with PA, a second LSPR was observed in the NIR and it shifted further red as more PA was spiked into the growth solution, confirming the role of PA in asymmetric growth.
Results
Anisotropic gold nanoparticle synthesis using PC30
Typically, HAuCl4 is the gold salt used to synthesize nanoparticles. However growth of GNPs using HAuCl4 with either L-α-phosphatidylcholine Type IV-S ≥ 30% (PC30) or a more pure form of lecithin, PC95, produces nanospheres as seen in the UV-Vis spectrum (Fig. 1). | ||
| Fig. 1 UV-Vis spectra of GNPs synthesized using HAuCl4 with ascorbic acid in a seed-mediated growth solution with no bromide. Nanoparticles were grown using PC30 (blue) or using PC95 (red). | ||
The effect of bromide concentration on anisotropic shape
In an initial study, anisotropic GNPs were synthesized by a seed-mediated route using PC30 and an in situ preparation of HAuBr4 as the gold source. Addition of KBr to a growth solution containing HAuCl4 and lecithin resulted in a color change from a bright yellow to dark orange, indicative of in situ formation of bromoauric acid.31 Following addition of fresh seed solution, ascorbic acid was used to reduce the orange Au(III) solution. The UV-Vis spectra of the resulting GNP solutions had an LSPR at 530 nm and a NIR LSPR near 700 nm (Fig. 2), consistent with anisotropic growth. | ||
| Fig. 2 Representative UV-Vis spectra of GNPs prepared using PC30, KBr, and HAuCl4. HAuCl4 was mixed with 4 (red), 6 (orange), 8 (green), 10 (blue), or 12 (purple) equivalents of KBr. | ||
To evaluate the effect of bromide on the formation of the NIR plasmon band, a series of syntheses were conducted with increasing KBr concentrations. KBr in a 4- to 12-fold excess was added to growth solutions containing a constant amount of HAuCl4 and PC30. With increasing concentrations of bromide there was a corresponding red-shift in the plasmon band from 690 to 720 nm and a decrease in the relative intensity of the plasmon band at 532 nm (Fig. 2).
Substituting bromoauric acid for HAuCl4 but adding no KBr resulted in an extinction spectrum with a sharper NIR plasmon band (Fig. 3) that was similar in wavelength to the KBr spiked nanoparticle growth solution containing a 12-fold excess of KBr. Avoiding any use of chloride limited competition among the ions binding to the GNP surface achieving a similar effect with less bromide. To further investigate this, a growth solution containing bromoauric acid was spiked with a 12-fold excess of either KCl or KBr. When the sample was spiked with KCl, the NIR plasmon was located at a lower wavelength (825 nm) and was broader in appearance (Fig. 4).
 | ||
| Fig. 3 UV-Vis spectra of GNPs grown in 12-fold excess of KBr spiked HAuCl4 growth solution (red) and HAuBr4 growth solution (blue). | ||
 | ||
| Fig. 4 UV-Vis spectra of GNPs prepared with growth solutions containing HAuBr4 spiked with a 12-fold excess of KBr (orange) or KCl (red) respectively. | ||
Effect of soy lecithin purity on anisotropic growth
To identify the components of soy lecithin responsible for asymmetric growth, a synthesis was performed with PC95 as the ligand source in the growth solution. While PC95 contains 95% PC, PC30 contains a complex mixture of compounds including phospholipids and those lipids are composed of approximately 55% PC and 20% phosphatidylethanolamine (PE). The remaining fraction of PC30 contains other lipids, saponins, carotenoids, and other organically soluble molecules.32 The formation of only symmetrical GNPs as indicated by a single LSPR at 532 nm was observed when GNPs were prepared with PC95 using the same conditions (Fig. 5). This suggested that other components in the PC30 were critical to the asymmetric growth.Stability of PC-coated GNPs
GNPs prepared with PC30 were stable for months while those prepared with PC95 and bromoauric acid aggregated within hours (Fig. 1). The LSPR intensity of the PC95 grown sample was also much lower than the PC30 syntheses. This demonstrates that the soy lecithin components other than PC are important for stability and preventing aggregation of the GNPs in aqueous media. Samples prepared using PC30 kept at 4 °C over 4 months showed no aggregation and were stable in both water and in buffered solutions (0.5× TBE buffer at pH 8.3). This is advantageous over other nanoparticle syntheses that require excess ligand and post-synthetic processing such as ligand exchange with lipids to prevent aggregation.13Transmission electron microscopy analysis
To confirm whether the NIR LSPR was caused by anisotropic growth, high-resolution TEM analysis was performed on the samples prepared with PC30. Representative TEM micrographs from crude samples reveal a variety of nanoparticle shapes and sizes (Fig. 6A). Growth of GNPs using PC30 results in the production of symmetrical GNPs including hexagonal and polyhedral GNPs and nanoprisms with a high degree of stacking are seen (Fig. 6B). The variability in shapes and sizes in the crude GNP sample made it difficult to correlate optical properties to shape, therefore separation of the different GNP shapes was necessary.Separation of nanoparticles by shape and size
Separation of GNPs by size and shape was achieved through preparative gel electrophoresis.33–35 A 0.5% agarose gel was prepared using 0.5× TBE buffer and the sample was run on a preparatory column at 300 V collecting 75 fractions (3 mL each) until the fractions had no color. It is important to also note that the eluted nanoparticles showed no aggregation even when dried at room temperature, suggesting retention of ligand.Select fractions collected from electrophoresis were analyzed by UV-Vis and TEM. The first fractions showed only symmetrical nanoparticles with an average diameter of 30.2 ± 8.3 nm and an LSPR at 532 nm (Fig. 7A). Analysis of the final fractions showed an increase in the population of triangular prismatic nanoparticles (Fig. 7B) with an increasingly intense NIR plasmon band in the range of 700 to 900 nm (Fig. 7C). This is consistent with later fractions being enriched in the large anisotropic nanoparticles. A NIR LSPR is consistent with previous reports of triangular prismatic nanoparticles and the nanoprisms are truncated as observed in previous syntheses of prisms.27 Selected area electron diffraction (SAED) (Fig. 8) reveals a single crystalline material with {220} diffraction spots seen down the [111] zone axis on the top face of the nanoparticle, consistent with fcc gold. As seen in Fig. 7B and Table 1, fractions with an intense LSPR in the NIR correspond to TEM images containing much larger quantities of the triangular prismatic nanoparticles. The average edge length from the last band was 94.7 ± 9 nm (Fig. 7B) in contrast to the first band with an average edge length of 54.6 ± 7.8 nm. Some symmetrical nanoparticles were still observed in the later fractions (Fig. 7B) as a single round of separation was not 100% efficient, however the separation did allow for analysis of the fractions by TEM and identification of nanoparticle types (Table 1). This confirms that the triangular prismatic nanoparticles are the source of the NIR LSPR in crude samples. As seen in Table 1, from the ratio of prisms to spheres after separation, a much larger fraction of triangular prismatic nanoparticles were present.
 | ||
| Fig. 8 (A) High resolution TEM showing uniform lattice of prism. (B) SAED showing [111] zone axis with {220} diffraction spots. | ||
| Shape | First fraction | Last fraction |
|---|---|---|
| Symmetrical GNPs | 1839 | 806 |
| Prismatic GNPs | 341 | 420 |
| Others | 33 | 63 |
| Average edge length | 54.6 nm | 94.7 nm |
Mass spectrometry of gel fractions
Every tenth fraction from the column was digested with KCN overnight. The lipids were extracted from these digests and re-dissolved in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (19
H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to perform LC/MS analysis. Molecules of PC with various tail groups were identified as well as PE. One peak at 669 m/z showed increasing abundance at later fractions and this was identified as PA (Fig. 9).
1) to perform LC/MS analysis. Molecules of PC with various tail groups were identified as well as PE. One peak at 669 m/z showed increasing abundance at later fractions and this was identified as PA (Fig. 9).
PA–PC95 growth solution
When a synthesis was performed using a growth solution of PC95 containing a spike of PA a second band appeared in the UV-Vis (Fig. 10) that was not present in the sample preparation using pure PC95 (Fig. 5). PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC95 growth solutions were made with various ratios and then analyzed using UV-Vis. As the PA concentration increased in relation to PC95, a shoulder appeared and shifted to the red (Fig. 10).
PC95 growth solutions were made with various ratios and then analyzed using UV-Vis. As the PA concentration increased in relation to PC95, a shoulder appeared and shifted to the red (Fig. 10).
As GNPs were prepared with increasing amounts of PA added to PC95, a red-shifted plasmon band becomes evident. At 50% PA, the LSPR wavelength is most similar to the LSPR in the PC30 samples, although the wavelength shifts further as more PA is added. At 95% PA in PC95, the red-shifted plasmon reaches a maximum intensity and shifts further red to 1050 nm (Fig. 11). When compared with the original PC30 growth solution, two things are apparent: first, the LSPR at 532 nm shows decreased intensity suggesting that there is a decreased relative population of symmetrical nanoparticles produced in this synthesis; second, the red-shifted plasmon is at a higher intensity and is shifted to a higher wavelength when compared to the PC30 growth solution. TEM taken of the 95% PA in PC95 GNPs shows a large increase in the number of prismatic GNPs to spherical GNPs (Fig. 12). An interesting property also found was that a spike of any PA into PC95 resulted in a large increase in stability over time, similar to that of a PC30 synthesis.
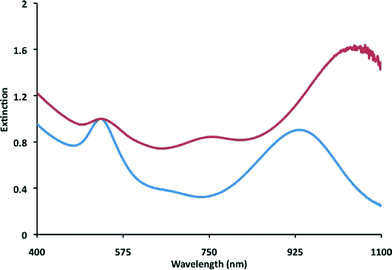 | ||
Fig. 11 UV-Vis spectra of GNPs prepared in PC30 (blue) and 95% PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% PC95 (red) showing difference in plasmon intensity ratios. Normalized at 532 nm. 5% PC95 (red) showing difference in plasmon intensity ratios. Normalized at 532 nm. | ||
Discussion
Because the role of ligands and additives in controlling shape is not fully understood, the synthesis of shape-controlled nanomaterials has proven challenging. Seed-mediated syntheses of anisotropic GNPs have been reported previously and typically use an additive to achieve shape control and most of these methods utilize CTAB as a ligand.21,36 From these methods it was recognized that the cationic surfactant plays an essential role in defining the shape of the nanoparticles. In addition, it has been shown that the counterion of the surfactant also plays an important role in shape control of GNPs.37 Here the fact that shape asymmetry was achieved without a cationic surfactant reveals that zwitterionic lipids (PC) or anionic lipids (PA) are also capable of replacing the cationic ammonium ligand. As CTAB micelles bound to gold atoms have been proposed as carriers of gold to the growing nanoparticle surface, we propose that liposomes containing PA and PC complex Au(I) atoms and carry these atoms to the less hindered facets of the growing prisms.While CTAB is one source of bromide, bromoauric acid is a suitable replacement for HAuCl4 for GNP synthesis and uses less material to achieve the same effect.37 Reduction of bromoauric acid results in different atomic packing in GNP seeds and thus different crystal structures of the nanoparticles.38 The NIR LSPR of GNPs produced with bromoauric acid is sharper compared to KCl-spiked bromoauric acid (Fig. 4). This supports the idea that the chloride competes for binding sites with bromide on the nanoparticle surface during growth.
The role of PA in producing triangular prismatic nanoparticles could arise from two effects. Binding of PA to planar gold39 and gold nanoparticles25,40 has been reported and PA may block growth on the [111] face of the gold seeds, similar to the role of CTAB in nanorod growth. Additionally, lipids with small headgroups, such as PA can induce curvature in membranes.41 The affinity of PA for highly curved regions of a membrane may facilitate templating around sharp edges of the nanostructures and improve the stability of the GNPs. The fact that similar shaped prisms are formed compared to those with CTAB, containing the same crystal packing, and having the same Au(111) facet on the top planar surface,31 suggests that a similar mechanism was responsible for their growth. Just as a bilayer of CTAB can block access of gold containing CTAB micelles, lipid vesicles laden with gold atoms can be blocked from the Au(111) surface by a bilayer coating that is closely packed on the Au(111) surface. Looser packing at the end caps in contrast allows for continued growth as new Au(I) is able to reach the apex of the prisms.
Having a molecular level understanding of what lipids cause asymmetric growth opens the possibility of controlling GNP shape using a rational approach based on the lipid composition of plant materials. Phospholipids such as PE, phosphatidylserine, phosphatidylinositol, and PC all originate from PA.42 The PA content in lipid extracts from a variety of plant extracts was recently reported to range from 0.1% for spinach to 43.6% for Japanese mustard spinach.43 This covers a range of PA comparable to the amount of PA used in Fig. 10 to tune the LSPR from 750 to 900 nm suggesting that lipids from different plant sources could produce different aspect ratios of triangular prismatic nanoparticles. Cabbage, one of the plants with the highest PA levels, also has high levels of phospholipase D, the enzyme that produces PA, and addition of cabbage juice to soy lipid results in a 6-fold increase in PA44 suggesting that nearly any lipid source could be tuned to provide the desired nanoparticle shapes.
Experimental
Materials
L-α-Phosphatidylcholine Type IV-S ≥ 30% TLC (PC30), L-ascorbic acid, potassium cyanide and Agarose Type II Medium EEO were purchased from Aldrich. 95% L-α-phosphatidylcholine (PC95) and L-α-phosphatidic acid (PA) were purchased from Avanti Polar Lipids. Hydrogen tetrachloroaurate(III) hydrate and hydrogen tetrabromoaurate(III) hydrate were from Strem Chemicals Inc. KBr was from Acros. Sodium borohydride, boric acid, and EDTA were from J. T. Baker. Milli-Q H2O at 18.1 mΩ resistance was used. Gel electrophoresis was performed on a Model 491 Prep Cell (Biorad). All other solvents used were reagent grade.Preparation of PC stock
PC stock solution was prepared by dissolving PC30 (200 mg, 0.080 mmol) or PC95 (63 mg, 0.080 mmol) in a minimal amount of cyclohexane. This was immediately dried by rotary evaporation for 1 hour to yield a thin film before use. The film was then re-suspended with H2O (20 mL) and the solution shaken until the solid was fully suspended.Preparation of PA–PC95 stock
PA–PC95 stock solution was prepared by dissolving PA (75 mg, 0.105 mmol) in H2O (4.5 mL) and adding varying amounts of PC95 stock into the solution.Preparation of gold seed using PC30
Gold chloride seed solution was freshly prepared prior to each experiment. Briefly, HAuCl4·xH2O or HAuBr4·xH2O (10 mM, 125 μL) was added to a PC30 stock (2 mL) and H2O (3 mL). With vigorous stirring, freshly prepared NaBH4 (10 mM, 300 μL) was quickly added and the resulting purple seed solution was allowed to react at least 5 min prior to use. Seed solutions were not used after 1 h.Nanoparticle growth in solutions with HAuCl4
A typical chloride growth solution was prepared by diluting an aqueous PC30 stock (2 mL) with H2O (15 mL). To this was added an aqueous solution of HAuCl4·xH2O (10 mM, 400 μL) and bromide derived PC30 gold seed (40 μL). The resulting yellow solution was vigorously stirred followed by a rapid addition of ascorbic acid (100 mM, 40 μL). The solution immediately became transparent and then darkened to a red/purple color within 5 min. The resulting solution was allowed to react 18 h prior to analysis.Nanoparticle growth in solutions with HAuCl4 and KBr
A typical growth solution was prepared by diluting an aqueous PC30 stock (2 mL) with H2O (15 mL). To this was added an aqueous solution of HAuCl4·xH2O (10 mM, 400 μL), KBr(aq) (1.65 M, 24.3 μL), and chloride derived PC30 gold seed (40 μL). The resulting orange solution was vigorously stirred, followed by rapid addition of ascorbic acid (100 mM, 40 μL). A transparent solution followed which darkened to a red color within 5 min. The solution was allowed to react for 18 h prior to analysis.Nanoparticle growth in solutions with HAuBr4
A typical bromide growth solution was prepared by diluting an aqueous PC30 or PC95 stock (2 mL) with H2O (15 mL). To this was added an aqueous solution of HAuBr4·xH2O (10 mM, 400 μL) and bromide derived PC gold seed (40 μL). The resulting orange solution was vigorously stirred followed by an immediate and rapid addition of ascorbic acid (100 mM, 40 μL). The solution immediately became transparent and then darkened to a red color within 5 min. The resulting solution was allowed to react 18 h prior to analysis.Nanoparticle growth in PA–PC95 solutions with HAuBr4
A typical bromide growth solution was prepared by diluting an aqueous PA–PC95 stock (1 mL) with H2O (7.5 mL). To this was added an aqueous solution of HAuBr4·xH2O (10 mM, 200 μL) and bromide derived PC gold seed (20 μL). The resulting orange solution was vigorously stirred followed by an immediate and rapid addition of ascorbic acid (100 mM, 20 μL). The solution immediately became transparent and then darkened to a red color within 20 min. The resulting solution was allowed to react 18 h prior to analysis.Nanoparticle separation by gel electrophoresis
Agarose (500 mg) was added to TBE buffer (0.5×, pH 8.3) and heated until dissolved. Using this, a gel was set in the preparatory column and additional 0.5× TBE buffer was added to the reservoirs. The GNP sample was concentrated by centrifugation and then loaded into the gel using a 50% glycerol solution. The resulting gel was run at 300 V for 2 h. The eluted particles were concentrated by evaporating the water at ambient pressure for 1 week prior to collecting a UV-Vis spectrum.Instrumentation
Extinction spectra were obtained on an Agilent 8453 UV-visible spectrophotometer with a diode array using a 1.0 cm path length quartz cell. TEM images were acquired on a Tecnai F-20 FEI microscope at an accelerating voltage of 200 kV using a CCD detector. Samples were prepared by drop casting (5 μL) aqueous solutions of nanoparticles onto carbon-coated (300 Å) Formvar films on copper. Samples were dried for at least 1 h before images were collected. For more dilute samples, multiple castings were performed on the same grid. Edge length was measured using ImageJ (http://rsbweb.nih.gov/ij/) software by drawing the smallest equilateral triangle that encompassed the GNP and then measuring the side of that triangle, neglecting truncation.Mass spectrometry analysis of GNP ligands
Samples were treated with KCN (300 mM in H2O) and allowed to react overnight. Samples were then lyophilized and organic matter extracted using cyclohexane. The samples were then dried under vacuum and re-dissolved in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (19
H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). LC/MS was obtained on a LTQ-Orbitrap mass spectrometer operating at a flow rate of 0.20 mL min−1. Mobile phases consisted of 95
1). LC/MS was obtained on a LTQ-Orbitrap mass spectrometer operating at a flow rate of 0.20 mL min−1. Mobile phases consisted of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 MeOH
5 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and remained constant for each run. The injection volume was 20 μL. The mass spectrometer was operated under positive electrospray ionization with a voltage 4000 V in positive mode, the heater was set at 300 °C, the curtain gas (N2) was kept constant at 50 psi. Quantitative analysis was monitored by total ion count and ESI results were analyzed on Xcalibur analysis software.
H2O and remained constant for each run. The injection volume was 20 μL. The mass spectrometer was operated under positive electrospray ionization with a voltage 4000 V in positive mode, the heater was set at 300 °C, the curtain gas (N2) was kept constant at 50 psi. Quantitative analysis was monitored by total ion count and ESI results were analyzed on Xcalibur analysis software.
Conclusion
Nanoparticles synthesized with soy lecithin and bromoauric acid produce triangular prismatic GNPs that are very stable. The LSPR of these GNPs is sensitive to bromide concentration and the purity of soy lecithin used. Different nanoparticle shapes can be separated out using preparative gel electrophoresis and TEM shows that larger nanoprisms are eluted as a later fraction. These triangular prismatic GNPs have a NIR plasmon band in the UV-Vis spectrum. Upon separation of the GNPs, followed by MS of organic extractions, it was found that PA is responsible for shape control. When PA is spiked into PC95, the number of prisms synthesized increases and this results in a UV-Vis spectrum with a large red-shifted plasmon band.Acknowledgements
We thank the Portland State University Center for Microscopy & Nanotechnology for assistance with TEM analysis. Funding from the NSF IGERT grant no. DGE-0549503 to BRA and Air Force Research Laboratory FA8650-05-1-5041 to SMR is acknowledged.References
- C. J. Murphy, A. M. Gole, S. E. Hunyadi, J. W. Stone, P. N. Sisco, A. Alkilany, B. E. Kinard and P. Hankins, Chem. Commun., 2008, 544 RSC.
- E. Ozbay, Science, 2006, 311, 189 CrossRef CAS PubMed.
- M. Valden, X. Lai and D. W. Goodman, Science, 1998, 281, 1647 CrossRef CAS.
- M. B. Mohamed, K. M. AbouZeid, V. Abdelsayed, A. A. Alijarash and M. S. El-Shall, ACS Nano, 2010, 4, 2766 CrossRef CAS PubMed.
- G. Garnweitner and M. Niederberger, J. Mater. Chem., 2008, 18, 1171 RSC.
- Y. Yu, S. Chang, C. Lee and C. R. C. Wang, J. Phys. Chem. B, 1997, 101, 6661 CrossRef CAS.
- J. Tanori and M. P. Pileni, Langmuir, 1997, 13, 639 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Chem. Commun., 1994, 801 RSC.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Commun., 2001, 617 RSC.
- B. Nikoobakht and M. A. El-Sayed, Langmuir, 2001, 17, 6368 CrossRef CAS.
- B. Nikoobakht, J. Wang and M. A. El-Sayed, Chem. Phys. Lett., 2002, 366, 17 CrossRef CAS.
- C. Lua, Q. Li, S. Chen, L. Zhao and Z. Zheng, Talanta, 2011, 85, 476 CrossRef PubMed.
- H. Takahashi, T. Niidome, A. Nariai, Y. Niidome and S. Yamada, Chem. Lett., 2006, 35, 500 CrossRef CAS.
- X. Kou, S. Zhang, C. K. Tsung, M. H. Yeung, Q. Shi, G. D. Stucky, L. Sun, J. Wang and C. Yan, J. Phys. Chem., 2006, 110, 16377 CAS.
- A. M. Gole and C. J. Murphy, Chem. Mater., 2004, 16, 3633 CrossRef CAS.
- J. E. Millstone, S. Park, K. L. Shuford, L. Qin, G. C. Schatz and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 5312 CrossRef CAS PubMed.
- K. Yong, Y. Sahoo, M. T. Swihart, P. M. Schneeberger and P. N. Prasad, Top. Catal., 2008, 47, 49 CrossRef CAS.
- D. E. Charles, M. Gara, D. Aherne, D. M. Ledwith, J. M. Kelly, W. J. Blau and M. E. Brennan-Fournet, Plasmonics, 2011, 6, 351 CrossRef CAS.
- J.-P. Clamme, S. Bernacchi, C. Vuilleumier, G. Duportail and Y. Mely, Biochim. Biophys. Acta, Biomembr., 2000, 1461, 347 CrossRef.
- H. Takahashi, Y. Niidome, T. Niidome, K. Kaneko, H. Kawasaki and S. Yamada, Langmuir, 2006, 22, 2 CrossRef CAS PubMed.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192 CrossRef CAS PubMed.
- C. Engelbrekt, K. H. Sørensen, J. Zhang, A. C. Welinder, P. S. Jensen and J. Ulstrup, J. Mater. Chem., 2009, 19, 7839 RSC.
- S. K. Nune, N. Chanda, R. Shukla, K. Katti, R. R. Kulkarni, S. Thilakavathy, S. Mekapothula, R. Kannan and K. V. Katti, J. Mater. Chem., 2009, 19, 2912 RSC.
- M. A. Markowitz, D. N. Dunn, G. M. Chow and J. Zhang, J. Colloid Interface Sci., 1999, 210, 73 CrossRef CAS PubMed.
- J. E. Hutchison, ACS Nano, 2008, 2, 395 CrossRef CAS PubMed.
- S. S. Shankar, A. Rai, B. Ankamwar, A. Singh, A. Ahmad and M. Sastry, Nat. Mater., 2004, 3, 482 CrossRef CAS PubMed.
- G. S. Ghodake, N. G. Deshpande, Y. P. Lee and E. S. Jin, Colloids Surf., B, 2010, 75, 584 CrossRef CAS PubMed.
- M. R. Mackiewicz, B. A. Ayres and S. M. Reed, Nanotechnology, 2008, 19, 115607 CrossRef PubMed.
- B. Chen, B. W. Pogue and T. Hasan, Expert Opin. Drug Delivery, 2005, 2, 477 CrossRef CAS PubMed.
- T. H. Ha, H. Koo and B. H. Chung, J. Phys. Chem. C, 2007, 111, 1123 CAS.
- Sigma Product Number P 3644 (L-α-Phosphatidylcholine) information sheet.
- M. Hanauer, S. Pierrat, I. Zins, A. Lotz and C. Sonnichsen, Nano Lett., 2007, 7, 2881 CrossRef CAS PubMed.
- S. Park, N. Sinha and K. Hamad-Schifferli, Langmuir, 2010, 26, 13071 CrossRef CAS PubMed.
- M. S. Wang and S. M. Reed, Proc. - IEEE Conf. Nanotechnol., 2011, 1652 Search PubMed.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389 CrossRef CAS.
- K. Park, PhD thesis, Georgia Institute of Technology, December 2006 Search PubMed.
- H. M. Chen, R. Liu, K. Asakura, L. Jang and J. Lee, J. Phys. Chem. C, 2007, 111, 18550 CAS.
- S. Sofou and J. L. Thomas, Biosens. Bioelectron., 2003, 18, 445 CrossRef CAS.
- S. K. Banerji and M. A. Hayes, Langmuir, 2007, 23, 3305 CrossRef CAS PubMed.
- J. Zimmerberg and M. Kozlov, Nat. Rev. Mol. Cell Biol., 2006, 7, 9 CrossRef CAS PubMed.
- K. Athenstaedt and G. Daum, Eur. J. Biochem., 1999, 266, 1 CrossRef CAS.
- T. Tanaka, A. Kassai, M. Ohmoto, K. Morito, Y. Kashiwada, Y. Takaishi, M. Urikura, J. Morishige, K. Satouchi and A. Tokumura, J. Agric. Food Chem., 2012, 60, 4156 CrossRef CAS PubMed.
- M. Urikura, J. Morishige, T. Tanaka and K. Satouchi, J. Agric. Food Chem., 2012, 60, 11359 CrossRef CAS PubMed.
Footnote |
| † Present address: Department of Chemistry, Reed College, Portland, Oregon 97202, USA. |
| This journal is © The Royal Society of Chemistry 2014 |






