 Open Access Article
Open Access ArticleApplication of chemical vapor generation systems to deliver constant gas concentrations for in vitro exposure to volatile organic compounds†
Ying-Hsuan
Lin
 a,
Kenneth G.
Sexton
a,
Ilona
Jaspers
bcde,
Ya-Ru
Li
a,
Jason D.
Surratt
a and
William
Vizuete
*a
a,
Kenneth G.
Sexton
a,
Ilona
Jaspers
bcde,
Ya-Ru
Li
a,
Jason D.
Surratt
a and
William
Vizuete
*a
aDepartment of Environmental Sciences and Engineering, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA. E-mail: airquality@unc.edu
bCenter for Environmental Medicine, Asthma and Lung Biology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
cCurriculum in Toxicology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
dDepartment of Pediatrics, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
eDepartment of Microbiology and Immunology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
First published on 22nd October 2014
Abstract
Exposure to volatile organic compounds from outdoor air pollution is a major public health concern; however, there is scant information about the health effects induced by inhalation exposure to photochemical transformed products of primary emissions. In this study, we present a stable and reproducible exposure method to deliver ppm–ppb levels of gaseous standards in a humidified air stream for in vitro cell exposure through a direct air–liquid interface. Gaseous species were generated from a diffusion vial, and coupled to a gas-phase in vitro exposure system. Acrolein and methacrolein, which are major first-generation photochemical transformation products of 1,3-butadiene and isoprene, respectively, were selected as model compounds. A series of vapor concentrations (0.23–2.37 ppmv for acrolein and 0.68–10.7 ppmv for methacrolein) were investigated to characterize the exposure dose–response relationships. Temperature and the inner diameter of the diffusion vials are key parameters to control the evaporation rates and diffusion rates for the delivery of target vapor concentrations. Our findings suggest that this exposure method can be used for testing a wide range of atmospheric volatile organic compounds, and permits both single compound and multiple compound sources to generate mixtures in air. The relative standard deviations (%RSD) of output concentrations were within 10% during the 4-hour exposure time. The comparative exposure-response data allow us to prioritize numerous hazardous gas phase air pollutants. These identified pollutants can be further incorporated into air quality simulation models to better characterize the environmental health risks arising from inhalation of the photochemical transformed products.
Environmental impactExposure to airborne volatile organic chemicals (VOCs) is a potential cause of various adverse health effects. Traditionally, assessments of in vitro toxicity of VOCs are usually performed by direct treatments of test chemicals dissolved in aqueous solutions, such as cell culture media or buffers, which may lead to significant loss of test chemicals during exposure due to evaporation or modifications of chemical composition when the test compound is unstable in water (e.g., susceptible to hydrolysis). Development of an effective and reproducible technique for in vitro exposure to gaseous air pollutants through an air–liquid interface as an alternative tool is needed to more closely represent the realistic exposure conditions. |
1. Introduction
Exposure to atmospheric air pollutants has been linked to various adverse health effects in epidemiologic studies.1 Recent evaluation by the International Agency for Research on Cancer (IARC) has also concluded that outdoor air pollution is a leading environmental cause of cancer deaths with sufficient evidence.2 Although health risks associated with ambient air pollution have been found, the causative agents responsible for the observed health effects and the underlying toxicological mechanisms remain unclear. One of the major challenges in identifying causative agents is the fact that many components of air pollutants are modified in the atmosphere due to photochemical reactions, and hence alter the observed health effects due to compositional changes.3 This has been illustrated by prior laboratory studies utilizing an outdoor smog chamber coupled to an in vitro human lung cell exposure system, showing that exposure to the mixture of photochemical transformation products from 1,3-butadiene (C4H6) and isoprene (2-methyl-1,3-butadiene; C5H8) significantly enhances the toxicological responses on cytotoxicity and proinflammatory mediator release compared to their precursor compounds.4,5 As a result, in addition to characterizing the health effects from primary volatile organic compound (VOC) emissions, there is a need to identify important hazardous secondary air pollutants that may be more harmful than their precursors.Acrolein (prop-2-enal; C3H4O) is one of the major first-generation photochemical transformation products formed in the gas phase from 1,3-butadiene.6 Due to its high vapor pressure (274 mm Hg at 25 °C),7 acrolein is highly volatile when it is produced in the atmosphere. Thus, inhalation is a major route of exposure. Inhaled acrolein is highly toxic and has been associated with asthma-like symptoms, chronic obstructive pulmonary disease, cystic fibrosis, and lung carcinogenesis.8–10 From a chemical point of view, acrolein is a strong electrophile possessing an unsaturated carbon–carbon double bond conjugated with an electron withdrawing carbonyl group.11 Such reactive functional groups can rapidly attack biological nucleophiles like thiol-containing glutathione, cysteine and lysine residues in protein that lead to conformational changes and impair protein functions,12,13 disrupt regulation of gene expression by direct modification of the DNA-binding domain of a transcription factor,14 and potentially bind with nucleophilic centers within DNA to form adducts and cross-links.15 Methacrolein (2-methylprop-2-enal; C4H6O), as a structural analog, is one of the major first-generation photooxidation products produced from isoprene in the gas phase. The vapor pressure of methacrolein is 155 mm Hg at 25 °C.16 In the atmosphere, methacrolein can further react with atmospheric oxidants through hydroxyl radical (OH) initiated oxidation or ozonolysis. The half-lives for these photochemical reactions are estimated to be around 11.5 hours and 10.5 days, respectively.17,18 Methacrolein has also been reported to cause sensory irritation after exposure through inhalation.19Table 1 lists some physicochemical and toxicological properties of acrolein and methacrolein.
| Test compound | Acrolein | Methacrolein |
|---|---|---|
| Molecular weight (g mol−1) | 56.06 | 70.09 |
| Formula | C3H4O | C4H6O |
| Structure |
|

|
| Boiling point | 53 °C | 69 °C |
| Vapor pressure (at 25 °C)7,16 | 274 mm Hg | 155 mm Hg |
| Solubility in water | 21.25 g per 100 mL | 6 g per 100 mL |
| Henry's law constant ([M atm−1])41 | 7.4 | 6.5 |
| LC50 (rat, inhalation, 4 h)35 | 20 mg m−3 | 560 mg m−3 |
Since acrolein and methacrolein are water soluble VOCs, current in vitro methods used to investigate acrolein and methacrolein toxicity often apply treatments by direct addition of chemical solutions into the cell medium, which does not maintain an air–liquid interface as found during in vivo exposures. This may result in significant loss of the test VOCs because of vapor evaporation from the cell medium or modification of the chemical composition (e.g., susceptible to hydrolysis) when the test compounds are dissolved in aqueous medium solutions. Therefore, an alternative method for in vitro gas phase exposure is needed to more closely simulate the in vivo exposure scenarios.20,21 To accomplish this goal, it requires a chemical generation system that can produce a stable and repeatable test atmosphere that permits the air–liquid interface for in vitro exposures to cultured cells.
The objective of this study is to develop an effective and reproducible method for generation of gaseous air pollutants for use in in vitro models through an air–liquid interface to more closely represent the realistic exposure conditions to VOCs, especially for the photochemical transformation products of volatile organic air toxics. We have developed an in vitro gas phase exposure method by coupling a diffusion vial to a gas phase in vitro exposure system (GIVES) that can generate continuous sources of acrolein and methacrolein capable of ventilating in vitro exposure samples with sufficient volume to overcome any losses to surfaces and tissue. This system maintains a steady vapor concentration over the course of exposure time, and provides sufficient excess material needed for chemical characterization or venting. Concentrations were shown to be stable and repeatable in both magnitude and stability. In addition, this chemical generation system is humidified to prevent desiccation of the in vitro models, but low enough to prevent condensation in any part of the system. The concentrations generated by this device can be easily adjusted to allow for in vitro exposure–dose–response studies and to determine the precision of exposure and toxicological processing. We demonstrated this system by investigation of gas phase acrolein and methacrolein exposure induced cytotoxicity and proinflammatory cytokine (interleukin 8) gene expression from A549 cells.
2. Materials and methods
2.1. Design of the in vitro exposure system
 | ||
| Fig. 1 A schematic diagram of the in vitro exposure system showing the connection of major components and the direction of airflows. | ||
The output flow of the chemical generator was well mixed with the humidified dilution air using a mixing flask consisting of a simple tee and midget impinger (ACE), with the goal of preventing condensation of water or chemical agents. Condensation needs to be avoided since it can absorb some chemical agents dramatically. A water trap was inserted in line in case condensation does occur. A distribution manifold consisting of a series of “tees” allows for the mixed stream to be shared and connected to the exposure devices, dew point monitor, chemical analyzers, and a vent line to maintain atmospheric pressure. While compounds we tested are easily maintained in a gaseous state at room temperature, if higher boiling point compounds are used or higher humidities needed, then the manifold and all further distribution and sample lines can be heated.
It should be noted that the external calibrations conducted with the outdoor smog chamber were operated under non-photochemical active conditions (dark or overcast conditions, with very low UV and total solar radiation detected) to minimize photochemical decomposition. In addition, the half-lives for acrolein and methacrolein against photochemical oxidation (i.e., hydroxyl radical-initiated oxidation) are 15–20 hours and 11.5 hours, respectively.17,18 Since the calibrations were completed within 1–2 hours, the photochemical decomposition would be negligible under given conditions.
2.2. Cell culture
For this study, the GIVES housed A549 human alveolar type II epithelial lung cell line, derived from human alveolar cell carcinoma of the lung. The A549 cell line was used here as an in vitro cell model because of its human pulmonary origin. Additionally, A549 cells have been reported to be sensitive to the inhaled gases for these alveolar epithelial cells lack the mucus layer for protection against inhaled air pollutants.4 This immortal epithelial-like cell line has been extensively used to access the toxicity of air pollutants, and it is known to produce cytokines capable of modulating immune cell activation.26,27 Investigation of exposure–dose–response relationships in a well-controlled bench scale exposure system facilitates testing toxicities of a number of gases to compare relative toxicity among these numerous atmospheric transformed products.A549 cells were seeded two days prior to exposure on 12-well cell culture plates with collagen-coated permeable membrane supports. A549 cells were grown at a density of 2.5 × 105 cells per well, and supplied with F12K medium plus 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA). Before exposure, the cell culture growth medium was replaced with serum-free F12K plus 1.5 μg mL−1 bovine serum albumin (BSA) and antibiotics. For each experiment set, six replicates of A549 cells were used for the exposure, while another six wells of A549 cells were maintained in a regular tissue-culture incubator at 37 °C with CO2 (5%) supply, served as unexposed controls. A549 cells were allowed to grow on the permeable membrane supports, and maintained in the GIVES exposure system at 37 °C with CO2 (5%) supply throughout the 4-hour exposure.
2.3. Toxicity endpoints
Lactate dehydrogenase (LDH) release was measured as the marker of cytotoxicity. Induced Interleukin-8 (IL-8) gene expression was measured as the indication of proinflammatory cytokine release. LDH Cytotoxicity Detection Kit (Takara Bio Inc., Japan) and Human IL-8 ELISA Set (BD Bioscience, San Diego, CA) were used to perform bioassays according to the manufacturers' protocols. For all experiments, the supernatants of the exposed cells were collected 9 hours post-exposure. Collected samples were stored at −20 °C until analysis.2.4. Statistical analysis
Statistical analysis was performed using GraphPad Prism software 4.0 (GraphPad, San Diego, CA, USA). All the cellular responses of exposure data were normalized to the incubator controls, and expressed as relative fold increases over controls, mean ± standard error of the mean (SEM), n = 6 for each experiment. Student's t test was performed for data analysis to compare if the exposure responses are significantly different from unexposed controls; p < 0.05 was considered to be statistically significant. A one-way ANOVA followed by Tukey's multiple comparison tests was used to interpret the results of concentration effects on cellular responses.3. Results and discussion
3.1. Quality control and assurance of the exposure method
In this study, A549 cells were exposed to gas-phase acrolein or methacrolein generated from a diffusion vial system with constant output vapor concentrations. Cells were allowed to grow post exposure for 9 hours prior to collection of the supernatants. Exposure induced cytotoxicity (LDH release) and proinflammatory cytokine (IL-8) gene expression were examined as toxicity endpoints. To ensure that the operation of exposure system would not affect the measured cellular responses, control experiments were conducted by exposing A549 cells to clean air flowing through this exposure system. Fig. 3 shows that no significant differences of LDH release and IL-8 gene expression levels were detected between clean air exposure and unexposed incubator controls. These results then confirm that the toxicological responses measured with this approach were actually induced by the target compounds.Fig. 4 shows the results of continuous GC/FID measurements over the 4-hour exposure duration. The relative standard deviation (%RSD) of the output vapor concentrations for both target compounds ranged between 1.0% and 9.5% over the 4-hour exposure time (Table 2). The diffusion vial system is a well-developed and reliable gas generator for low-concentration calibration gases, consisting of a liquid-containing reservoir and a diffusing capillary with a uniform inner diameter. The diffusion technique has been widely used for generating standard gas sources.28 Specifically, reliability of gaseous standard production from liquid or by headspace diffusion of aqueous standards has been reported.29 Similar to the use of permeation tubes, the operation of the diffusion vial also experiences the saturation (dynamic), steady-state equilibrium (kinetic), and depletion stages.29,30 Constant temperature and flow rates are critical for controlling precise standard gas generation.31,32 The sample emission rates in the steady state are very stable. Thus, gases for exposure can be generated continuously for longer periods of time, and it can be easily generated in a wide range of concentrations. This system has advantages over direct addition of chemical solutions into the cell medium for it characterizes a realistic exposure route of inhalation. The continuous and stable supply of source generation is helpful to quantify accurate levels among different exposure settings. Moreover, this approach is capable of further studying multiple compounds of interest. Nevertheless, it is worth noting that this method is not suitable for substances with extremely high or low vapor pressure or substances with decomposability, hygroscopicity or polymerizability.
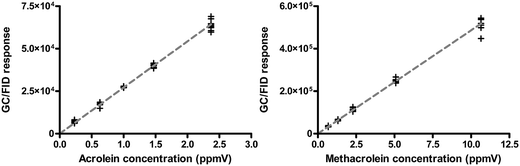 | ||
| Fig. 4 Measurements of the gas phase acrolein and methacrolein concentrations over the 4-hour exposure time. Vapor concentrations were monitored in a 30 minute interval throughout the experiments. | ||
| # | Test species | Measured concentrations (ppmv) | Relative standard deviation (RSD) |
|---|---|---|---|
| 1 | Clean air | 0.00 | — |
| 2 | Acrolein | 0.23 | 9.51% |
| 3 | Acrolein | 0.63 | 6.83% |
| 4 | Acrolein | 1.00 | 1.02% |
| 5 | Acrolein | 1.47 | 3.08% |
| 6 | Acrolein | 2.37 | 4.99% |
| 7 | Methacrolein | 0.68 | 7.99% |
| 8 | Methacrolein | 1.31 | 4.26% |
| 9 | Methacrolein | 2.29 | 6.81% |
| 10 | Methacrolein | 5.09 | 3.81% |
| 11 | Methacrolein | 10.70 | 8.49% |
3.2. Exposure induced cellular responses
In all experiments, exposure parameters and conditions were identical including the delivery flow rates (1 L min−1), the exposure duration (4 hours), and the exposure surface area per well (1.12 cm2; 12-well plate). Using the gas generator, five different concentrations of acrolein and methacrolein were created for exposures as shown in Table 2. Details of statistical results for concentration effects on cellular responses are provided in ESI (Table S1–S4†).LDH leakage is a widely used biomarker to measure chemically induced cytotoxicity of cellular membrane rupture and severe irreversible cell damage.33Fig. 5 shows the concentration dependent cytotoxic cellular response for acrolein and methacrolein exposure. The concentration-dependent cytotoxic effects have been observed for both compounds. The potency of these chemicals, however, is very different. The cytotoxic response for acrolein exposure remains insignificant compared to unexposed controls at the low dose range, until a concentration of 0.63 ppmv where LDH responses rapidly increase, and the fold change peaks at 15 at a dose of 2.37 ppmv. The cytotoxic response for methacrolein did not show a significant increase until a concentration of 10.7 ppmv. When cells are under low dose exposure, cells usually can adapt to the environment and survive. At high dose exposure when homeostasis is disrupted, pathways of cell death could be triggered. The sigmoid dose–response curve of acrolein exposure shows that there is a threshold at low dose level, while the biological gradient increases significantly as the acrolein dose increases above the point of departure (0.63 ppmv). This illustrates the value of the precision provided by the exposure system to detect these important changes in responses. Acrolein was also more toxic than methacrolein by inhalation in the rat (Table 1).
A second biomarker assessed in this study was IL-8, a proinflammatory mediators produced by epithelial cells. Some epidemiological studies have suggested occupational asthma associated with IL-8 increases.27,34Fig. 6 shows acrolein and methacrolein induced IL-8 gene expression at different vapor concentrations. Detailed statistical outputs are provided in the ESI (Table S3 and S4†). Unlike the LDH response with acrolein, IL-8 levels did not increase in doses beyond 0.63 ppmv. It should be noted that cell death due to exposure strongly influenced the capacity for IL-8 expression. From the acrolein LDH responses, it is clear that with increasing doses there were larger fractions of the original cells no longer viable and thus not capable of expressing IL-8. As a result, the low levels of IL-8 expression at high dose exposures were likely only coming from a smaller population of living cells. The proinflammatory effects at high dose exposures would have been more pronounced on a per cell basis. On the contrary, with less cytotoxic effects and more viable cells post exposure, methacrolein showed an increased response of IL-8 as exposure concentrations increased. This is consistent with current published toxicological data showing that acrolein is a much more severe irritant.35
Several studies investigated the possible pathways of acrolein induced toxicity. Thompson and Burcham reported a study using microarray analysis to investigate transcriptional responses of human lung A549 cells to acrolein, and their results indicate that acrolein dysregulated a broad range of cellular pathways including those involved in apoptosis, cell cycle control, transcription, cell signaling, and protein biosynthesis.36 Roy et al. reported a dose dependent study of A549 cells exposed to acrolein, and concluded that antiapoptosis processes dominate at low dose and shorter exposure times to acrolein, whereas proapoptotic processes dominate at high dose and longer exposure times.10 These findings are consistent with the observations in this study that IL-8 responses were only elevated at low dose levels (0–0.63 ppmv), whereas LDH release significantly increased at high levels (0.63–2.37 ppmv) that suggest apoptosis has been triggered and dominates the cellular responses. Importantly, results from this study provide the information for a gas-phase dosimetry more relevant to the inhalation exposure route. Furthermore, this well-controlled exposure method will be capable to be used for the purposes of fast toxicity screening to prioritize numerous air toxics in a complex mixture.
4. Conclusions
Taken together, this exposure method demonstrates an alternative approach to investigate in vitro exposure to VOCs, especially for water-soluble secondary organic gases that are produced in a complex photochemical reaction mixture. The findings in this study indicate that acrolein significantly contributes to exposure induced cytotoxic responses, which supports the observations by Doyle et al., showing enhanced cytotoxicity from butadiene photooxidation products.4 Methacrolein induced less cytotoxic effects on the basis of exposure concentrations, but increased response of IL-8 at concentrations greater than 5 ppmv was observed. Other gaseous components produced in the complex mixture of isoprene photooxidation products, such as recently identified gas-phase epoxide intermediates including isoprene epoxydiols (IEPOX) and methacrylic acid epoxide (MAE), are worthy of further investigations through this approach.37–40 Because we used short exposure times (i.e. 4 hours), high concentrations are needed to achieve an adequate exposure level to observe toxic responses. Although the concentrations in this study are higher than ambient levels, the comparative exposure–dose–response profiles will allow us to prioritize numerous air toxics in a complex mixture. These identified pollutants can be further incorporated into air quality models to characterize the environmental health risks arising from inhalation of the photochemical transformed products. Additionally, this same system and experimental protocols should be also applicable to studying indoor gaseous air pollutants in homes and workplace.Conflict of interest
No conflicts of interest.Acknowledgements
This project was supported by a Gillings Innovation Laboratory award funded by the Gillings gift.References
- L. Curtis, W. Rea, P. Smith-Willis, E. Fenyves and Y. Pan, Environ. Int., 2006, 32, 815–830 CrossRef CAS PubMed.
- K. Straif, A. Cohen and J. M. Samet, International Agency for Research on Cancer, Geneva, 2013 Search PubMed.
- K. G. Sexton, H. E. Jeffries, M. Jang, R. M. Kamens, M. Doyle, I. Voicu and I. Jaspers, Inhalation Toxicol., 2004, 16, 107–114 CrossRef CAS PubMed.
- M. Doyle, K. Sexton, H. Jeffries, K. Bridge and I. Jaspers, Environ. Health Perspect., 2004, 112, 1488–1495 CrossRef CAS.
- M. Doyle, K. Sexton, H. Jeffries and I. Jaspers, Chem.–Biol. Interact., 2007, 166, 163–169 CrossRef CAS PubMed.
- X. Liu, H. Jeffries and K. Sexton, Atmos. Environ., 1999, 33, 3005–3022 CrossRef CAS.
- ATSDR, U.S. Department of Health and Human Service, Public Health Service, 1990.
- G. Leikauf, Environ. Health Perspect., 2002, 110, 505–526 CrossRef CAS.
- I. Rahman and I. M. Adcock, Eur. Respir. J., 2006, 28, 219–242 CrossRef CAS PubMed.
- J. Roy, P. Pallepati, A. Bettaieb, A. Tanel and D. A. Averill-Bates, Chem.–Biol. Interact., 2009, 181, 154–167 CrossRef CAS PubMed.
- C. Thompson and P. Burcham, Chem. Res. Toxicol., 2008, 21, 2245–2256 CrossRef CAS PubMed.
- J. Stevens and C. Maier, Mol. Nutr. Food Res., 2008, 52, 7–25 CAS.
- A. Furuhata, M. Nakamura, T. Osawa and K. Uchida, J. Biol. Chem., 2002, 277, 27919–27926 CrossRef CAS PubMed.
- C. Lambert, J. Li, K. Jonscher, T. Yang, P. Reigan, M. Quintana, J. Harvey and B. Freed, J. Biol. Chem., 2007, 282, 19666–19675 CrossRef CAS PubMed.
- P. J. O'Brien, A. G. Siraki and N. Shangari, Crit. Rev. Toxicol., 2005, 35, 609–662 CrossRef.
- C. L. Yaws, Handbook of Vapor Pressure: C1 to C4 compounds, Gulf Pub. Co., Houston, TX, 1994 Search PubMed.
- R. Atkinson, Chem. Rev., 1986, 86, 69–201 CrossRef CAS.
- R. Atkinson, W. P. Carter, S. M. Aschmann, J. N. Pitts and A. M. Winer, Atmospheric Fates of Organic Chemicals: Prediction of Ozone and Hydroxyl Radical Reaction Rates and Mechanisms, U.S. Environmental Protection Agency, Atmospheric Sciences Research Laboratory, 1985 Search PubMed.
- S. T. Larsen and G. D. Nielsen, Toxicol. Lett., 2000, 114, 197–202 CrossRef CAS.
- S. Bakand, C. Winder, C. Khalil and A. Hayes, J. Environ. Monit., 2006, 8, 100–105 RSC.
- F. Pariselli, M. Sacco and D. Rembges, Exp. Toxicol. Pathol., 2009, 61, 33–39 CrossRef CAS PubMed.
- G. O. Nelson, Controlled test atmospheres: principles and techniques, Ann Arbor Science Publishers, 1971 Search PubMed.
- S. Ebersviller, K. Lichtveld, K. G. Sexton, J. Zavala, Y.-H. Lin, I. Jaspers and H. E. Jeffries, Atmos. Chem. Phys., 2012, 12, 12277–12292 CAS.
- K.-H. Kim, Anal. Chim. Acta, 2006, 566, 75–80 CrossRef CAS PubMed.
- K.-H. Kim and H. T. Nguyen, J. Sep. Sci., 2007, 30, 367–374 CrossRef CAS.
- I. Jaspers, E. Flescher and L. C. Chen, Am. J. Physiol.: Lung Cell. Mol. Physiol., 1997, 272, L504–L511 CAS.
- S. E. Anderson, J. Wells, A. Fedorowicz, L. F. Butterworth, B. Meade and A. E. Munson, Toxicol. Sci., 2007, 97, 355–363 CrossRef CAS PubMed.
- M. Gautrois and R. Koppmann, J. Chromatogr. A, 1999, 848, 239–249 CrossRef CAS.
- Y.-H. Kim and K.-H. Kim, Anal. Chem., 2013, 85, 5087–5094 CrossRef CAS PubMed.
- D. P. Lucero, Anal. Chem., 1971, 43, 1744–1749 CrossRef CAS.
- J. Susaya, K.-H. Kim, J. W. Cho and D. Parker, J. Chromatogr. A, 2011, 1218, 9328–9335 CrossRef CAS PubMed.
- J. Susaya, K.-H. Kim, J. Cho and D. Parker, J. Chromatogr. A, 2012, 1225, 8–16 CrossRef CAS PubMed.
- M. Cho, A. Niles, R. Huang, J. Inglese, C. Austin, T. Riss and M. Xia, Toxicol. In Vitro, 2008, 22, 1099–1106 CrossRef CAS PubMed.
- J. J. Zhang, J. E. McCreanor, P. Cullinan, K. F. Chung, P. Ohman-Strickland, I.-K. Han, L. Järup and M. J. Nieuwenhuijsen, Research report, Health Effects Institute, 2009, 5–109, discussion 111–123 Search PubMed.
- D. Arntz, M. Höpp, S. Jacobi, J. Sauer, T. Ohara, T. Sato, N. Shimizu, G. Prescher, H. Schwind and O. Weiberg, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- C. A. Thompson and P. C. Burcham, Chem. Res. Toxicol., 2008, 21, 2245–2256 CrossRef CAS PubMed.
- F. Paulot, J. D. Crounse, H. G. Kjaergaard, A. Kürten, J. M. St. Clair, J. H. Seinfeld and P. O. Wennberg, Science, 2009, 325, 730–733 CrossRef CAS PubMed.
- J. D. Surratt, A. W. H. Chan, N. C. Eddingsaas, M. N. Chan, C. L. Loza, A. J. Kwan, S. P. Hersey, R. C. Flagan, P. O. Wennberg and J. H. Seinfeld, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6640–6645 CrossRef CAS PubMed.
- Y.-H. Lin, Z. Zhang, K. S. Docherty, H. Zhang, S. H. Budisulistiorini, C. L. Rubitschun, S. L. Shaw, E. M. Knipping, E. S. Edgerton, T. E. Kleindienst, A. Gold and J. D. Surratt, Environ. Sci. Technol., 2012, 46, 250–258 CrossRef CAS PubMed.
- Y.-H. Lin, H. Zhang, H. O. T. Pye, Z. Zhang, W. J. Marth, S. Park, M. Arashiro, T. Cui, S. H. Budisulistiorini, K. G. Sexton, W. Vizuete, Y. Xie, D. J. Luecken, I. R. Piletic, E. O. Edney, L. J. Bartolotti, A. Gold and J. D. Surratt, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 6718–6723 CrossRef CAS PubMed.
- R. Sander, Max-Planck Institute of Chemistry, Air Chemistry Department Mainz, Germany, 1999.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4em00465e |
| This journal is © The Royal Society of Chemistry 2014 |




