Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assessing trichloromethane formation and control in algal-stimulated waters amended with nitrogen and phosphorus
Clinton A.
Mash
a,
Byron A.
Winston
b,
David A.
Meints II
a,
Ashley D.
Pifer
a,
J. Thad
Scott
b,
Wen
Zhang
a and
Julian L.
Fairey
*a
aDepartment of Civil Engineering, University of Arkansas, 4190 Bell, Fayetteville, AR 72701, USA. E-mail: julianf@uark.edu; Fax: +1 (479) 575-7168; Tel: +1 (479) 575-4023
bDepartment of Crop, Soil, and Environmental Sciences, University of Arkansas, USA
First published on 21st January 2014
Abstract
Nitrogen (N) and phosphorus (P) enrichments can stimulate algal growth in drinking water sources, which can cause increased production of disinfection byproduct (DBP) precursors. However, the effect of systematic N and P enrichments on DBP formation and control has not been adequately studied. In this work, we enriched samples from a drinking water source – sampled on April 5, May 30, and August 19, 2013 – with N and P to stimulate algal growth at N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios covering almost five orders of magnitude (0.2–4429). To simulate DBP-precursor removal processes at drinking water treatment plants (DWTPs), the samples were treated with ClO2 followed by alum coagulation prior to free chlorine addition to assess the DBP formation potential (FP). Trichloromethane (TCM) was the predominant DBP formed and the TCMFP was the highest at intermediate N
P ratios covering almost five orders of magnitude (0.2–4429). To simulate DBP-precursor removal processes at drinking water treatment plants (DWTPs), the samples were treated with ClO2 followed by alum coagulation prior to free chlorine addition to assess the DBP formation potential (FP). Trichloromethane (TCM) was the predominant DBP formed and the TCMFP was the highest at intermediate N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P molar ratios (∼10 to 50), which corresponded with the peak in algal biomass, as measured by chlorophyll-a (Chl-a). Algal biomass was P-limited throughout the study period, and co-limited by N for the August 19 sampling set. The differences in TCMFP between the raw and treated waters decreased with increasing P amendment, indicating that ClO2 and alum coagulation became less effective for TCM precursor removal as algal biomass increased. This study highlights the impact of nutrient enrichments on TCM formation and control and has implications for nutrient management strategies related to source water protection and for DWTPs that use source waters increasingly enriched with N and P.
P molar ratios (∼10 to 50), which corresponded with the peak in algal biomass, as measured by chlorophyll-a (Chl-a). Algal biomass was P-limited throughout the study period, and co-limited by N for the August 19 sampling set. The differences in TCMFP between the raw and treated waters decreased with increasing P amendment, indicating that ClO2 and alum coagulation became less effective for TCM precursor removal as algal biomass increased. This study highlights the impact of nutrient enrichments on TCM formation and control and has implications for nutrient management strategies related to source water protection and for DWTPs that use source waters increasingly enriched with N and P.
Environmental impactThe experiments presented here demonstrate that nutrient-driven increases in algal biomass reduced the effectiveness of two common disinfection byproduct control measures, ClO2 oxidation and alum coagulation. For nutrient amended raw waters, algal biomass, measured as chlorophyll-a, was a maximum at molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of ∼10 to 50, which following chlorination corresponded to an increase in trichloromethane. Across the experimental P-gradient, the differences in trichloromethane between the raw and treated waters decreased with increasing P amendment, indicating the algal biomass exerted a demand for ClO2 and alum. These results demonstrate that current treatment strategies for trichloromethane control may become less effective as waters become more algal-impaired. P ratios of ∼10 to 50, which following chlorination corresponded to an increase in trichloromethane. Across the experimental P-gradient, the differences in trichloromethane between the raw and treated waters decreased with increasing P amendment, indicating the algal biomass exerted a demand for ClO2 and alum. These results demonstrate that current treatment strategies for trichloromethane control may become less effective as waters become more algal-impaired.
|
Introduction
Despite the discovery of disinfection byproducts (DBPs) in chlorinated waters almost four decades ago,1 DBP control at drinking water treatment plants (DWTPs) remains an ongoing challenge. DBPs are formed by reactions between disinfectants (e.g., free chlorine and chlorine dioxide) and natural organic matter (NOM). While over 600 individual DBPs have been identified,2 only 11 are regulated by the United States Environmental Protection Agency under the Stage 2 Disinfectants/DBP Rule – four trihalomethanes (THMs), five haloacetic acids, chlorite, and bromate.DWTPs can draw from a two-pronged approach to curb formation of regulated DBPs: (1) increase NOM removal, by processes such as enhanced coagulation in which more coagulant is added than is necessary for turbidity removal,3,4 and (2) switch primary and/or secondary disinfectants. One common primary disinfectant for DWTPs seeking to curb DBPs is chlorine dioxide (ClO2), which can improve NOM coagulation5 and does not react with NOM to form THMs.6 However, the use of ClO2 necessitates the addition of a secondary disinfectant, like free chlorine, to maintain a residual throughout the distribution system. As such, DBPs such as THMs can still form, but only after some NOM removal has occurred through the coagulation process. The drawbacks of chlorine dioxide addition are that it is reduced to chlorite,7,8 a regulated DBP that can be removed by the addition of ferrous salts, and that it may lyse algal cells and release intracellular organic matter, a potential source of DBP precursors.9
It has long been recognized that DBP formation is impacted by nutrient loadings to source waters. As urban and agricultural land use intensifies, nitrogen (N) and phosphorus (P) enrichments can cause increases in algal biomass and productivity,10–12 decreasing the availability of pristine water supplies. Increased algal biomass and extracellular products13 can react with disinfectants to form DBPs.14–17 In addition to elevated nutrients increasing algal biomass, the ratio of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P can influence the type of algae growing in lakes,18,19 which also has consequences for water quality. Eutrophic waters often have high algal productivity and lower N
P can influence the type of algae growing in lakes,18,19 which also has consequences for water quality. Eutrophic waters often have high algal productivity and lower N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios,20 which favor nitrogen-fixing cyanobacteria, and can deteriorate water quality through the production of toxins and taste-and-odor forming compounds.21 On the other hand, oligotrophic lakes are often characterized by low productivity and high N
P ratios,20 which favor nitrogen-fixing cyanobacteria, and can deteriorate water quality through the production of toxins and taste-and-odor forming compounds.21 On the other hand, oligotrophic lakes are often characterized by low productivity and high N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios, conditions under which cyanobacteria are rare and diatoms typically dominate the phytoplankton community composition.
P ratios, conditions under which cyanobacteria are rare and diatoms typically dominate the phytoplankton community composition.
Despite these previous research efforts, comparatively little is known about DBP formation and control in waters enriched across environmentally relevant gradients of N and P. Such work is important to help guide nutrient management strategies and to assist DWTPs in adapting DBP control processes for increasingly impaired water sources. The research objective of this work was to assess the effect of algal growth driven by N and P enrichments on DBP formation and control. Source water was sampled in the spring and summer 2013 from Beaver Lake near a DWTP intake (Lowell, AR) and amended with N and P at various N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios to stimulate biomass growth. To simulate DBP-precursor removal processes at DWTPs, these waters were subjected to ClO2 oxidation and alum coagulation. After each treatment, the samples were filtered and various DBP-precursor surrogate parameters were measured.22 The raw and treated waters were chlorinated to assess the DBP formation potential (DBPFP) as a function of N and P amendments, and correlations were sought between DBPFP and the various precursor surrogate parameters.
P ratios to stimulate biomass growth. To simulate DBP-precursor removal processes at DWTPs, these waters were subjected to ClO2 oxidation and alum coagulation. After each treatment, the samples were filtered and various DBP-precursor surrogate parameters were measured.22 The raw and treated waters were chlorinated to assess the DBP formation potential (DBPFP) as a function of N and P amendments, and correlations were sought between DBPFP and the various precursor surrogate parameters.
Materials and methods
Sampling location and nutrient enrichment experiments
Source waters were collected from the transition zone of Beaver Lake Reservoir (Lowell, AR) near the Beaver Water District (BWD) DWTP intake structure and used as an algae seed culture. This reservoir provides drinking water and recreation opportunities for the Northwest Arkansas region. It has an average depth of 18 m and an average hydraulic retention time of 1.5 years. Trophic conditions range from eutrophic at the mouth of the White River to oligotrophic near the dam. The reservoir is also fed by Richland Creek, War Eagle Creek, and Brush Creek, and comprises a total hydraulic catchment area of 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ha of largely forested (69%) and agricultural (26%) land.23
000 ha of largely forested (69%) and agricultural (26%) land.23
Beaver Lake water was collected from a boat in the spring and summer of 2013 on April 5, May 30, and August 19. On each day, a 120 L composite sample was collected from across the photic zone and transported to the University of Arkansas for bioassay experiments. Samples were mixed and dispensed in 3 L aliquots into 4 L acid-washed plastic cubitainers. For each sampling date, a total of 36 cubitainers were used for a nutrient enrichment experiment. The nutrient enrichment bioassay experiment on each date was intended to create various nutrient-amendment rates and various N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios. A P enrichment gradient of 0, 0.025, 0.05, 0.075, 0.1, and 0.2 mg L−1 P as disodium hydrogen phosphate (Na2HPO4) along with 2 mg L−1 nitrogen as potassium nitrate (KNO3) was created to achieve 6 triplicate N
P ratios. A P enrichment gradient of 0, 0.025, 0.05, 0.075, 0.1, and 0.2 mg L−1 P as disodium hydrogen phosphate (Na2HPO4) along with 2 mg L−1 nitrogen as potassium nitrate (KNO3) was created to achieve 6 triplicate N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of ∼4429, 442, 177, 89, 44, and 22 by moles, respectively. A separate N enrichment gradient of 0, 0.1, 0.25, 0.5, and 1 mg L−1 N (as KNO3) along with 0.2 mg L−1 P (as Na2HPO4) was created to achieve 5 triplicate molar N
P ratios of ∼4429, 442, 177, 89, 44, and 22 by moles, respectively. A separate N enrichment gradient of 0, 0.1, 0.25, 0.5, and 1 mg L−1 N (as KNO3) along with 0.2 mg L−1 P (as Na2HPO4) was created to achieve 5 triplicate molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of ∼0.22, 1.1, 2.8, 5.5, and 11.1, respectively. As such, the combined N
P ratios of ∼0.22, 1.1, 2.8, 5.5, and 11.1, respectively. As such, the combined N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio gradient spanned almost five orders of magnitude, while the N and P enrichment gradients spanned more than one order of magnitude each.
P ratio gradient spanned almost five orders of magnitude, while the N and P enrichment gradients spanned more than one order of magnitude each.
After N and P amendment, samples were placed in a 30 °C water bath under artificial lighting. Lights were controlled by a 12 hour on/off timer and measured to be 500 μmol photons m−2 s−1 during illumination. The cubitainers used were transparent and were inverted during incubation to prevent shading from the opaque lids. Each cubitainer was opened to the atmosphere and shaken daily by hand to aid in aeration and minimize attached growth. Algal biomass was estimated daily as raw water fluorescence measurements using a Turner Design Trilogy fluorometer (Turner Designs, Sunnyvale, CA) at 880 nm. Once the samples had achieved their maximum biomass (∼4 days), the cubitainers were shaken vigorously and 2 L were poured into prepared HDPE containers. These containers were stored in the dark at 4 °C for DBPFP experiments. The remaining cubitainer volume was divided evenly for analyses of phytoplankton biomass and particulate nutrients. Aliquots were filtered onto Whatman glass fiber filters (GFFs) and stored frozen for measurement of phytoplankton biomass as extracted chlorophyll-a (Chl-a).
Chl-a was measured to estimate phytoplankton biomass according to Standard Methods 10200H,24 with modifications. One filter from each sample was protected from light and transferred to a 15 mL test tube containing 7 mL of 90% acetone solution. The samples were placed in a dark freezer for 24 hours to further enhance pigment extraction. In a dark room, 3 mL of each sample extract were then transferred into disposable test tubes and were analyzed using the Turner Design fluorometer at 880 nm. To adjust for the chlorophyll degradation product pheophytin, each sample was re-measured 90 seconds after addition of 0.1 mL of 0.1 N HCl.
Water quality tests
Laboratory glassware and plastic ware were prepared in accordance with previous work.25 All stock chemicals used were ACS grade, and aqueous solutions were made with Milli-Q water (18.2 MΩ-cm) generated by a Millipore Integral 3 (Billerica, MA) water purification system. The pH and turbidity of the raw waters were measured using equipment and methods described previously.25 Prior to measurement of dissolved organic carbon (DOC) and ultraviolet (UV) absorbance, samples were filtered through prepared 0.45 μm nominal pore size polyethersulfone (PES) membranes. These filters were prepared by rinsing with 500 mL of Milli-Q water prior to use.8 The first 25 mL of filtered sample was wasted for each new filter, to minimize organic carbon adsorption. Filtered samples were then stored in 250 mL amber glass screw top bottles in the dark at 4 °C. DOC analysis was performed on a Sievers 900 Portable Total Organic Carbon Analyzer (GE Analytical Instruments, Boulder, CO). UV absorbance scans from 600 to 225 nm were performed on a Shimadzu UV-Vis 2450 (Kyoto, Japan) spectrophotometer using a 1 cm path length low volume quartz cell.Chlorine dioxide preparation
Chlorine dioxide was generated using methods described previously.26 Before dosing, raw water samples were poured into prepared 1 L amber glass screw top bottles and placed in a water bath at 24 °C. The stock chlorine dioxide concentration was measured by absorptivity at 360 nm after dilution with Milli-Q water, using an assumed molar absorptivity of 1225 M−1 cm−1. The nutrient amended samples generated from source water collected on May 30, 2013 were dosed with chlorine dioxide at 1 mg L−1, whereas the August 19 samples were dosed at 2 mg L−1. After dosing, samples were capped headspace-free and placed in the dark at room temperature for 24 hours.Alum coagulation jar tests
After the chlorine dioxide dosing and hold time, 500 mL aliquots of each sample water were alum coagulated in square-bottom plastic jars equipped with 5 cm magnetic PTFE stir bars with ring-collared ends on an eight-position magnetic stir plate (Challenge Technology, Springdale, AR). Samples were mixed at 200 rpm to simulate rapid mix conditions prior to the simultaneous addition of alum (aluminum sulfate octadecahydrate) as a coagulant and sodium carbonate to aid in pH control. May 30 samples were dosed with 40 mg L−1 alum and 25 mg L−1 sodium carbonate, while August 19 samples were dosed with 80 mg L−1 alum and 85 mg L−1 sodium carbonate. After 30 seconds of rapid mix (∼200 rpm), the jars were moved to an adjacent eight-position magnetic stir plate for flocculation at 40 rpm for 30 minutes. The samples were then allowed to settle quiescently for at least 30 minutes before decanting. The supernatant was characterized and filtered as described in the Water Quality Tests, then used for subsequent experiments as detailed in the remainder of this section.Fluorescence measurements
Fluorescence excitation–emission matrices (EEMs) were collected for every raw and treated water sample (244 EEMs). Excitation wavelengths ranged from 225 to 400 nm in 1 nm step sizes and emission data was collected from 270 to 600 nm in 1 nm step sizes, resulting in a total of 58256 fluorescence intensity values, IEx/Em, per EEM. Scatter correction methods used were described previously.25,27 For the group of 244 EEMs, each IEx/Em pair was regressed against the DBPFP data using an in-house MATLAB® code.In addition to the pair-picking procedure, EEM data was modeled with PARAFAC analysis, following methods described previously.25 Of the 244 EEM sample set, one sample was classified as an outlier and removed from the dataset based on high leverage and apparent measurement error.28 A 5-component model was validated using split-halves analysis as detailed previously,25 and fluorescence maximum (FMAX) values from each component and EEM were used in DBPFP regression analyses.
Disinfection byproducts
The DBPFP was measured following Standard Methods 5710 B.24 Filtered samples were poured into 125 mL amber glass bottles and buffered with a phosphate solution to pH 7.0 ± 0.2. Sodium hypochlorite stock solution was standardized following Standard Methods 4500-Cl B, and then diluted to a lower concentration (between 2 and 4 g L−1 as Cl2) for dosing with a micropipette. The free chlorine dose required to achieve 7 day chlorine residuals of 3 to 5 mg L−1 as Cl2 was estimated based on raw water DOC. Free chlorine doses were stair-stepped with nutrient loading and ranged from 9 to 22 mg L−1 as Cl2. After addition of free chlorine, samples were capped headspace-free and placed in the dark at room temperature. After seven days, the chlorine residual was measured. Standards of free chlorine were prepared and analyzed with DPD total chlorine reagent powder pillows (Hach Company) and a spectrophotometer (Shimadzu UV-Vis 2450) across a measurement range of 1 to 7 mg L−1 as Cl2 (n = 5, r2 = 0.99, data not shown). An aliquot of sample was wasted before gently inverting the bottle three times, to minimize possible sample stratification. Precisely 5 mL of sample was pipetted into 5 mL of Milli-Q water for measurement of chlorine residual to measure high residuals.Precisely 30 mL of the remaining sample was withdrawn for DBPFP testing as described previously,29 with modifications. Two additional standard curve concentrations (150 μg L−1 and 200 μg L−1) were added to encompass higher trichloromethane (TCM) yields. Blanks and check standards were analyzed every 18 injections for quality control and 90% of check standards were within ±20% of the standard concentration, and all check standards were within ±25%, which is considered to be acceptable based on EPA 551.1.
Results and discussion
Algal biomass, nutrient concentrations, and N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) P ratios
P ratios
Algal biomass, measured as Chl-a, increased proportionally along the P enrichment gradient when N availability was high in experiments from all three months (Fig. 1a). Similarly, algal biomass increased along the N enrichment gradient when P availability was high in the August 19 experiment only (Fig. 1b). As a result, there was an obvious pattern in algal biomass along the experimental N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P gradient (Fig. 1c). For the May 30 and August 19 samples, algal biomass was greatest at intermediate N
P gradient (Fig. 1c). For the May 30 and August 19 samples, algal biomass was greatest at intermediate N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (∼5–50 by moles) and decreased substantially when the molar N
P (∼5–50 by moles) and decreased substantially when the molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio exceeded ∼80, indicating P-limiting conditions. These results indicate that P at least partially controlled algal biomass in Beaver Lake throughout the summer of 2013. Nitrogen exerted little control on algal biomass in spring, but partially controlled algal biomass in August (Fig. 1b). These results are consistent with previously reported patterns showing the seasonal transition between P- and N-limited algal growth in southern U.S. river impoundment reservoirs.30,31
P ratio exceeded ∼80, indicating P-limiting conditions. These results indicate that P at least partially controlled algal biomass in Beaver Lake throughout the summer of 2013. Nitrogen exerted little control on algal biomass in spring, but partially controlled algal biomass in August (Fig. 1b). These results are consistent with previously reported patterns showing the seasonal transition between P- and N-limited algal growth in southern U.S. river impoundment reservoirs.30,31
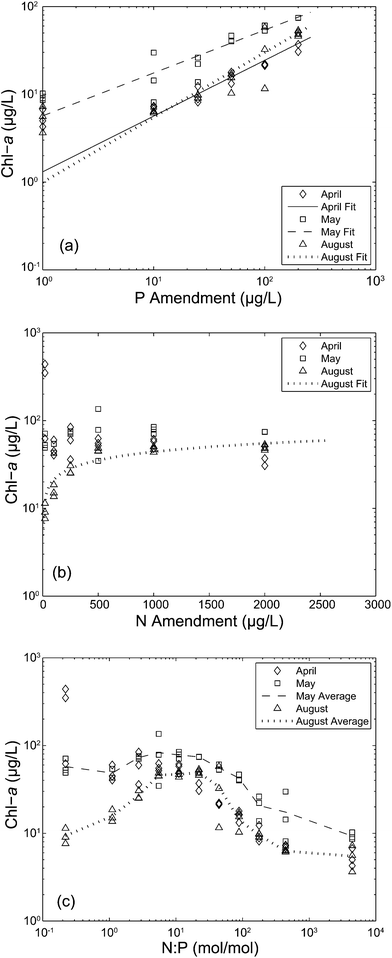 | ||
Fig. 1 Chlorophyll-a (Chl-a) of the raw water samples as a function of the (a) P amendment gradient with constant N (2000 μg L−1) on a log–log basis, (b) N amendment gradient with constant P (200 μg L−1) on a semi-log basis, and (c) molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of all samples on a log–log basis. Lines in panels (a) and (b) represent the least squares best fit and lines in panel (c) represent triplicate averages for the May 30 and August 19 sample collection. See Table 1 for details on N P ratio of all samples on a log–log basis. Lines in panels (a) and (b) represent the least squares best fit and lines in panel (c) represent triplicate averages for the May 30 and August 19 sample collection. See Table 1 for details on N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio. P ratio. | ||
Water quality tests
Raw water quality results for the April 5 sample collection are shown in Table 1. DOC increased with P dose from an average of 2.26 to 2.77 mg L−1 as C, suggesting the increased algal biomass (Fig. 1a) augmented the DOC by release of extracellular organic matter. While UV254 increased with P dose, the average SUVA decreased from 1.89 to 1.81 mg L−1 m−1, indicating the DOC produced was not enriched with aromatic carbon. This is a noteworthy result given the aromatic carbon fraction has been shown to be a significant source of THM precursors.32 In contrast with the trends in P dose, DOC, UV254, and SUVA did not change across the range of N doses. Taken together, these results suggest P-limited growth for the April 5 sampling set, which is consistent with the biomass data (Fig. 1). The free chlorine residuals after 7 days (FC-7d) were between 4 and 7 mg L−1 as Cl2, with no trends based on the N or P dose.| N dose (μg L−1) | P dose (μg L−1) | N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (mol mol−1) P (mol mol−1) |
DOC (mg L−1) | UV254 (m−1) | SUVA (mg L−1 m−1) | FC dose/FC-7d (mg L−1 as Cl2) |
|---|---|---|---|---|---|---|
a Values are averages ± standard deviations. N = nitrogen added as KNO3; P = phosphorus added as Na2HPO4; DOC = dissolved organic carbon; UV254 = ultraviolet absorbance at 254 nm; SUVA = specific UV254 (UV254/DOC); FC = free chlorine; FC-7d = free chlorine residual after a 7 day hold time; N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = molar nitrogen to phosphorus ratio based on amended doses, with the exception of two values (4429 and 0.2) which were calculated using the initial background concentrations of 2700 μg N L−1 and 11 μg P L−1 (initial molar N P = molar nitrogen to phosphorus ratio based on amended doses, with the exception of two values (4429 and 0.2) which were calculated using the initial background concentrations of 2700 μg N L−1 and 11 μg P L−1 (initial molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 539); NA = not applicable. Note: free chlorine was dosed after all other reported measurements. P = 539); NA = not applicable. Note: free chlorine was dosed after all other reported measurements.
|
||||||
| 0 | 0 | NA | 2.31 | 4.3 | 1.86 | 9/5.22 |
| 2000 | 0 | 4429 | 2.26 ± 0.02 | 4.3 ± 0.1 | 1.89 ± 0.04 | 9/5.59 ± 0.13 |
| 2000 | 10 | 442.3 | 2.37 ± 0.05 | 4.5 ± 0.1 | 1.89 ± 0.06 | 10/6.02 ± 0.04 |
| 2000 | 25 | 176.9 | 2.44 ± 0.03 | 4.6 ± 0.0 | 1.89 ± 0.02 | 11/6.34 ± 0.16 |
| 2000 | 50 | 88.5 | 2.50 ± 0.07 | 4.7 ± 0.1 | 1.87 ± 0.04 | 12/6.64 ± 0.24 |
| 2000 | 100 | 44.2 | 2.56 ± 0.05 | 4.6 ± 0.2 | 1.81 ± 0.03 | 12/6.59 ± 0.11 |
| 2000 | 200 | 22.1 | 2.77 ± 0.10 | 5.0 ± 0.0 | 1.81 ± 0.06 | 13/6.85 ± 0.17 |
| 0 | 200 | 0.2 | 2.87 ± 0.07 | 5.0 ± 0.1 | 1.76 ± 0.03 | 9/4.30 ± 0.07 |
| 100 | 200 | 1.1 | 2.83 ± 0.09 | 5.0 ± 0.1 | 1.77 ± 0.02 | 10/5.00 ± 0.13 |
| 250 | 200 | 2.8 | 2.80 ± 0.05 | 5.0 ± 0.1 | 1.77 ± 0.01 | 9/4.44 ± 0.08 |
| 500 | 200 | 5.5 | 2.82 ± 0.09 | 5.1 ± 0.1 | 1.80 ± 0.05 | 12/6.09 ± 0.24 |
| 1000 | 200 | 11.1 | 2.87 ± 0.07 | 5.0 ± 0.1 | 1.75 ± 0.02 | 13/6.48 ± 0.32 |
Raw and treated water quality results for the May 30 sample collection are shown in Table 2. Similar to the April results, raw water DOC increased with P dose from an average of 3.99 to 4.91 mg L−1 as C and did not increase uniformly with N dose, indicating P-limited growth. For all twelve N and P doses, ClO2 treatment increased the average DOC and decreased the average SUVA, suggesting algal cells were lysed by ClO2 oxidation and released intracellular organic matter with relatively low aromatic carbon content, similar to previous results.33 Subsequent alum coagulation decreased the average DOC below their corresponding raw waters in all 6 cases across the P gradient, but only in 3 of 5 cases across the N gradient. This indicates that DOC produced by N enrichment was more resistant to removal by alum coagulation. It is worth noting that the average FC-7d residuals in Table 2 were between 10 and 16 mg L−1 as Cl2, above the target window of 3–5 mg L−1 as Cl2 for the DBPFP tests. Ongoing experiments in our laboratory suggest these higher residuals will enhance formation of chlorinated THMs at the expense of bromine-substituted species and haloacetonitriles.
| Sample type | N dose (μg L−1) | P dose (μg L−1) | pH | Turbidity (NTU) | DOC (mg L−1) | UV254 (m−1) | SUVA (mg L−1 m−1) | FC Dose/FC-7d (mg L−1 as Cl2) |
|---|---|---|---|---|---|---|---|---|
a Values are averages ± standard deviations. Initial background concentrations in raw water were 724 μg N L−1 and 13 μg P L−1 (initial molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 125); N = nitrogen added as KNO3; P = phosphorus added as Na2HPO4; DOC = dissolved organic carbon; UV254 = ultraviolet absorbance at 254 nm; SUVA = specific UV254 (UV254/DOC); FC = free chlorine; FC-7d = free chlorine residual after a 7 day hold time; C = chlorine dioxide dosed at 1 mg L−1 as Cl2; CA = chlorine dioxide dosed at 1 mg L−1 as Cl2 and alum coagulation at 40 mg L−1 as alum; R = nutrient amended raw water; NM = not measured. Note: free chlorine was dosed after all other reported measurements. P = 125); N = nitrogen added as KNO3; P = phosphorus added as Na2HPO4; DOC = dissolved organic carbon; UV254 = ultraviolet absorbance at 254 nm; SUVA = specific UV254 (UV254/DOC); FC = free chlorine; FC-7d = free chlorine residual after a 7 day hold time; C = chlorine dioxide dosed at 1 mg L−1 as Cl2; CA = chlorine dioxide dosed at 1 mg L−1 as Cl2 and alum coagulation at 40 mg L−1 as alum; R = nutrient amended raw water; NM = not measured. Note: free chlorine was dosed after all other reported measurements.
|
||||||||
| R | 0 | 0 | 8.18 | 12.00 | 4.05 | 10.0 | 2.47 | 18/13.54 |
| C | 0 | 0 | 7.79 | 8.50 | 4.52 | 8.6 | 1.90 | 18/13.56 |
| CA | 0 | 0 | NM | NM | 3.31 | 4.6 | 1.39 | 18/15.66 |
| R | 2000 | 0 | 8.14 ± 0.02 | 9.23 ± 0.15 | 3.99 ± 0.06 | 9.5 ± 0.0 | 2.38 ± 0.03 | 18/13.74 ± 0.23 |
| C | 2000 | 0 | 7.80 ± 0.03 | 8.70 ± 0.44 | 4.37 ± 0.05 | 8.6 ± 0.1 | 1.97 ± 0.02 | 18/13.53 ± 0.19 |
| CA | 2000 | 0 | NM | NM | 3.02 ± 0.13 | 4.1 ± 0.1 | 1.37 ± 0.05 | 18/15.69 ± 0.45 |
| R | 2000 | 10 | 9.07 ± 0.08 | 9.60 ± 0.00 | 4.08 ± 0.04 | 9.4 ± 0.1 | 2.30 ± 0.03 | 19/14.00 ± 0.33 |
| C | 2000 | 10 | 8.22 ± 0.09 | 10.33 ± 0.29 | 4.56 ± 0.28 | 8.7 ± 0.0 | 1.91 ± 0.11 | 19/14.49 ± 0.50 |
| CA | 2000 | 10 | NM | NM | 3.37 ± 0.15 | 4.4 ± 0.2 | 1.31 ± 0.02 | 19/15.82 ± 0.23 |
| R | 2000 | 25 | 9.37 ± 0.08 | 9.67 ± 0.83 | 4.18 ± 0.08 | 9.5 ± 0.2 | 2.28 ± 0.05 | 20/14.52 ± 0.17 |
| C | 2000 | 25 | 8.76 ± 0.12 | 10.83 ± 0.76 | 4.67 ± 0.10 | 9.1 ± 0.2 | 1.95 ± 0.01 | 20/14.56 ± 0.45 |
| CA | 2000 | 25 | NM | NM | 3.66 ± 0.18 | 4.8 ± 0.2 | 1.31 ± 0.02 | 20/15.97 ± 0.26 |
| R | 2000 | 50 | 9.84 ± 0.04 | 11.33 ± 0.58 | 4.32 ± 0.03 | 9.7 ± 0.2 | 2.24 ± 0.05 | 21/14.60 ± 0.64 |
| C | 2000 | 50 | 9.44 ± 0.06 | 10.50 ± 0.87 | 4.89 ± 0.04 | 9.4 ± 0.1 | 1.93 ± 0.03 | 21/14.15 ± 0.49 |
| CA | 2000 | 50 | NM | NM | 3.75 ± 0.04 | 6.2 ± 0.5 | 1.66 ± 0.12 | 21/15.66 ± 0.08 |
| R | 2000 | 100 | 10.07 ± 0.04 | 11.00 ± 0.00 | 4.55 ± 0.15 | 10.1 ± 0.2 | 2.21 ± 0.03 | 21/14.09 ± 0.27 |
| C | 2000 | 100 | 9.73 ± 0.02 | 11.67 ± 0.29 | 5.17 ± 0.12 | 9.7 ± 0.1 | 1.87 ± 0.03 | 21/12.91 ± 1.05 |
| CA | 2000 | 100 | NM | NM | 4.56 ± 0.42 | 9.3 ± 0.4 | 2.05 ± 0.10 | 21/15.38 ± 0.25 |
| R | 2000 | 200 | 10.26 ± 0.01 | 11.75 ± 0.35 | 4.91 ± 0.13 | 10.6 ± 0.2 | 2.15 ± 0.01 | 22/13.93 ± 0.07 |
| C | 2000 | 200 | 9.78 ± 0.03 | 11.40 ± 5.09 | 6.79 ± 1.77 | 9.9 ± 0.1 | 1.50 ± 0.40 | 22/12.36 ± 0.72 |
| CA | 2000 | 200 | NM | NM | 4.52 ± 0.45 | 8.9 ± 0.6 | 1.96 ± 0.06 | 22/14.12 ± 0.27 |
| R | 0 | 200 | 10.11 ± 0.20 | 12.67 ± 0.58 | 4.66 ± 0.17 | 9.8 ± 0.4 | 2.11 ± 0.09 | 18/11.54 ± 0.25 |
| C | 0 | 200 | 9.67 ± 0.17 | 7.13 ± 3.35 | 5.45 ± 0.38 | 9.5 ± 0.1 | 1.74 ± 0.12 | 18/11.18 ± 0.27 |
| CA | 0 | 200 | NM | NM | 5.75 ± 0.72 | 7.5 ± 1.2 | 1.32 ± 0.26 | 18/12.69 ± 0.80 |
| R | 100 | 200 | 10.19 ± 0.08 | 11.67 ± 0.58 | 6.58 ± 3.31 | 10.1 ± 0.4 | 1.75 ± 0.65 | 19/11.90 ± 1.85 |
| C | 100 | 200 | 9.78 ± 0.11 | 15.33 ± 2.08 | 7.20 ± 3.29 | 9.6 ± 0.1 | 1.50 ± 0.54 | 19/9.95 ± 1.78 |
| CA | 100 | 200 | NM | NM | 6.07 ± 2.67 | 7.8 ± 0.7 | 1.47 ± 0.61 | 19/12.27 ± 1.91 |
| R | 250 | 200 | 10.25 ± 0.10 | 12.00 ± 0.00 | 4.72 ± 0.09 | 10.1 ± 0.3 | 2.14 ± 0.05 | 20/12.53 ± 1.32 |
| C | 250 | 200 | 9.71 ± 0.08 | 12.33 ± 0.58 | 5.14 ± 0.03 | 9.6 ± 0.1 | 1.88 ± 0.02 | 20/11.89 ± 0.52 |
| CA | 250 | 200 | NM | NM | 4.12 ± 0.20 | 7.8 ± 0.6 | 1.90 ± 0.07 | 20/13.89 ± 0.26 |
| R | 500 | 200 | 10.28 ± 0.01 | 12.00 ± 0.00 | 4.66 ± 0.09 | 9.9 ± 0.2 | 2.13 ± 0.04 | 21/13.55 ± 0.18 |
| C | 500 | 200 | 9.82 ± 0.06 | 14.33 ± 1.15 | 5.21 ± 0.03 | 9.8 ± 0.1 | 1.87 ± 0.02 | 21/11.97 ± 0.27 |
| CA | 500 | 200 | NM | NM | 4.70 ± 0.18 | 9.6 ± 0.2 | 2.05 ± 0.12 | 21/13.46 ± 0.44 |
| R | 1000 | 200 | 10.29 ± 0.07 | 11.33 ± 0.58 | 4.98 ± 0.55 | 9.9 ± 0.1 | 2.01 ± 0.20 | 22/14.42 ± 0.68 |
| C | 1000 | 200 | 9.85 ± 0.04 | 13.33 ± 0.58 | 5.09 ± 0.02 | 9.7 ± 0.1 | 1.91 ± 0.01 | 22/12.97 ± 0.09 |
| CA | 1000 | 200 | NM | NM | 4.68 ± 0.43 | 10.1 ± 0.4 | 2.16 ± 0.19 | 22/13.33 ± 0.29 |
Raw and treated water quality results for the August 19 sample collection are shown in Table 3. For the P-gradient, the raw water DOC ranged from 2.96 to 3.35 mg L−1 as C, but in contrast to April and May samples only increased for the two highest P doses (100 and 200 μg L−1). No discernible trends in average DOC were apparent across the N gradient, although Fig. 1b indicates N was co-limiting for the August 19 samples. ClO2 treatment increased the average DOC and decreased the average SUVA, supporting the previous results (Table 2) that lysis of algal cells occurred and released DOC depleted in aromatic carbon. Subsequent alum coagulation decreased the average DOC relative to their corresponding raw waters for all 11 nutrient amended samples. The ranges of the average SUVA for raw, ClO2-treated only, and ClO2 + alum coagulated waters were 1.54–1.70 mg L−1 m−1, 1.20–1.36 mg L−1 m−1, and 1.28–1.61 mg L−1 m−1. The modest increase in SUVA following alum coagulation of ClO2-treated waters for all 11 samples was unexpected and suggests that alum coagulation preferentially removed the less aromatic DOC. FC-7d residuals ranged from 5 to 9 mg L−1 as Cl2, more inline with the target residual for the DBPFP tests (3–5 mg L−1 as Cl2) compared to the April samples (Table 2), but nevertheless relatively high, which, as stated previously, favors the formation of chlorinated THMs.
| Sample type | N dose (μg L−1) | P dose (μg L−1) | pH | Turbidity (NTU) | DOC (mg L−1) | UV254 (m−1) | SUVA (mg L−1 m−1) | FC Dose/FC-7d (mg L−1 as Cl2) |
|---|---|---|---|---|---|---|---|---|
a Values are averages ± standard deviations. Initial background concentrations in raw water were 1900 μg N L−1 and 20 μg P L−1 (initial molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 214); N = nitrogen added as KNO3; P = phosphorus added as Na2HPO4; DOC = dissolved organic carbon; UV254 = ultraviolet absorbance at 254 nm; SUVA = specific UV254 (UV254/DOC); FC = free chlorine; FC-7d = free chlorine residual after a 7 day hold time; C = chlorine dioxide dosed at 2 mg L−1 as Cl2; CA = chlorine dioxide dosed at 2 mg L−1 as Cl2 and alum coagulation at 80 mg L−1 as alum; R = nutrient amended raw water. Note: free chlorine was dosed after all other reported measurements. P = 214); N = nitrogen added as KNO3; P = phosphorus added as Na2HPO4; DOC = dissolved organic carbon; UV254 = ultraviolet absorbance at 254 nm; SUVA = specific UV254 (UV254/DOC); FC = free chlorine; FC-7d = free chlorine residual after a 7 day hold time; C = chlorine dioxide dosed at 2 mg L−1 as Cl2; CA = chlorine dioxide dosed at 2 mg L−1 as Cl2 and alum coagulation at 80 mg L−1 as alum; R = nutrient amended raw water. Note: free chlorine was dosed after all other reported measurements.
|
||||||||
| R | 0 | 0 | 8.63 | 3.20 | 3.10 | 4.8 | 1.55 | 9/5.36 |
| C | 0 | 0 | 7.94 | 2.70 | 3.47 | 4.2 | 1.21 | 9/5.23 |
| CA | 0 | 0 | 8.23 | 0.90 | 3.23 | 3.3 | 1.02 | 9/6.11 |
| R | 0 | 0 | 8.83 ± 0.03 | 1.53 ± 0.06 | 3.09 ± 0.06 | 4.8 ± 0.0 | 1.56 ± 0.03 | 10/6.47 ± 0.04 |
| C | 0 | 0 | 8.12 ± 0.06 | 1.83 ± 0.12 | 3.18 ± 0.07 | 3.9 ± 0.1 | 1.24 ± 0.03 | 10/6.43 ± 0.07 |
| CA | 0 | 0 | 8.32 ± 0.04 | 0.43 ± 0.03 | 2.72 ± 0.07 | 3.5 ± 0.1 | 1.29 ± 0.05 | 10/7.01 ± 0.10 |
| R | 2000 | 0 | 8.94 ± 0.17 | 1.80 ± 0.26 | 3.09 ± 0.03 | 5.0 ± 0.1 | 1.61 ± 0.03 | 10/6.58 ± 0.15 |
| C | 2000 | 0 | 8.21 ± 0.25 | 1.80 ± 0.26 | 3.25 ± 0.03 | 4.0 ± 0.1 | 1.24 ± 0.01 | 10/6.23 ± 0.36 |
| CA | 2000 | 0 | 8.28 ± 0.03 | 0.42 ± 0.05 | 2.77 ± 0.03 | 3.6 ± 0.2 | 1.30 ± 0.06 | 10/7.11 ± 0.20 |
| R | 2000 | 10 | 8.92 ± 0.13 | 1.53 ± 0.15 | 3.10 ± 0.01 | 5.0 ± 0.0 | 1.61 ± 0.01 | 11/7.91 ± 0.51 |
| C | 2000 | 10 | 8.23 ± 0.12 | 1.77 ± 0.21 | 3.18 ± 0.05 | 3.9 ± 0.1 | 1.22 ± 0.00 | 11/7.41 ± 0.24 |
| CA | 2000 | 10 | 8.26 ± 0.01 | 0.40 ± 0.08 | 2.69 ± 0.14 | 3.6 ± 0.2 | 1.32 ± 0.04 | 11/8.51 ± 0.49 |
| R | 2000 | 25 | 9.25 ± 0.01 | 2.43 ± 0.23 | 3.06 ± 0.03 | 4.9 ± 0.1 | 1.61 ± 0.03 | 11/7.80 ± 0.34 |
| C | 2000 | 25 | 8.61 ± 0.05 | 2.13 ± 0.42 | 3.34 ± 0.02 | 4.3 ± 0.1 | 1.28 ± 0.01 | 11/7.25 ± 0.54 |
| CA | 2000 | 25 | 8.34 ± 0.03 | 0.83 ± 0.32 | 2.70 ± 0.05 | 3.8 ± 0.1 | 1.40 ± 0.00 | 11/8.11 ± 0.39 |
| R | 2000 | 50 | 9.36 ± 0.03 | 2.87 ± 0.57 | 2.96 ± 0.04 | 5.0 ± 0.1 | 1.70 ± 0.01 | 12/8.75 ± 0.26 |
| C | 2000 | 50 | 8.78 ± 0.05 | 3.20 ± 0.20 | 3.40 ± 0.06 | 4.4 ± 0.1 | 1.29 ± 0.02 | 12/8.25 ± 0.38 |
| CA | 2000 | 50 | 8.36 ± 0.01 | 0.83 ± 0.32 | 2.77 ± 0.06 | 3.8 ± 0.1 | 1.37 ± 0.05 | 12/9.33 ± 0.29 |
| R | 2000 | 100 | 9.55 ± 0.28 | 4.23 ± 0.75 | 3.24 ± 0.03 | 5.1 ± 0.1 | 1.58 ± 0.04 | 12/7.81 ± 0.88 |
| C | 2000 | 100 | 9.00 ± 0.28 | 4.53 ± 0.64 | 3.76 ± 0.51 | 4.7 ± 0.4 | 1.27 ± 0.09 | 12/7.25 ± 0.82 |
| CA | 2000 | 100 | 8.37 ± 0.03 | 0.77 ± 0.15 | 3.12 ± 0.39 | 4.2 ± 0.4 | 1.34 ± 0.11 | 12/8.21 ± 0.54 |
| R | 2000 | 200 | 9.80 ± 0.12 | 5.40 ± 0.53 | 3.35 ± 0.08 | 5.3 ± 0.1 | 1.57 ± 0.01 | 13/7.83 ± 0.27 |
| C | 2000 | 200 | 9.28 ± 0.16 | 5.57 ± 0.25 | 3.73 ± 0.05 | 5.1 ± 0.2 | 1.36 ± 0.03 | 13/7.71 ± 0.07 |
| CA | 2000 | 200 | 8.60 ± 0.11 | 1.27 ± 0.29 | 3.03 ± 0.08 | 4.9 ± 0.7 | 1.61 ± 0.20 | 13/8.81 ± 0.19 |
| R | 0 | 200 | 9.34 ± 0.01 | 2.23 ± 0.25 | 3.27 ± 0.03 | 5.2 ± 0.2 | 1.60 ± 0.03 | 10/6.96 ± 0.17 |
| C | 0 | 200 | 8.71 ± 0.04 | 2.30 ± 0.17 | 3.43 ± 0.07 | 4.1 ± 0.1 | 1.20 ± 0.02 | 10/6.26 ± 0.10 |
| CA | 0 | 200 | 8.43 ± 0.04 | 0.77 ± 0.21 | 2.88 ± 0.03 | 3.7 ± 0.1 | 1.28 ± 0.04 | 10/7.10 ± 0.16 |
| R | 100 | 200 | 9.56 ± 0.01 | 3.30 ± 0.62 | 3.17 ± 0.03 | 5.1 ± 0.1 | 1.62 ± 0.02 | 11/7.47 ± 0.10 |
| C | 100 | 200 | 9.06 ± 0.05 | 3.63 ± 0.15 | 3.72 ± 0.06 | 4.5 ± 0.1 | 1.22 ± 0.04 | 11/6.69 ± 0.11 |
| CA | 100 | 200 | 8.49 ± 0.03 | 0.87 ± 0.31 | 3.06 ± 0.08 | 4.3 ± 0.2 | 1.39 ± 0.02 | 11/7.63 ± 0.20 |
| R | 250 | 200 | 9.67 ± 0.02 | 3.53 ± 0.50 | 3.24 ± 0.02 | 5.1 ± 0.1 | 1.58 ± 0.01 | 12/8.35 ± 0.15 |
| C | 250 | 200 | 9.20 ± 0.01 | 4.07 ± 0.45 | 3.84 ± 0.05 | 4.7 ± 0.1 | 1.23 ± 0.00 | 12/7.10 ± 0.07 |
| CA | 250 | 200 | 8.52 ± 0.02 | 0.97 ± 0.21 | 3.12 ± 0.01 | 4.7 ± 0.1 | 1.50 ± 0.03 | 12/8.47 ± 0.05 |
| R | 500 | 200 | 9.70 ± 0.06 | 3.73 ± 0.06 | 3.30 ± 0.05 | 5.3 ± 0.1 | 1.59 ± 0.00 | 13/7.32 ± 0.29 |
| C | 500 | 200 | 9.14 ± 0.08 | 4.33 ± 0.31 | 3.78 ± 0.08 | 5.0 ± 0.1 | 1.32 ± 0.01 | 13/7.23 ± 0.10 |
| CA | 500 | 200 | 8.47 ± 0.02 | 1.03 ± 0.21 | 3.01 ± 0.05 | 4.5 ± 0.1 | 1.48 ± 0.01 | 13/8.41 ± 0.15 |
| R | 1000 | 200 | 9.76 ± 0.10 | 4.27 ± 0.64 | 3.33 ± 0.09 | 5.1 ± 0.1 | 1.54 ± 0.03 | 14/8.37 ± 0.23 |
| C | 1000 | 200 | 9.24 ± 0.05 | 4.67 ± 0.58 | 3.89 ± 0.05 | 5.2 ± 0.1 | 1.33 ± 0.01 | 14/7.95 ± 0.24 |
| CA | 1000 | 200 | 8.42 ± 0.05 | 1.00 ± 0.26 | 3.03 ± 0.05 | 4.8 ± 0.1 | 1.57 ± 0.03 | 14/9.09 ± 0.02 |
DBPFP tests
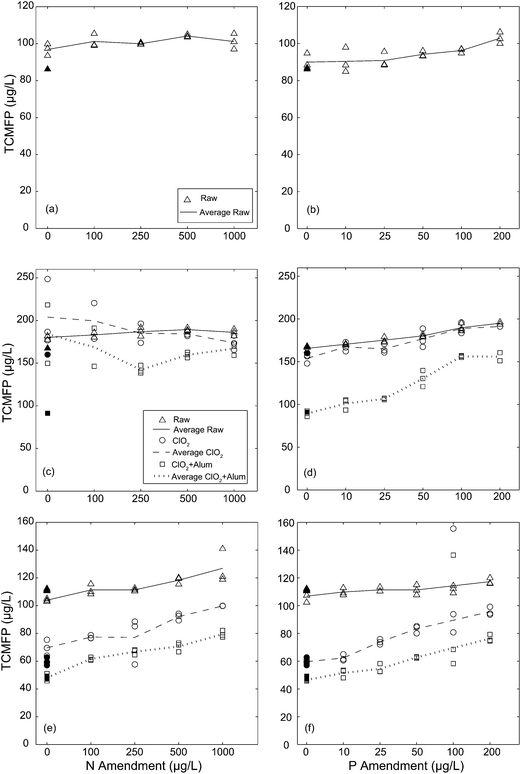
As expected based on the high free chlorine residuals (Tables 1–3) trichloromethane (TCM) was the predominant DBP formed, comprising 89–98% by mass of the total THMs (data not shown). Additionally, other DBPs quantified as part of EPA 551.1, such as dichloroacetonitrile, formed at relatively low concentrations (below 1.76 μg L−1) and, as a result, further discussion is focused on TCM only. TCMFP results are presented in Fig. 2, organized by sample month (April 5, May 30, and August 19) and nutrient amendment (N or P). The relatively high raw water TCMFP concentrations for the May 30 samples (approximately 50 μg L−1 higher than the April 5 and August 19 samples) are likely due to the comparatively high FC-7d values (Tables 1–3), rather than a greater abundance of TCM precursors. For the April 5 samples, the average TCMFP did not change across the N amendment (Fig. 2a), but increased 13% across the P amendment (from 90.0 to 102.8 μg L−1, Fig. 2b). For the May 30 samples, the average TCMFP in raw waters showed similar trends, with no increase across the N amendment (Fig. 2c), and an increase of 15% across the P amendment (from 165.7 to 195.1 μg L−1, Fig. 2d). For the August 19 samples, by contrast, the average TCMFP in the raw waters increased 18% across N amendment (from 103.9 to 126.9 μg L−1, Fig. 2e), and 9% across the P amendment (from 106.8 to 117.3 μg L−1, Fig. 2f). For the raw water samples, TCMFP was greatest at intermediate values of the experimental N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P gradient (∼10 to 50 by moles, Fig. 3a), which corresponded with the greatest algal biomass across all experiments (Fig. 1c). Thus, TCMFP was positively correlated with algal biomass as Chl-a in all experiments, with the steepest and strongest relationship occurring for the May 30 samples (Fig. 3b).
P gradient (∼10 to 50 by moles, Fig. 3a), which corresponded with the greatest algal biomass across all experiments (Fig. 1c). Thus, TCMFP was positively correlated with algal biomass as Chl-a in all experiments, with the steepest and strongest relationship occurring for the May 30 samples (Fig. 3b).
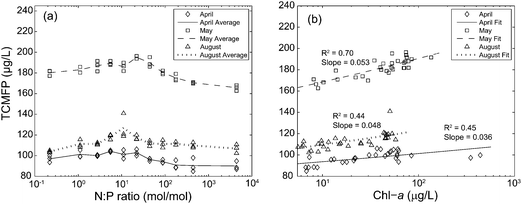 | ||
Fig. 3 Trichloromethane formation potential (TCMFP) for the raw water samples amended with nitrogen (N) and phosphorus (P) for the April 5, May 30, and August 19 samples as a function of the (a) log-molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio, where N and P represent the applied doses and (b) chlorophyll-a (Chl-a). Lines in panel (a) represent triplicate averages for each sample collection and lines in panel (b) represent the least squares best fit. See Table 1 for details on N P ratio, where N and P represent the applied doses and (b) chlorophyll-a (Chl-a). Lines in panel (a) represent triplicate averages for each sample collection and lines in panel (b) represent the least squares best fit. See Table 1 for details on N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio. P ratio. | ||
Treatment of raw waters occurred for the samples collected on May 30 and August 19 only. The May 30 samples were treated with ClO2 at 1 mg L−1 and an alum dose of 40 mg L−1; to achieve greater TCM precursor removal, both of these doses were doubled for the August 19 samples. Fig. 2c shows that treatment with 1 mg L−1 ClO2 increased the average TCMFP relative to the raw waters for the lowest two N amendments, and was similar to the raw waters for the higher N doses. Fig. 2d shows this same dose of ClO2 had little impact on TCMFP across the P amendment. This result indicates that the aromatic carbon depleted DOC released by ClO2 treatment (Table 2 – DOC and SUVA), was not a significant source of TCM precursors. For August 19 samples, a ClO2 dose of 2 mg L−1 decreased the average TCMFP by 20–30 μg L−1 across the N amendments (Fig. 2e) and 2–47 μg L−1 across the P amendments (Fig. 2f). Further, Fig. 2f shows that the differences in TCMFP between the raw and ClO2 treated samples decreased with increasing P amendment, presumably because the biomass produced (Fig. 1a) exerted a demand for ClO2, more so than directly contributing to the TCM precursor pool.
Alum coagulation following ClO2 treatment lowered the average TCMFP, an expected result based on previous research.26 The one exception to this trend occurred for the May 30 samples at an N amendment of 1000 μg L−1 (Fig. 2c), in which the average TCMFP values were similar for both treatments. Fig. 2d shows that alum coagulation decreased the average TCMFP by 34–64 μg L−1 compared to ClO2-only, but the difference between treatments decreased as the P amendment increased. For the August 19 samples, alum coagulation decreased TCMFP by 10–20 μg L−1 relative to ClO2-only for both nutrient amendments (Fig. 2e and f). The implication of this result for DWTPs is that ClO2 pre-oxidation and alum coagulation may be less effective for removal of TCM precursors as source waters become more nutrient enriched.
To further explain the TCMFP data, correlations were sought with known TCM precursor surrogate parameters (e.g., UV254, DOC, IEx/Em, and PARAFAC component FMAX values). For this dataset, I344/425 and FMAX from component 2 (Table 4) were the most strongly correlated fluorescence metrics (IEx/Em correlation results not shown). Fig. 4 shows correlations (p < 0.001) between TCMFP and (i) DOC (r2 = 0.72, Fig. 4a), (ii) UV254 (r2 = 0.88, Fig. 4b), (iii) I344/425 (r2 = 0.62, Fig. 4c), and (iv) C2 FMAX (r2 = 0.61, Fig. 4d). A weaker correlation was found between TCMFP and SUVA (r2 = 0.57, data not shown), an expected result given that SUVA is an intensive property. Data presented in Fig. 4 includes all samples and treatments except seven samples (out of 244) that were determined to be outliers – five of these samples had TCM concentrations that were 150% greater (e.g., 300–700 μg L−1) than the highest value in the GC standard curve, one sample had no measurable FC-7d residual, and the other sample was determined to be an outlier during the PARAFAC modeling process. The comparatively strong TCMFP:DOC correlation (r2 = 0.72, Fig. 4a) was unexpected because ClO2 treatment increased DOC (Tables 2 and 3) but decreased TCMFP (Fig. 2). The high TCMFP:UV254 correlation (r2 = 0.88, Fig. 4b) is in agreement with prior research,34 supporting the contention that released DOC from nutrient stimulated biomass was both low in aromatic carbon and did not contribute significantly to the pool of TCM precursors. The comparatively weak correlations between TCMFP and the fluorescence metrics (Fig. 4c and d) were unexpected based on previous research26,29 and suggest that dissolved species present in the samples from the nutrient enrichments (e.g., algal extrudates and intracellular organic matter) may have interfered with fluorescence measurements more so than UV254.
| Component | Excitation maxima (nm) | Emission maxima (nm) | r 2 (TCMFP:FMAX) |
|---|---|---|---|
| a Values in parentheses are secondary and tertiary maxima; r2 values describe the linear correlations between trichloromethane formation potential (TCMFP) and the fluorescence maximum values (FMAX) for each parallel factor (PARAFAC) component. | |||
| C1 | 235 (325, 386) | 422 (476) | 0.55 |
| C2 | 337 (237) | 375 (423) | 0.61 |
| C3 | 267 (367) | 456 | 0.52 |
| C4 | 226 (280) | 355 | 0.18 |
| C5 | 400 (370, 309) | 490 (394) | 0.47 |
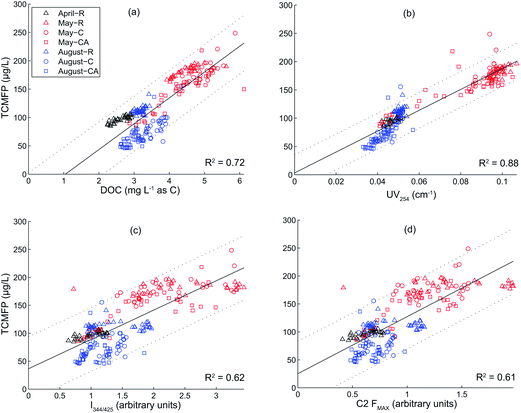 | ||
| Fig. 4 Correlations between trichloromethane formation potential (TCMFP) and (a) DOC, (b) UV254, (c) I344/425, (d) C2 FMAX. Linear best-fit models (solid lines) were determined based on least-squares analyses of raw (R), chlorine dioxide treated (C), and chlorine dioxide treated and alum coagulated (CA) waters from the April 5, May 30, and August 19 sampling collections. Dashed lines encompass the upper and lower 95% prediction intervals for the linear models. DOC is the dissolved organic carbon, UV254 is the ultraviolet absorbance at 254 nm, I344/425 is the fluorescence intensity at an excitation of 344 nm and an emission of 425 nm, and C2 FMAX is the maximum fluorescence intensity for PARAFAC component 2 (see Table 4 for description of the fluorescence–PARAFAC components). Seven samples (out of 244) were excluded from this figure because they were determined to be outliers as described in the Results and discussion – DBPFP tests section. | ||
Conclusions
The experiments presented here demonstrate that nutrient-driven increases in algal biomass reduced the effectiveness of two common DBP-control measures, ClO2 oxidation and alum coagulation. Algal biomass in nutrient amended waters was shown to be P-limited for the April 5, May 30, and August 19 sampling sets, with an N co-limitation for the August 19 samples. For the nutrient amended raw waters, algal biomass, measured as Chl-a, was a maximum at molar N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of ∼10 to 50, which following chlorination corresponded to a measurable increase in the TCMFP. Oxidation of the sample waters with chlorine dioxide increased the DOC with aromatic-depleted compounds that were not significant TCM precursors. Across the experimental P-gradient, the differences in TCMFP between the raw and ClO2 + alum coagulated waters decreased with increasing P amendment, indicating the algal biomass exerted a demand for ClO2 and alum. Results from this study can be used to guide nutrient management strategies for source water protection and can be used by DWTPs to assess the impact of N and P enrichments on TCM formation and control.
P ratios of ∼10 to 50, which following chlorination corresponded to a measurable increase in the TCMFP. Oxidation of the sample waters with chlorine dioxide increased the DOC with aromatic-depleted compounds that were not significant TCM precursors. Across the experimental P-gradient, the differences in TCMFP between the raw and ClO2 + alum coagulated waters decreased with increasing P amendment, indicating the algal biomass exerted a demand for ClO2 and alum. Results from this study can be used to guide nutrient management strategies for source water protection and can be used by DWTPs to assess the impact of N and P enrichments on TCM formation and control.
Acknowledgements
The authors gratefully acknowledge the support of the Arkansas Water Resources Center (AWRC) for funding this work.References
- J. J. Rook, J. – Am. Water Works Assoc., 1976, 68, 168–172 CAS.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Mutat. Res., Rev. Mutat. Res., 2007, 636, 178–242 CrossRef CAS PubMed.
- J. K. Edzwald and J. E. Tobiason, Water Sci. Technol., 1999, 40, 63–70 CrossRef CAS.
- S. W. Krasner and G. Amy, J. – Am. Water Works Assoc., 1995, 87, 93–107 CAS.
- W. P. Cheng and F. H. Chi, Chemosphere, 2003, 53, 773–778 CrossRef CAS.
- R. J. Miltner, The Effect of Chlorine Dioxide on Trihalomethanes in Drinking Water, University of Cincinnati, 1976 Search PubMed.
- C. Korn, R. C. Andrews and M. D. Escobar, Water Res., 2002, 36, 330–342 CrossRef CAS.
- T. Karanfil, I. Erdogan and M. A. Schlautman, J. – Am. Water Works Assoc., 2003, 95, 86–100 CAS.
- J. D. Plummer and J. K. Edzwald, J. Water Supply: Res. Technol.–AQUA, 2002, 51, 307–318 CAS.
- V. H. Smith, G. D. Tilman and J. C. Nekola, Environ. Pollut., 1999, 100, 179–196 CrossRef CAS.
- J. J. Elser, M. E. S. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin and J. E. Smith, Ecol. Lett., 2007, 10, 1135–1142 CrossRef PubMed.
- S. R. Carpenter, N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley and V. H. Smith, Ecol. Appl., 1998, 8, 559–568 CrossRef.
- S. M. Myklestad, Sci. Total Environ., 1995, 165, 155–164 CrossRef CAS.
- J. Y. Fang, X. Yang, J. Ma, C. Shang and Q. A. Zhao, Water Res., 2010, 44, 5897–5906 CrossRef CAS PubMed.
- J. Jack, T. Sellers and P. A. Bukaveckas, Can. J. Fish. Aquat. Sci., 2002, 59, 1482–1491 CrossRef CAS PubMed.
- R. C. Hoehn, D. B. Barnes, B. C. Thompson, C. W. Randall, T. J. Grizzard and P. T. B. Shaffer, J. – Am. Water Works Assoc., 1980, 72, 344–350 CAS.
- N. J. D. Graham, V. E. Wardlaw, R. Perry and J. Q. Jiang, Water Sci. Technol., 1998, 37, 83–89 CrossRef CAS.
- D. W. Schindler, Science, 1974, 184, 897–899 CAS.
- V. H. Smith, Science, 1983, 221, 669–671 CAS.
- J. A. Downing and E. McCauley, Limnol. Oceanogr., 1992, 37, 936–945 CrossRef CAS.
- Harmful Cyanobacteria, ed. J. Huisman, H. C. P. Matthijs and P. M. Visser, Springer, The Netherlands, 2005 Search PubMed.
- A. D. Pifer, S. L. Cousins and J. L. Fairey, J. Water Supply: Res. Technol.–AQUA, 2013 DOI:10.2166/aqua2013.122.
- S. Sen, B. E. Haggard, I. Chaubey, K. R. Brye, T. A. Costello and M. D. Matlock, Water, Air, Soil Pollut., 2007, 179, 67–77 CrossRef CAS.
- Standard methods for the examination of water and wastewater, ed. A. D. Eaton, L. S. Clesceri, E. W. Rice and A. E. Greenberg, APHA-AWWA-WEF, Washington, D.C., 21 edn., 2005 Search PubMed.
- A. D. Pifer, D. R. Miskin, S. L. Cousins and J. L. Fairey, J. Chromatogr. A, 2011, 1218, 4167–4178 CrossRef CAS PubMed.
- C. W. Granderson, A. D. Pifer and J. L. Fairey, J. – Am. Water Works Assoc., 2013, 105, 45–46 CrossRef CAS.
- R. G. Zepp, W. M. Sheldon and M. A. Moran, Mar. Chem., 2004, 89, 15–36 CrossRef CAS PubMed.
- C. A. Stedmon and R. Bro, Limnol. Oceanogr.: Methods, 2008, 6, 572–579 CrossRef CAS.
- A. D. Pifer and J. L. Fairey, Water Res., 2012, 46, 2927–2936 CrossRef CAS PubMed.
- J. T. Scott, R. D. Doyle, S. J. Prochnow and J. D. White, Ecol. Appl., 2008, 18, 805–819 CrossRef.
- J. T. Scott and E. M. Grantz, Freshwater Science, 2013, 32, 849–861 CrossRef.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS.
- L. Li, N. Y. Gao, Y. Deng, J. J. Yao and K. J. Zhang, Water Res., 2012, 46, 1233–1240 CrossRef CAS PubMed.
- J. K. Edzwald, W. C. Becker and K. L. Wattier, J. – Am. Water Works Assoc., 1985, 77, 122–132 CAS.
| This journal is © The Royal Society of Chemistry 2014 |