Potential beneficial uses of coalbed natural gas (CBNG) water†
K. J.
Reddy
*a,
Ashley J.
Whitman
a and
Andrew R.
Kniss
b
aDepartment of Ecosystem Science and Management, University of Wyoming, Laramie, Wyoming, USA. E-mail: katta@uwyo.edu
bDepartment of Plant Sciences, University of Wyoming, Laramie, Wyoming, USA
First published on 30th October 2013
Abstract
The CBNG well water is typically managed by discharging into nearby disposal ponds. The CBNG well water could potentially be very useful in the water-limited regions (e.g., arid and semi-arid), but beneficial uses may be hindered by water quality problems. Objectives of this research were to: (1) examine trend analysis of nine years of CBNG well water at discharge (outfall) points and in corresponding disposal ponds, (2) evaluate geochemical processes, (3) identify potential water quality issues, and (4) find potential beneficial uses. The CBNG well water at discharge points and in corresponding disposal ponds was measured on-site for pH and electrical conductivity (EC). These water samples were also analyzed in the laboratory for calcium (Ca), sodium (Na), magnesium (Mg), potassium (K), iron (Fe), aluminum (Al), copper (Cu), arsenic (As), selenium (Se), cadmium (Cd), and barium (Ba). Total dissolved solids (TDS) were calculated from EC measurements. The sodium adsorption ratio (SAR) was calculated from Na, Ca, and Mg concentrations. Trend analyses of outfalls and disposal ponds were conducted separately so that the differences in trends could be compared. Trends in CBNG well water at discharge points are not always the same as trends in CBNG disposal ponds: environmental and geochemical processes play an important role in the water quality of these well waters. Overall trend analyses suggest that CBNG well water at discharge points in all basins of the Powder River Basin meets beneficial use criteria, except for SAR and to some extent EC, for aquatic life, livestock and wildlife watering, and irrigation. The CBNG well water in disposal ponds across all basins meets criteria for all beneficial uses except for As, pH, SAR, and to some extent EC for irrigation, aquatic life, and livestock and wildlife watering.
Environmental impactCoalbed natural gas (CBNG) exploration and development are expanding worldwide, due to increased demand for a clean and economical source of energy. Methane is a main constituent of coalbed natural gas. Extraction of methane from coalbed deposits is facilitated with extraction wells. Water is a by-product of these extraction wells. The quality of CBNG well water can vary significantly from one geologic formation to the next. In conjunction with CBNG energy development, effective management of well water is indispensable. In this study we examined water quality trend analysis on nine years' well water data and identified geochemical processes to predict water quality issues and potential beneficial uses. |
Introduction
Natural gas exploration and development are expanding worldwide, due to increased demand for a clean and economical source of energy. Methane is a main constituent of coalbed natural gas (CBNG). Coalbed natural gas is the fastest-growing source of natural gas supply in the United States (US) as well as in other countries including Canada, Australia, China, and New Zealand.1 In US it accounts for more than 7.5% of US natural gas production.2 Rocky Mountain States, Wyoming, Colorado, Montana, New Mexico, and Utah are further utilizing their coal resources by extracting CBNG to supplement the US energy demands.3 Wyoming has one-third of the recoverable CBNG reserves of the US and is projected to contain approximately 31.7 TCF (Trillion Cubic Feet) of recoverable methane.4 The CBNG reserves in Wyoming are found in Powder River Basin (PRB), Wind River Basin (WRB), and Green River Basin (GRB). Among these basins, PRB has experienced a rapid expansion in CBNG production.5Methane, the main constituent of CBNG, is formed in deep confined coalbeds over geologic time as a result of natural chemical, physical, and biological processes. The natural gas remains trapped in the coalbed by confining pressure of the groundwater at an approximate depth of 160 meters. Methane is commonly extracted from the coalbed by depressurizing and pumping large quantities of groundwater, called CBNG well water. Each CBNG well can generate 8 to 80 liters of well water per minute, depending upon the number of wells in the area. To contain this water, 2 to 10 CBNG wells are combined together in a manifold system to allow well water flow into a single discharge.1 The Wyoming State Geological Survey (WSGS) has projected that approximately 7.01 billon m3 of well water will be discharged from CBNG development in the PRB, Wyoming.
The CBNG well water is managed by discharging well water into nearby disposal ponds (impoundments) and stream channels and dispersing into the air or re-injecting into the aquifer.6 Among these options, discharging CBNG well water into nearby disposal ponds is a common practice in the PRB. However, this practice then provides an opportunity for nearby livestock and wildlife as well as migrating birds to use these disposal ponds.
In a water-limited environment such as the western US, there is a tremendous appeal in turning CBNG well water from oil and gas operations into a useful product. Developing beneficial uses for well water could also reduce the costs of CBNG operations. In addition, the well water could potentially be used for developing livestock and wildlife habitat and providing water for agriculture, industry, and other uses to offset problems created by near-record drought conditions.7 The Powder River Basin, WY, is a semi-arid rangeland characteristic of the northern mixed grass prairie. There is ample vegetation for grazing, but water availability is limited. CBNG well water can substantially increase the amount of water available for livestock if the water quality is good enough to meet the health requirements. However, the quality of the well water and its effects must be understood because of its potential impacts on soil properties, wetland vegetation, aquatic life, livestock and wildlife.
Several studies have evaluated the quality of CBNG well water at the discharge points in the PRB, Wyoming, indicating that CBNG well water is dominated by sodium (Na+) and bicarbonate (HCO3−) ions.6,8–10 Furthermore these studies suggested that salt concentration and sodium adsorption ratio (SAR), a measure of irrigation water quality, of CBNG well water changes significantly within the PRB. SAR is a measure of sodium concentration in relation to the concentrations of calcium and magnesium. The following equation is commonly used to compute SAR, where the ion concentrations are expressed as meq. L−1.23
Additionally, a number of other studies have shown that quality of CBNG well water changes substantially from discharge points to disposal ponds due to environmental (rainfall, evaporation) and geochemical processes (mineral dissolution, ion complexation, and mineral precipitation).11–18 For example, these references have shown a significant increase in pH from discharge points to corresponding disposal ponds due to degassing of CO2 from CBNG well water.18 Thus, it is critical to assess the quality of both CBNG well water at discharge points and in corresponding disposal ponds. Such information could potentially help understand CBNG well water impacts on natural ecosystems, and help develop beneficial uses for CBNG well water.
We examined nine years' water data from CBNG well water at discharge points and in corresponding disposal ponds in the PRB. We initially established fourteen CBNG sites in three sub-basins (CHR, BFR, and LPR) of the PRB consisting of both well water discharge points and corresponding disposal ponds, and monitored sites for water quality for two years.11 Subsequently, twelve more sites from two additional sub-basins (PR, TR) were added to the study (total twenty-six), and continued with monitoring of these sites for water quality for three more years.13 We continued further monitoring of these sites for four more years.18
One of the major goals of long-term monitoring of water chemistry of CBNG well water at discharge points and in corresponding disposal ponds is to predict the changes in water quality. Such information could help regulatory agencies, landowners, and industry in developing beneficial uses for the CBNG well water. Thus, specific objectives of this research were to: (1) conduct trend analyses on the water quality of both CBNG well water at discharge points and in corresponding disposal ponds in the PRB, based on nine-years' monitoring data, (2) understand geochemical processes, (3) identify potential water quality issues, and (4) find potential beneficial uses for well water.
Materials and methods
Study area description
Study area of the PRB, sampling sites, and sampling site numbers are shown in Fig. 1. The PRB extends across southeast Montana and northeastern Wyoming, with about two-thirds of the basin in Wyoming. The PRB is bordered by the Yellowstone River to the north, Black Hills to the east, Hartville Uplift to the south, and Big Horn Mountains to the west. The PRB consists of five sub-basins: Cheyenne River (CHR), Belle Fourche River (BFR), Little Powder River (LPR), Powder River (PR), and Tongue River (TR), all of which are tributaries of the Missouri River. Powder River Basin is a semi-arid basin with an annual precipitation that ranges from 300 to 600 mm, mostly in the form of snow with occasional summer thunderstorms. The basin is dominated by a variety of loamy soils ranging from sandy clay loams to clay loams. Clay minerals in the loamy soils are dominantly smectite type clays, which are very reactive with sodium. The substratum is typically gravel and sand. The soils in this area usually have a pH of about 7.2 and are classified as moderate to well draining soils.5 | ||
| Fig. 1 Map of CBNG well water discharge and disposal pond study sites. Tongue River (TR) is to the northwest and Cheyenne River (CHR) is to the south of the PRB, WY (not to scale). | ||
The landscape is broken up by hills and deep gullies. Vegetation varies with the topography but consists mainly of northern mixed grass prairie. Associated vegetation is a mixture of cool and warm season grasses and shrubs. Cool season grasses thrive in early spring with the melting of the winter snowdrifts and warm season grasses respond to the mid-summer thunderstorms. Dominant grasses include but are not limited to needle and thread (Hesperostipa comata (Trin. & Rupr.) Barkw.), intermediate wheatgrass (Elymus hispidus (P. Opiz) Melderis), and western wheatgrass (Elymus smithii (Rydb.) Gould).19 The dominant shrub is big sagebrush (Artemisia tridentata Nutt.), and the more elevated topography is dominated by ponderosa pine (Pinus ponderosa P. & C. Lawson) and Rocky Mountain juniper (Juniperus scopulorum Sarg.).
Sample collection and analysis
The CBNG well water from discharge points and disposal ponds was sampled in July and August of 2008 and July of 2009. In 2008, ten discharge points and seventeen disposal ponds were sampled; in 2009, six discharge points and sixteen disposal ponds were sampled. The discharge points and disposal ponds not sampled in 2009 were either not flowing or dry, respectively. All sample bottles were either acid washed or new and washed with de-ionized (DI) water before use. A Thermo Five-Star Field Probe, which was calibrated on days of use, was used to measure on-site pH and EC before the water samples were collected. From EC measurements, the TDS was calculated.23 The disposal pond samples were collected at a sufficient distance from the running discharge points.13 A trip blank of DI (distilled de-ionized) water from the lab was taken to the field and was included in each cooler along with samples.Water sampling procedures were in accordance with the WYDEQ Standard Operating Procedures for Sample Collection and Analysis;20 the sample bottles were immersed in the disposal pond or discharge point water three times before collecting the sample without any headspace in the bottle. From each discharge point and disposal pond site, two duplicate water samples were taken.
The procedures for laboratory preparation and analysis followed standard laboratory procedures outlined in 40 Code of Federal Regulations, Part I, Chapter 36 procedures.20 Water samples were transferred into a cooler (2 °C), protected from light and transported to the University of Wyoming Water Quality Lab, where samples were kept refrigerated before analyses. The samples were filtered through a 0.45 μm Fisher Millipore filter and divided into two parts for analysis of cations and anions. The sample analyzed for cations was acidified with trace metal grade nitric acid to a concentration of 1%, which ensured a pH of less than 2. The calcium, sodium, magnesium, potassium, iron, aluminum, chromium, manganese, lead, copper, zinc, arsenic, selenium, molybdenum, cadmium, barium, and boron were measured with inductively coupled plasma-mass spectrometry (Agilent ICP-MS-7500c).20 Ion chromatography (IC, Dionex DX-500) was used to analyze sulfate, chloride, nitrate, and phosphate in un-acidified samples.20 Alkalinity was determined by an automatic titrator (702 SM Titrino), which acidified the sample to a pH of 4.5 using 1 N hydrochloric acid. Two years' water quality-monitoring data from this study along with seven-year water quality monitoring data from other studies were used in trend analysis.11,13,18
Statistical analyses
Trend analyses of discharge points and disposal ponds were conducted separately so that the differences in trends could be compared. Any discharge point/disposal pond was removed from the trend analysis if less than three observations were available. The variable “Year” was subtracted by 1999 to set the first observation year to zero so that the intercept of the trend line would represent initial levels in 1999. Each discharge point and disposal pond was plotted to identify outliers. Outliers in regional geochemical datasets are common due to the variability of environmental factors.21 Data removed prior to analysis were as follows: site 166 in the BFR had a spike in arsenic concentration of 146.3 μg L−1 in 2004, and therefore the pond was shut down until the cause of the spike could be identified.17 This spike was not mirrored in the broader trend in arsenic concentrations; so this site was removed prior to trend analysis. Aluminum concentrations from 2003 were two orders of magnitude greater than any other observation in the dataset. All other data were included in the analysis.Trend analysis was conducted for each basin using a linear mixed-effects model.22 Where a basin was considered a fixed effect, year as a linear covariate and site within the basin were considered a random effect to account for the similarity of repeated observations from each site. A separate linear mixed-effects model was fit to each measured or calculated variable (pH, Na, As, TDS, etc.). Significant interactions between year and basin, or significant main effects of either variable were interpreted where appropriate (alpha = 0.05). If the year and basin interaction was not statistically significant (p > 0.05), the model was systematically simplified by first removing the interaction term and then either the year or basin term in an effort to get the most parsimonious linear mixed-effects model. The intercepts of the trend lines represent the initial levels of the variable in 1999, and the slopes quantify the amount of increase/decrease of that particular element per year. In addition to trends in CBNG well water quality, nine-year water quality data for discharge points and disposal ponds were averaged and compared to beneficial use criteria.
Results and discussion
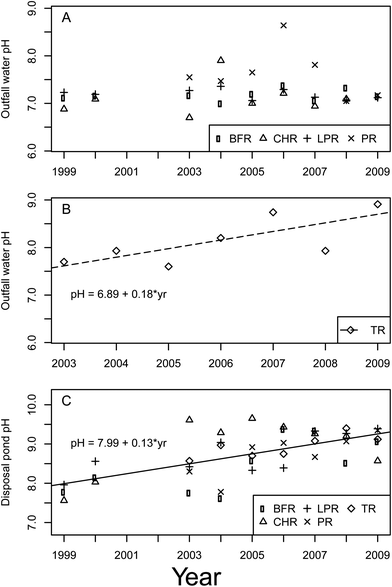
The CBNG well water pH, TDS, SAR, and some trace elements have shown significant trends as a function of time due to soil chemical and physical properties.pH
A significant year and basin interaction was observed for pH in CBNG well water at discharge points (p = 0.0004, df = 4.89, F = 5.65) (Fig. 2A). This was similar to the findings of other studies.11,13 These studies reported that pH was statistically different between all basins, and increased from the CHR basin in the south, to the LPR basin in the north. The BFR, CHR, LPR, and PR basins had discharge points with no trend in pH over time (p > 0.60). Discharge points in the TR basin showed an increasing pH trend of 0.18 per year (Fig. 2B). The well water coming from the discharge point increased in pH when it interacted with the disposal ponds.18 An overall increasing trend in pH of disposal ponds was observed across all basins (p < 0.0001, df = 1.126, F = 60.51) (Fig. 2C). The pH in disposal ponds was increasing at a rate of 0.13 per year.Our results somewhat differed from those found by others, since these reported a significant difference in the pH of disposal ponds among different basins, whereas disposal pond pH was similar among basins in this analysis.13 The increase in pH over time was due to the degassing of CO2(g) from disposal ponds because the discharge point water consists of high dissolved CO2, which diffuses to the atmosphere. The pH increase could have also been influenced by aquatic vegetation and evaporation. The pH of these ponds was expected to eventually plateau since it was high, which would draw CO2 from the atmosphere back into the disposal ponds and thus buffer the pH. It is possible that this plateau has already occurred, explaining the lack of differences between basins.15,18
TDS
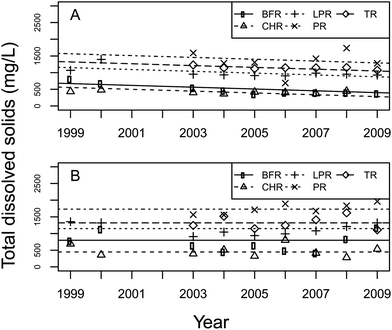
The total dissolved solids (TDS) in the discharge points were statistically different among basins (p = 0.0026, df = 4.15, F = 6.74) and an overall decreasing trend was observed over time (p = 0.0040, df = 1.89, F = 8.74) (Fig. 3A). TDS in discharge points decreased at a constant rate across all basins by 27.2 mg per L per year. Discharge point TDS were generally greater in the LPR, PR, and TR basins compared with those in BFR and CHR. In the disposal ponds, TDS were different among basins (p = <0.0001, df = 4.18, F = 13.03), but no trend was observed over time (p > 0.70) (Fig. 3B). These differences are supported by the findings of other studies, which reported that TDS only increased from CHR to the LPR basin. Our data followed a similar pattern.11–13Sodium, calcium, and magnesium
The trend analyses for sodium showed a significant year and basin interaction (p < 0.01, df = 4.89, F = 13.21). The BFR and LPR basins showed decreasing trends of sodium concentrations in the discharge points at rates of 4.4 mg per L per year and 9.6 mg L−1 (Fig. 4A). The CHR, PR, and TR all showed an increasing trend for sodium concentrations. The PR basin in particular was increasing by 61.2 mg per L per year, and the TR basin was increasing by 14.9 mg per L per year. The CHR basin was not increasing by a significant amount. These results were similar to the findings of other studies as the sodium concentrations increase from CHR to TR (Fig. 1).11–13 The sodium concentrations in the disposal ponds also showed no increasing/decreasing trend, which again could have been due to the variability in sodium concentrations within the disposal ponds (Fig. 4B). Sodium levels were significant by the basin effect (p < 0.01, df = 4.18, F = 15.38).The trend results for calcium concentrations in the discharge points had a significant effect of the basin only (p < 0.01, df = 4.15, F = 19.48), which meant that the calcium concentrations of the discharge points varied by basin but that no trend was found over time (Table 1). These findings were consistent with one study in this basin11 and differed with another study,13 which reported significant increases of calcium in the LPR. Our results indicated that over time the concentrations of calcium fluctuated greatly that it resulted in no net increase or decrease. The calcium concentrations in the pond once again showed no increasing/decreasing trend over time, which was due to the fluctuations in the calcium concentrations. The linear mixed-effects model showed a significant basin effect (p = 0.01, df = 4.18, F = 4.47).
| Basin | Discharge point Ca (mg L−1) | Disposal pond Ca (mg L−1) |
|---|---|---|
| Belle Fourche River (BFR) | 20.1 (3.0) | 31.3 (5.9) |
| Cheyenne River (CHR) | 15.3 (3.7) | 12.8 (6.4) |
| Little Powder River (LPR) | 34.1 (1.9) | 27.8 (4.1) |
| Powder River (PR) | 16.1 (2.8) | 9.3 (5.3) |
| Tongue River (TR) | 4.8 (3.2) | 6.9 (5.6) |
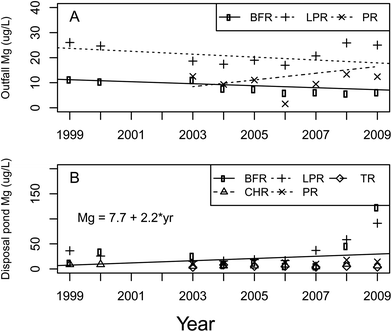
The trend analyses for magnesium showed a significant year and basin interaction (p = 0.04, df = 4.89, F = 2.69) (Fig. 5). The BFR, CHR, and LPR basins all showed decreasing magnesium concentrations whereas the PR and TR basins showed increasing magnesium concentrations. These results agree with other studies, which reported similar results.11,13 The rates at which the BFR, CHR and LPR were decreasing varied but all were quite small. The TR basin was found to be increasing but not meaningfully. The PR basin, on the other hand, was increasing at a rate of 1.1 mg per L per year. The trends for magnesium in the disposal ponds were statistically significant by basin (p = 0.04, df = 4.18, F = 3.15) and year effects (p = 0.03, df = 1,132, F = 4.62) (Fig. 5), but the interaction term was not significant. The increase in magnesium concentrations in all basins was not meaningful.
SAR
Irrigation water quality is often quantified by the SAR, because it will indicate how water will react with the surrounding soils. SAR in the discharge points was affected by basin (p < 0.0001, df = 4.15, F = 86.48) and year effects (p = 0.0335, df = 1.89, F = 4.67) (Fig. 6A), but the interaction between basin and year was not significant (p = 0.1557). The TR and PR basins had greater SAR in discharge points compared with the BFR, CHR, and LPR basins. These results agree with the findings of other studies in the PRB,11,13,18 which suggested that SAR increases from CHR to TR. As an example, the BFR basin had an average SAR value in 1999 of 7.32, and for the TR basin it was 43.9. Although the trend analysis indicated that the SAR of the discharge points was decreasing by 0.086 per year across all basins, the decrease was too small to be of much practical significance.The trend results for SAR in the disposal ponds showed significant effects of basin (p < 0.01, df = 4.18, F = 52.04) and year (p = 0.0001, df = 1,126, F = 17.63) (Fig. 6B). Similar to the discharge point results, the statistically significant trend of 0.23 per year for SAR is not practically meaningful at this time. Trends of increasing SAR in the disposal ponds were similar to the other study in the PRB.13
Trace metals
Trace elements such as chromium, cadmium, lead, selenium, and zinc did not exhibit significant trends in the discharge points or disposal ponds, and concentrations of some of these elements remained very low in all CBNG discharge points or disposal ponds. For example, selenium and lead concentrations in CBNG discharge points or disposal ponds were <1.5 and <2 μg L−1, respectively. The arsenic, copper, and barium results are discussed here. The aluminum and iron results are discussed in the ESI.†The discharge point concentrations of arsenic were different among basins (p < 0.0001, df = 4.15, F = 12.46) (Fig. 7A). Disposal pond arsenic concentrations were marginally affected by basin (p = 0.0575, df = 4.17, F = 2.83) and a strong year effect (p < 0.0001, df = 1.121, F = 19.28) (Fig. 7B). All discharge points were producing ≤2.53 μg L−1 arsenic over time with no observable trend. Several studies found that concentrations of arsenic in the discharge points remained about the same but arsenic had higher concentration in the disposal ponds.12,14 Our results are similar, indicating an increase of 0.4 μg per L per year averaged across basins in disposal ponds (Fig. 7B). Disposal pond 166 (LPR basin) was removed from the trend analysis as an outlier since in 2004 the disposal pond had an arsenic concentration of 146.3 μg L−1, and the pond was subsequently shut down until the cause of the spike in arsenic could be determined.17 The increase in dissolved arsenic concentrations in disposal ponds is attributed to the high pH, which is caused by the degassing of CO2 from the ponds. Under high pH conditions, the predominant dissolved arsenic species will be HAsO42−. Since this species is an anion it could desorb from mineral surfaces (e.g., iron oxides or hydroxides), due to high pH, and become more soluble and mobile in alkaline environments such as the PRB.24
Copper is another trace metal of environmental concern, although the trend analyses showed that copper concentrations were decreasing in the CBNG discharge points and disposal ponds across all basins. The trends for the discharge points showed a significant year and basin interaction (p = 0.02, df = 4.89, F = 3.09) (Fig. S1A†). All basins were decreasing but the rates varied from 0.8 μg per L per year to 2.5 μg per L per year. The copper concentrations in the disposal ponds were also decreasing over time, by an average of 1.2 μg per L per year (p < 0.0001, df = 1.126, F = 21.6) (Fig. S1B†). These trends agree with other studies,14 which found that copper concentration significantly increased from the discharge point to the disposal pond but decreased over time in the disposal ponds.
Barium concentrations in the discharge points showed a significant year and basin interaction (p = 0.03, df = 4.89, F = 2.91). Only the PR basin showed an increasing trend for barium concentrations in the discharge points and had a significant rate of increase of 104.3 μg per L per year (Fig. S2A†). Barium concentration in discharge points was decreasing in the BFR basin at a rate of 12.4 μg per L per year. Barium tends to accumulate in CBNG well water in the absence of sulfate in solution.16 Once the well water entered the oxidizing environment of the disposal ponds, the barium precipitated out of the solution as barite (BaSO4).14,18 Barium concentrations in the disposal ponds also showed a significant year and basin interaction (p = 0.0124, df = 4.126, F = 3.34). Although barium was increasing in the discharge point of the PR basin over time, no trend for barium concentration was observed in the disposal pond in the PR basin (p = 0.48) (Fig. S2B†). The BFR, CHR, and LPR basins had decreasing barium in disposal ponds at rates of 29.2 μg L−1, 24.4 μg L−1, and 29.3 μg per L per year, respectively (Fig. S2B†). Recent studies have shown that the combination of high pH, due to degassing of CO2, and calcareous nature of CBNG disposal ponds will enhance precipitation of sparingly soluble minerals. These studies attributed decrease in barium concentrations in the disposal ponds to the precipitation of BaCO3 (witherite).24 However, disposal pond barium concentration increased in the TR basin at a rate of 14.6 μg per L per year. These trends differed with findings of another study in the PRB.14 This study reported that disposal pond concentrations of barium increased between years in the BFR, LPR, PR, and TR basins, while the CHR basins decreased in barium concentrations indicating that barium concentrations fluctuated from year to year.
Beneficial uses for CBNG well water
It is important to determine the suitability of using CBNG well water for beneficial uses such as livestock and wildlife watering, aquatic life, and irrigation. We determined whether the CBNG well water was of “good” enough quality as livestock and wildlife drinking water following the local Wyoming standards. These local Wyoming standards were developed based on an extensive literature review.25The CBNG well water from the discharge points in all basins (Table 2) met all criteria and therefore could be used. The CBNG well water in the disposal ponds in all five basins (Table 3) meet the criteria for arsenic, barium, molybdenum, nitrate, selenium, sodium, and sulfate for the drinking water standards for livestock and wildlife, except for pH in CHR and TR, which is approaching the upper limit of 9.0. The CBNG well water in disposal ponds of CHR and TR may or may not be suitable for livestock and wildlife since the effects of high pH water are relatively unknown. However with time, since the pH is approaching 9.0, the atmospheric CO2 is expected to enter the disposal ponds and buffer the pH to between 8.0 and 8.5. This pH stabilization can be explained by the following reaction:18
| (CBNG discharge ponds)Ca2+ + CO2(g)↓ + H2O → CaCO3(calcite)↓ + 2H+ |
| Basin | Component | Discharge points | Limit | Yes/no |
|---|---|---|---|---|
| CHR | pH | 7.1 ± 0.26 | 5.5–9.0 | Yes |
| Arsenic | 0.0016 ± 0.00063 | 1 | Yes | |
| Barium | 0.3063 ± 0.07978 | 10 | Yes | |
| Molybdenum | 0.0002 ± 0.00028 | 0.3 | Yes | |
| Nitrate | 0.1 ± 0.15 | 500 | Yes | |
| Selenium | 0.0006 ± 0.00029 | 0.1 | Yes | |
| Sodium | 131.6 ± 8.2 | 1000 | Yes | |
| Sulfate | 0.2 ± 0.22 | 1800 | Yes | |
| BFR | pH | 7.2 ± 0.11 | 5.5–9.0 | Yes |
| Arsenic | 0.0008 ± 0.00047 | 1 | Yes | |
| Barium | 0.3629 ± 0.0558 | 10 | Yes | |
| Molybdenum | 0.0003 ± 0.00061 | 0.3 | Yes | |
| Nitrate | 0.2 ± 0.29 | 500 | Yes | |
| Selenium | 0.0007 ± 0.0005 | 0.1 | Yes | |
| Sodium | 143.5 ± 24.63 | 1000 | Yes | |
| Sulfate | 6.2 ± 11.37 | 1800 | Yes | |
| PR | pH | 7.7 ± 0.50 | 5.5–9.0 | Yes |
| Arsenic | 0.0008 ± 0.00056 | 1 | Yes | |
| Barium | 0.5282 ± 0.2217 | 10 | Yes | |
| Molybdenum | 0.0006 ± 0.00035 | 0.3 | Yes | |
| Nitrate | 0.3 ± 0.22 | 500 | Yes | |
| Selenium | 0.001 ± 0.00097 | 0.1 | Yes | |
| Sodium | 476.9 ± 109.85 | 1000 | Yes | |
| Sulfate | 6.3 ± 5.89 | 1800 | Yes | |
| LPR | pH | 7.2 ± 0.11 | 5.5–9.0 | Yes |
| Arsenic | 0.0004 ± 0.00029 | 1 | Yes | |
| Barium | 0.7123 ± 0.1857 | 10 | Yes | |
| Molybdenum | 0.0007 ± 0.00158 | 0.3 | Yes | |
| Nitrate | 1.2 ± 2.92 | 500 | Yes | |
| Selenium | 0.0008 ± 0.00056 | 0.1 | Yes | |
| Sodium | 319.4 ± 40.38 | 1000 | Yes | |
| Sulfate | 2.3 ± 2.10 | 1800 | Yes | |
| TR | pH | 8.1 ± 0.44 | 5.5–9.0 | Yes |
| Arsenic | 0.0005 ± 0.0003 | 1 | Yes | |
| Barium | 0.2466 ± 0.055 | 10 | Yes | |
| Molybdenum | 0.0002 ± 0.00015 | 0.3 | Yes | |
| Nitrate | 0.2 ± 0.21 | 500 | Yes | |
| Selenium | 0.0006 ± 0.00031 | 0.1 | Yes | |
| Sodium | 434.9 ± 58.66 | 1000 | Yes | |
| Sulfate | 67.1 ± 110.3 | 1800 | Yes |
| Basin | Component | Disposal ponds | Limit | Yes/no |
|---|---|---|---|---|
| a Approaching the limit. | ||||
| CHR | pH | 8.9 ± 0.73 | 5.5–9.0 | |
| Arsenic | 0.0055 ± 0.00323 | 1 | Yes | |
| Barium | 0.2279 ± 0.10576 | 10 | Yes | |
| Molybdenum | 0.0033 ± 0.00297 | 0.3 | Yes | |
| Nitrate | 0.6 ± 0.77 | 500 | Yes | |
| Selenium | 0.0009 ± 0.00033 | 0.1 | Yes | |
| Sodium | 156.7 ± 69.66 | 1000 | Yes | |
| Sulfate | 7.7 ± 11.32 | 1800 | Yes | |
| BFR | pH | 8.5 ± 0.67 | 5.5–9.0 | Yes |
| Arsenic | 0.0031 ± 0.00179 | 1 | Yes | |
| Barium | 0.236 ± 0.11799 | 10 | Yes | |
| Molybdenum | 0.0016 ± 0.00125 | 0.3 | Yes | |
| Nitrate | 0.9 ± 0.94 | 500 | Yes | |
| Selenium | 0.0008 ± 0.00059 | 0.1 | Yes | |
| Sodium | 193.0 ± 53.24 | 1000 | Yes | |
| Sulfate | 155.9 ± 224.29 | 1800 | Yes | |
| PR | pH | 8.6 ± 0.49 | 5.5–9.0 | Yes |
| Arsenic | 0.0051 ± 0.00169 | 1 | Yes | |
| Barium | 0.2646 ± 0.08812 | 10 | Yes | |
| Molybdenum | 0.0028 ± 0.00089 | 0.3 | Yes | |
| Nitrate | 0.5 ± 0.26 | 500 | Yes | |
| Selenium | 0.0017 ± 0.00026 | 0.1 | Yes | |
| Sodium | 684.5 ± 125.27 | 1000 | Yes | |
| Sulfate | 13.4 ± 10.28 | 1800 | Yes | |
| LPR | pH | 8.5 ± 0.74 | 5.5–9.0 | Yes |
| Arsenic | 0.0065 ± 0.00718 | 1 | Yes | |
| Barium | 0.3338 ± 0.14225 | 10 | Yes | |
| Molybdenum | 0.0023 ± 0.00219 | 0.3 | Yes | |
| Nitrate | 1.4 ± 2.37 | 500 | Yes | |
| Selenium | 0.0011 ± 0.00054 | 0.1 | Yes | |
| Sodium | 353.7 ± 34.97 | 1000 | Yes | |
| Sulfate | 123.2 ± 166.52 | 1800 | Yes | |
| TR | pH | 8.9 ± 0.26 | 5.5–9.0 | |
| Arsenic | 0.0024 ± 0.00157 | 1 | Yes | |
| Barium | 0.1359 ± 0.03764 | 10 | Yes | |
| Molybdenum | 0.0028 ± 0.00182 | 0.3 | Yes | |
| Nitrate | 1.0 ± 0.89 | 500 | Yes | |
| Selenium | 0.0012 ± 0.00041 | 0.1 | Yes | |
| Sodium | 503.3 ± 93.50 | 1000 | Yes | |
| Sulfate | 92.0 ± 102.0 | 1800 | Yes | |
However, the above natural process could take longer time to stabilize the pH of the CBNG well water in the disposal ponds. Another option would be to speed up this process by aerating these ponds, which would increase CO2 interaction with water and lower pH.
The CBNG well water at a discharge point met the criteria for aquatic life (Table 4). The CBNG well water in disposal ponds in CHR and TR basins may not be suitable for aquatic life (Table S1†). In these basins, the pH of the disposal pond water was approaching the pH limit of 9.0. In addition, in the LPR basin the arsenic concentrations were approaching the upper limit of 7.0 μg L−1 for aquatic life. We also observed that the CHR, BFR, LPR, and TR basins contained individual disposal ponds with arsenic concentrations approaching 15.0 μg L−1 and therefore individual disposal ponds should be evaluated before using for aquatic life. Arsenic concentrations may be of concern since several species of birds were seen feeding in these ponds during our water sampling. The CBNG well water in disposal ponds in all basins were well below the limits for cadmium, chromium, copper, selenium and zinc; therefore they are not likely to become a problem within the life of these ponds.
| Basin | Component | Discharge points | Limit | Yes/no |
|---|---|---|---|---|
| CHR | pH | 7.1 ± 0.26 | 6.5–9.0 | Yes |
| Arsenic | 1.6 ± 0.63 | 7 | Yes | |
| Cadmium | 0.1 ± 008 | 4 | Yes | |
| Chromium | 10.3 ± 14.2 | 100 | Yes | |
| Copper | 6.2 ± 4.04 | 1000 | Yes | |
| Selenium | 0.6 ± 0.29 | 50 | Yes | |
| Zinc | 7.0 ± 6.52 | 5000 | Yes | |
| BFR | pH | 7.2 ± 0.11 | 6.5–9.0 | Yes |
| Arsenic | 0.8 ± 0.47 | 7 | Yes | |
| Cadmium | 0.1 ± 0.07 | 4 | Yes | |
| Chromium | 12.9 ± 19.49 | 100 | Yes | |
| Copper | 7.4 ± 4.46 | 1000 | Yes | |
| Selenium | 0.1 ± 0.5 | 50 | Yes | |
| Zinc | 6.9 ± 5.42 | 5000 | Yes | |
| PR | pH | 7.7 ± 0.5 | 6.5–9.0 | Yes |
| Arsenic | 0.8 ± 0.56 | 7 | Yes | |
| Cadmium | 0.0 ± 0.03 | 4 | Yes | |
| Chromium | 8.8 ± 5.67 | 100 | Yes | |
| Copper | 12.5 ± 7.16 | 1000 | Yes | |
| Selenium | 1.0 ± 0.97 | 50 | Yes | |
| Zinc | 6.7 ± 4.20 | 5000 | Yes | |
| LPR | pH | 7.2 ± 0.11 | 6.5–9.0 | Yes |
| Arsenic | 0.4 ± 0.29 | 7 | Yes | |
| Cadmium | 0.2 ± 0.28 | 4 | Yes | |
| Chromium | 26.3 ± 35.81 | 100 | Yes | |
| Copper | 11.4 ± 8.86 | 1000 | Yes | |
| Selenium | 0.8 ± 0.56 | 50 | Yes | |
| Zinc | 11.3 ± 12.16 | 5000 | Yes | |
| TR | pH | 8.1 ± 0.44 | 6.5–9.0 | Yes |
| Arsenic | 0.5 ± 0.3 | 7 | Yes | |
| Cadmium | 0.3 ± 0.52 | 4 | Yes | |
| Chromium | 6.4 ± 4.18 | 100 | Yes | |
| Copper | 9.6 ± 4.15 | 1000 | Yes | |
| Selenium | 0.6 ± 0.31 | 50 | Yes | |
| Zinc | 7.4 ± 7.84 | 5000 | Yes |
To determine whether the CBNG well water at the discharge points and in disposal ponds has the potential to cause soil degradation, results of nine-year water quality data are compared with the irrigation water quality guidelines27 (Table S2†). Typically, the water quality standards for irrigation are based on EC and SAR, but specific ions can be included as well such as chloride, boron, and nitrate. We did not include chloride, boron, and nitrate in the table since the standards are more directed to the plant interactions rather than the water effects on the soil.27
The EC is a measure of the water salinity hazard, which directly relates to crop productivity. Values for EC are broken down into classes of suitability for irrigation: class 1 – excellent (≤0.25 dS m−1), class 2 – good (0.25–0.75 dS m−1), class 3 – permissible (0.76–2.00 dS m−1), class 4 – doubtful (2.01–3.00 dS m−1), and class 5 – unsuitable (≥3.00 dS m−1) (Table S2†). The CBNG well water from the discharge points ranged from class 2 to class 4 depending upon the basin (Table S3†). Based on the EC classification, the CHR basin discharge points were class 2 waters. The BFR, LPR, and TR discharge points were class 3 waters. The PR basin discharge points were class 4 with a high EC of 2.0 ± 0.48 (dS m−1). According to these classifications, the CHR basin can use the discharge point well water for irrigation without a problem; whereas, the LPR, BFR, and TR basins may need treatment before use. The PR basin discharge point water requires a careful management, if this water is used for irrigation. The CBNG well waters from the discharge points had lower EC when compared to the corresponding disposal ponds. The CBNG well water in the disposal ponds in all basins were between 3 and 4 classifications; thus use of this water for irrigation will require careful management.
The SAR is a measure of the sodium concentration in relation to the concentrations of calcium and magnesium in the water. Irrigation water with a high SAR will tend to replace calcium on soil exchange sites with sodium, thus reducing the fertility of the soil. Values for SAR are rated as low hazard (1–9), medium hazard (10–17), high hazard (18–25), and very high hazard (≥26) (Table S2†). The SAR values of the CBNG well water from the discharge points varied considerably (Table S3†). The CHR and BFR basins are rated as low hazard with SAR values of 6.90 ± 0.96 and 6.8 ± 0.65, respectively. The LPR basin was rated as medium hazard with a SAR value of 10.6 ± 1.09; and the PR and TR were rated as high and very high hazard with a SAR value of 23.6 ± 1.96, respectively. The CBNG well waters from the disposal ponds have generally higher SAR values when compared to the discharge point waters, except for the TR basin (Table S3†). This is due to evapotranspiration. Treatment of CBNG well water in these basins with clinoptilolite, a locally available zeolite or electrodialysis reversal (EDR) could help lower SAR and increase potential beneficial use of CBNG well water for irrigation.29,30
Conclusions
This study monitored water quality of the same CBNG discharge points and disposal ponds in 2008 and 2009 as in previous studies.11,13,18 Our trend analyses were the summation of 9 years' water quality data. Some of the most significant conclusions of this study include the following:• Trends in CBNG well water at discharge points were different when compared with the CBNG water in disposal ponds. Geochemical processes played an important role in the water quality of well waters in the disposal ponds.
• pH was not significantly changing in discharge points of the BFR, CHR, LPR, or PR basins, but the TR basin discharge points were increasing by 0.18 per year. pH in all disposal ponds was increasing by 0.13 per year.
• Trends in the discharge points varied with the basin. For instance, barium concentrations were increasing in the PR basin but decreasing in all others. Iron, copper, and chromium concentrations were decreasing in all basins. Arsenic and selenium concentrations were not increasing or decreasing in CBNG well water discharge points.
• Trends in the disposal ponds also varied by the basin. Barium concentrations were increasing in the PR and TR basins but decreasing in the BFR, CHR, and LPR basins. Iron concentrations were increasing in the BFR, CHR and TR basins and decreasing in the LPR and PR basins. Copper and chromium were decreasing in all basins. Arsenic concentrations were increasing in all basins at a slow rate.
• CBNG well water at discharge points was suitable for livestock and wildlife drinking and aquatic life in all basins. CBNG well water at discharge points was suitable for irrigation in the BFR, CHR, and LPR basins. The CBNG well water from the disposal ponds was only suitable for irrigation in the BFR and CHR basins.
• In general, CBNG well water's suitability for beneficial uses depended upon the pH, SAR, EC, and arsenic. All other water quality components (e.g. barium, selenium) were typically at low concentrations and well below recommended standards for common beneficial uses (livestock watering, irrigation).
Our long-term water quality monitoring studies suggest that the CBNG well water at discharge points and disposal ponds has independent water chemistries that fluctuate over time. This information is essential in determining the longevity of the beneficial uses of well water. For instance, arsenic concentrations in all disposal ponds are increasing and if this trend continues the arsenic concentrations could surpass the limit for aquatic life in the next few years. Overall, results of water quality trend analysis of CBNG well water at discharge points and corresponding disposal ponds will help landowners, land managers, state (DEQ) and federal agencies (BLM, EPA) in developing better management options for the CBNG well water in the PRB.
Acknowledgements
We would like to thank School of Energy Resources, University of Wyoming, Laramie, WY for providing funds for this research. We also thank anonymous reviewers for their valuable suggestions, which improved the manuscript.References
- Coalbed Natural Gas: Energy and Environment. Energy Science, Engineering, and Technology Series, ed. K. J. Reddy, Nova Science Publishers, Inc., Hauppauge, NY 11788, 2010, p. 511 Search PubMed.
- U. S. Geological Survey, Coalbed Methane and Coal Stratigraphy Research, http://energy.cr.usgs.gov/oilgas/cbmethane/, accessed 28 February 2012 Search PubMed.
- U. S. Geological Survey, Coal-Bed Gas Resources of the Rocky Mountain Region, USGA Fact sheet 0110-01, 2005.
- R. H. De Bruin, R. M. Lyman, R. W. Jones and L. W. Cook, Information Pamphlet 7 (second revision), Wyoming State Geological Survey, Laramie, WY, 2004, pp. 1–23 Search PubMed.
- K. J. Reddy, Q. D. Skinner and B. H. Hulin, Geochemical processes governing trace elements in CBNG-produced water, in Trace Elements in the Environment Biogeochemistry, Biotechnology, and Bioremediation, ed. M. Prasad, K. S. Sajwan and R. Naidu, CRC Press, Boca Raton, FL, 2006, pp. 125–144 Search PubMed.
- J. Wheaton and T. Donato, Coalbed-Methane Basics: Powder River Basin, Montana, Montana Bureau of Mines and Geology Information Pamphlet 5, 2004 Search PubMed.
- C. E. Clark and J. A. Veil, Produced water volumes and management practices in the United States, U. S. Department of Energy, Office of Scientific and Technical Information, Oak Ridge, TN, 2009 Search PubMed.
- C. A. Rice, M. S. Ellis and J. H. Bullock Jr, Water co-produced with coalbed methane in the Powder River Basin, Wyoming: Preliminary compositional data, Open-File Report 00-372, US Geological Survey, Denver, CO, 2000 Search PubMed.
- W. A. Van Voast, Geochemical nature of formation water associated with coalbed methane, AAPG Bull., 2003, 87, 667–676 CrossRef CAS.
- K. G. Dahm, K. L. Guerra, P. Xu and J. E. Drewes, Composite geochemical database for coalbed methane produced water quality in the Rocky Mountain Region, Environ. Sci. Technol., 2011, 45, 7655–7663 CrossRef CAS PubMed.
- I. H. McBeth, K. J. Reddy and Q. D. Skinner, Coalbed methane product water chemistry in three Wyoming watersheds, J. Am. Water Resour., 2003, 39, 575–585 CrossRef CAS.
- I. H. McBeth, K. J. Reddy and Q. D. Skinner, Chemistry of trace elements in coalbed methane product water, Water Res., 2003, 37, 884–890 CrossRef CAS PubMed.
- R. E. Jackson and K. J. Reddy, Geochemistry of coalbed natural Gas (CBNG) produced water in the Powder River Basin, Wyoming: Salinity and sodicity, Water, Air, Soil Pollut., 2007, 184, 49–61 CrossRef CAS.
- R. E. Jackson and K. J. Reddy, Trace element chemistry of coal bed natural gas produced water in the Powder River Basin, Wyoming, Environ. Sci. Technol., 2007, 41, 5953–5959 CrossRef CAS PubMed.
- M. J. Patz, K. J. Reddy and Q. D. Skinner, Chemistry of coalbed methane discharge water interacting with semi-arid ephemeral stream channels, J. Am. Water Resour. Assoc., 2004, 40, 1247–1255 CrossRef CAS.
- E. L. Brink, J. I. Drever and C. D. Frost, The geochemical evolution of water coproduced with coalbed natural gas in the Powder River Basin, Wyoming, Environ. Geosci., 2008, 15, 152–171 Search PubMed.
- J. T. Sowder, T. J. Kelleners and K. J. Reddy, The origin and fate of arsenic in coalbed natural gas produced water ponds, J. Environ. Qual., 2010, 39, 1604–1615 CrossRef CAS PubMed.
- C. Milligan, K. J. Reddy and D. Legg, Monitoring geochemistry of CBNG produced water outfalls, disposal ponds, and sediments in Powder River Basin, Wyoming, in Coalbed Methane: Energy and Environment, ed. K. J. Reddy, Nova Science Publishers, NY, 2010, ch. 8 Search PubMed.
- J. Stubbendieck, S. L. Hatch and L. M. Landholt, North American wildland plants – A field guide, University of Nebraska Press, Lincoln, 2003 Search PubMed.
- Wyoming Department of Environmental Quality/Water Quality Division, Manual of standard operating procedures for sample collection and analysis, Wyoming Department of Environmental Quality and Water Quality Division, Cheyenne, WY, 2004 Search PubMed.
- C. Reimann, P. Filzmoser, R. Garrett and R. Dutter, Statistical Data Analysis Explained-Applied Environmental Statistics with R, John Wiley & Sons Ltd., West Sussex, England, 2008 Search PubMed.
- J. C. Pinheiro and D. M. Bates, Mixed-Effect Models in S and S-PLUS, Springer Verlag, NY, 2000 Search PubMed.
- B. Hanson, S. R. Grattan and A. Fulton, Agricultural Salinity and Drainage: A Handbook for Water Managers, University of California Irrigation Program, Davis, CA, 1993 Search PubMed.
- K. J. Reddy, C. Milligan, A. Whitman and D. Legg, Geochemical processes controlling trace elemental mobility in coalbed natural gas (CBNG) disposal ponds in the Powder River Basin, WY, Int. J. Coal Geol., 2013, Search PubMed in final revisions.
- M. F. Raisbeck, S. L. Riker, C. M. Tate, R. Jackson, M. A. Smith, K. J. Reddy and J. R. Zygmunt, Water Quality for Wyoming Livestock & Wildlife: A Review of the Literature Pertaining to Health Effects of Inorganic Contaminants, Wyoming Department of Environment Quality, Cheyenne, WY, 2008 Search PubMed.
- Wyoming Department of Environmental Quality, SAP: Water Quality Rules and Regulations, Department of Environmental Quality and Water Quality Division, Cheyenne, WY, 2001, ch. 1, http://deq.state.wy.us/wqd/WQDrules/Chapter_01.pdf Search PubMed.
- T. A. Bauder, R. M. Waskom and J. G. Davis, Irrigation Water Quality Criteria, Crop Series, Colorado State University Cooperative Extension, CO, 2007 Search PubMed.
- G. K. Ganjegunte, G. F. Vance and L. A. King, Soil chemical changes resulting from irrigation with water co-produced with coalbed natural gas, J. Environ. Qual., 2005, 34, 2217–2227 CrossRef CAS PubMed.
- J. A. Brant, E. Sajtar and D. M. Bagley, Ion exchange and membrane treatment processes for produced waters from oil and gas development, in Coalbed Methane: Energy and Environment, ed. K. J. Reddy, Nova Science Publishers, NY, 2010, ch. 13 Search PubMed.
- G. F. Vance and G. K. Ganjegunte, Utilization of coalbed natural gas water: Issues, implications and management, in Coalbed Methane: Energy and Environment, ed. K. J. Reddy, Nova Science Publishers, NY, 2010, ch. 14 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3em00255a |
| This journal is © The Royal Society of Chemistry 2014 |