Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantitative passive soil vapor sampling for VOCs- part 2: laboratory experiments†
Todd
McAlary
*ab,
Hester
Groenevelt
a,
Suresh
Seethapathy
b,
Paolo
Sacco
c,
Derrick
Crump
d,
Michael
Tuday
e,
Brian
Schumacher
f,
Heidi
Hayes
g,
Paul
Johnson
h and
Tadeusz
Górecki
b
aGeosyntec Consultants, Inc., 130 Research Lane, #2, Guelph, Ontario N1G 5G3, Canada. E-mail: tmcalary@geosyntec.com; Fax: +1(519) 822 3151; Tel: +1(519) 515 0861
bUniversity of Waterloo, Waterloo, Ontario, Canada
cFondazione Salvatore Maugeri, Padova, Italy
dCranfield University, Cranfield, UK
eColumbia Analytical Services, Simi Valley, CA, USA
fUnited States Environmental Protection Agency, Las Vegas, NV, USA
gEurofins/Air Toxics, Inc. (formerly Air Toxics Ltd.), Folsom, CA, USA
hArizona State University, Tempe, AZ, USA
First published on 24th January 2014
Abstract
Controlled laboratory experiments were conducted to demonstrate the use of passive samplers for soil vapor concentration monitoring. Five different passive samplers were studied (Radiello, SKC Ultra, Waterloo Membrane Sampler, ATD tubes and 3M OVM 3500). Ten different volatile organic compounds were used of varying classes (chlorinated ethanes, ethanes, and methanes, aliphatics and aromatics) and physical properties (vapor pressure, solubility and sorption). Samplers were exposed in randomized triplicates to concentrations of 1, 10 and 100 ppmv, with a relative humidity of ∼80%, a temperature of ∼24 °C, and a duration of 30 minutes in a chamber with a face velocity of about 5 cm min−1. Passive samplers are more commonly used for longer sample durations (e.g., 8 hour workday) and higher face velocities (>600 cm min−1), so testing to verify the performance for these conditions was needed. Summa canister samples were collected and analyzed by EPA Method TO-15 to establish a baseline for comparison for all the passive samplers. Low-uptake rate varieties of four of the samplers were also tested at 10 ppmv under two conditions; with 5 cm min−1 face velocity and stagnant conditions to assess whether low or near-zero face velocities would result in a low bias from the starvation effect. The results indicate that passive samplers can provide concentration measurements with accuracy (mostly within a factor of 2) and precision (RSD < 15%) comparable to conventional Summa canister samples and EPA Method TO-15 analysis. Some compounds are challenging for some passive samplers because of uncertainties in the uptake rates, or challenges with retention or recovery.
Environmental impactSoil vapor intrusion to indoor air is an important pathway of potential human exposure to volatile chemicals at contaminated sites, but assessment is challenging using conventional indoor air and soil gas sampling methods because of spatial and temporal variability. This research demonstrates the use of an alternative sampling approach (passive diffusive samplers) for soil vapor monitoring via controlled laboratory experiments including 10 compounds, five sampler types, a range of flow rates, exposure durations, and concentrations to provide a robust characterization of the capabilities and limitations of this approach. |
Introduction
Volatile organic compounds (VOCs) including chlorinated solvents and petroleum hydrocarbons are common contaminants in soil and groundwater. Subsurface vapor intrusion to indoor air is a pathway of concern in human health risk assessments, which creates a need for effective monitoring technologies for VOC soil vapor concentrations. Quantitative passive samplers have been used for four decades for measuring concentrations of volatile organic compounds (VOCs) and other chemicals in indoor air quality monitoring (ambient and personal), outdoor air quality and industrial hygiene applications;1–5 however, passive soil vapor sampling has not yet been shown to provide reliable quantification of soil vapor concentrations.6–8 As a result, many regulatory guidance documents caution that passive soil vapor sampling is not quantitative and should only be used as a screening tool.9,10 This paper describes controlled laboratory experiments designed to demonstrate the performance of passive samplers under conditions that would be typical for soil vapor monitoring. This research team has related articles on mathematical modeling11 and field experiments.12Passive samplers are defined here as devices that contain a sorbent medium and uptake VOC vapors passively by diffusion or permeation. Concentrations are calculated using eqn (1):
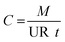 | (1) |
The mass (M) of VOC sorbed and sample duration (t) are measured typically with accuracy of 10% or better, so the key factor controlling the accuracy of passive diffusive sampler concentrations is the uptake rate (UR) of the sampler.
Uptake rates for quantitative passive samplers can be obtained in three main ways: (1) supplied by the vendor based on controlled exposure chamber tests, (2) interpolated from the uptake rates of similar compounds based on ratios of diffusion or permeation coefficients or (3) field-verified via side-by-side contemporaneous duplicate samples collected using a conventional sampling method (“field-verified uptake rates”). The passive diffusive samplers included in this study all have experimentally measured vendor-supplied uptake rates, which distinguishes these devices from qualitative or semi-quantitative passive samplers (e.g., Petrex tubes™,13,14 EMFLUX Cartridges™,7 Beacon BeSure Passive Soil Gas Technology™,15 and Gore™ Modules (formerly known as the Gore-Sorber®),8 and similar devices that are not specifically designed to control the uptake rate). Where vendor-supplied uptake rates were not available for some of the compounds in this study, they were interpolated from values for compounds of similar structure and mass (and collectively referred to hereafter simply as “uptake rates”). No adjustments were made for temperature or pressure because all tests were performed at 24 °C and atmospheric pressure.
Several of the samplers in this study are available in more than one variety with different uptake rates. High uptake rates allow lower concentrations to be measured in a shorter period of time. However, if the uptake rate is too high relative to the face velocity of air near the sampler, then the sampler may cause a localized reduction in the vapor concentrations, and an associated low bias in the measured concentration, which is referred to as the “starvation effect”. For soil gas sampling, there is a greater risk of starvation compared to indoor or outdoor air sampling because soil gas flow rates are typically very low or negligible, and replenishment of vapors to the vicinity of the passive sampler occurs primarily by diffusion.11
This testing program focused on different compounds, concentrations and samplers (uptake rates, sorbent and extraction method). Test protocols for evaluating occupational indoor air quality monitors16 were considered, but not employed because they address variables such as temperature, humidity and sampling duration, but in the subsurface, the humidity is almost always high, the temperature is insulated to some extent and long sample durations were not needed to quantify the concentration range of this study for most of the compounds tested for most of the samplers. Most of the tests were conducted using a steady gas velocity of 5 cm min−1 (flowrate of 100 mL min−1 in a 5 cm diameter cylinder) through the exposure chamber to minimize the starvation effect in order to focus on the performance of the passive samplers for different compounds and different concentrations in a high humidity environment. Water can be adsorbed by carbon-based sorbents and this can cause poor retention of weakly sorbed analytes or interference during analysis, so the high humidity typical of soil gas was considered likely to pose challenges for some samplers. The gas velocity of 5 cm min−1 was very low compared to typical indoor air velocities (600 to 3000 cm min−1 is a common range of air flow velocities for testing passive samplers designed for indoor air quality monitoring17), in keeping with the intent of assessing the performance of the passive samplers under conditions approximating soil vapor sampling. A series of samples collected under stagnant conditions was also included.
Experimental design and sampling methods
A concentration range of 1 to 100 parts per million by volume (ppmv) was tested to evaluate the performance of the samplers over a sufficiently wide range to assess whether their response is linear with concentration. Ten VOCs were included in the supply gas mixture, spanning a range of chemical families (chlorinated ethenes, ethanes and methanes, aliphatic hydrocarbon and aromatic hydrocarbon) and properties (vapor pressure, solubility and solid phase partitioning) to represent the range of VOCs typically encountered in assessing contaminated land (Table 1). Two standard J-size cylinders were custom-filled with these compounds at concentrations of 10 and 100 ppmv in N2. These were prepared by Air Liquide America Specialty Gases LLC of Santa Fe Springs, CA. Naphthalene (NAPH) and 1,2,4-trimethylbenzene (124TMB) have much lower vapor pressures than the other compounds, and to avoid potential condensation issues, NAPH was added at a concentration of about 1 ppmv in the 10 ppmv supply gas and neither compound was included in the 100 ppmv supply gas mixture. For the test at 1 ppmv concentrations, the 10 ppmv supply gas was diluted 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with ultra pure nitrogen using a mass flow controller to deliver 9 mL min−1 of the supply gas and a needle-valve to deliver about 90 mL min−1 of nitrogen (verified periodically with a soap-bubble flowmeter). For the 10 and 100 ppmv tests, the supply gasses were delivered undiluted at a flow rate of about 100 mL min−1, controlled using a mass flow controller and verified using a soap-bubble flow meter.
1 with ultra pure nitrogen using a mass flow controller to deliver 9 mL min−1 of the supply gas and a needle-valve to deliver about 90 mL min−1 of nitrogen (verified periodically with a soap-bubble flowmeter). For the 10 and 100 ppmv tests, the supply gasses were delivered undiluted at a flow rate of about 100 mL min−1, controlled using a mass flow controller and verified using a soap-bubble flow meter.
| Analyte | Organic carbon partitioning coefficient Koc (mL g−1) | Henry's law constant @ 25 °C (dim) | Vapor pressure (atm) | Pure component maximum vapor concentration (ppmv) | Water solubility (g L−1) |
|---|---|---|---|---|---|
| 1,1,1-Trichloroethane (111TCA) | 43 | 0.70 | 0.16 | 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
| 1,2,4-Trimethylbenzene (124TMB) | 614 | 0.25 | 0.0020 | 2000 | 0.057 |
| 1,2-Dichloroethane (12DCA) | 39 | 0.048 | 0.11 | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8.6 |
| 2-Butanone (MEK) | 4.5 | 0.0023 | 0.10 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
220 |
| Benzene (BENZ) | 146 | 0.23 | 0.13 | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.8 |
| Carbon tetrachloride (CTET) | 43.9 | 1.1 | 0.15 | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.79 |
| Naphthalene (NAPH) | 1540 | 0.18 | 0.00012 | 120 | 0.031 |
| n-Hexane (NHEX) | 132 | 74 | 0.20 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0095 |
| Tetrachloroethene (PCE) | 94.9 | 0.72 | 0.024 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.21 |
| Trichloroethene (TCE) | 61 | 0.40 | 0.095 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.3 |
The following samplers were used in this study
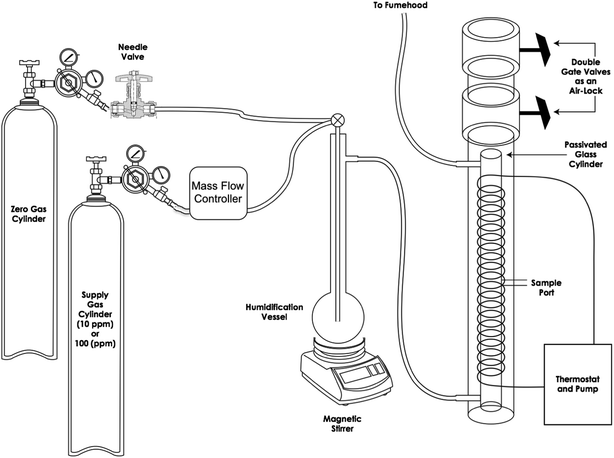
The laboratory apparatus consisted of a 1 m long × 5 cm diameter glass cylinder with three side ports (influent at the bottom, effluent at the top and a sampling port in the middle). A schematic diagram of the apparatus is shown in Fig. 1. The interior surface of the glass cylinder was passivated using a silanization process. The outer wall of the cylinder was wrapped with 1.6 cm diameter Tygon tubing, which was used to circulate water for temperature control. The cylinder and tubing were placed inside a 10 cm diameter clear acetate tube for structural support and mounted to a frame for stability. Two PVC and stainless steel gate valves were secured to the top of the acetate pipe by friction with Teflon™ tape acting as a seal. The gate valves formed an air-lock, to allow samplers to enter and exit the chamber with minimal disruption to the concentrations inside. A supply of gas containing known concentrations of selected VOCs was humidified and fed through the apparatus. When deployed in the exposure chamber, the badge samplers (3M and SKC) had their face vertical, the WMS and ATD samplers faced down and the Radiello was aligned near vertical.
Stainless steel and nylon tubing were used to deliver the supply gas to the exposure chamber, with compression fittings used at all connections. All fittings were leak-tested by connecting the apparatus to a 100 mL min−1 flow of pure helium and monitoring all the fittings with a helium meter. Adjustments were made as necessary until there were no measurable helium leaks in the regions immediately outside of the fittings.
Three identical humidification vessels were used (one for each concentration) and the water in each vessel was spiked with a mixture containing each of the 10 neat liquid VOCs mixed in proportions such that after dissolving into the water in the humidification vessel, the water would be approximately in equilibrium with the supply gas according to Henry's Law (Table 2). Each humidification vessel contained about 1 L of distilled, deionized water and a Teflon-coated magnetic stir bar. The stir bars operated continuously and the supply gas was delivered to the bottom of the humidification vessel through 1/4-inch glass tubing with a porous ceramic cup at the bottom to generate a large number of small gas bubbles. This apparatus consistently delivered steady source vapor concentrations with a relative humidity of about 80%.
| Compound | Molecular weight | Gas phase concentration corresponding to 100 ppmv in μg L−1 | Henry's constant at 22 °C | Aqueous concentration (μg L−1) | Density of pure liquid (g mL−1) | Volume (μL) to dose 1000 mL of water |
|---|---|---|---|---|---|---|
| 111TCA | 133.41 | 557 | 0.65 | 857 | 1.320 | 649 |
| 124TMB | 120.2 | 502 | 0.2 | 2508 | 0.876 | 2863 |
| 12DCA | 98.96 | 413 | 0.059 | 7001 | 1.253 | 5587 |
| MEK | 72.11 | 301 | 0.004 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 244 |
0.805 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 471 471 |
| BENZ | 78.11 | 326 | 0.2 | 1630 | 0.877 | 1860 |
| CTET | 153.8 | 642 | 0.99 | 648 | 1.587 | 409 |
| NAPH | 128.2 | 54 | 0.018 | 2973 | 1.140 | 2608 |
| NHEX | 86.18 | 360 | 50 | 7 | 0.655 | 11 |
| PCE | 165.8 | 692 | 0.65 | 1065 | 1.622 | 656 |
| TCE | 131.4 | 548 | 0.39 | 1406 | 1.460 | 963 |
All three supply-gas systems were set up simultaneously (Fig. 1 shows only one for simplicity) and allowed to run continuously for a week at about 100 mL min−1 and monitored periodically with a MiniRae 1000 photoionization detector (PID) and sampled using an active (pumped) sorbent tube filled with Anasorb 747 and analyzed by solvent extraction GC/MS to document the attainment of stable conditions prior to the experiments. The temperature and relative humidity were monitored using a Madgetech RHTemp101A datalogger.
Testing was performed starting with the concentrations at 1 ppmv, followed by 10 ppmv and 100 ppmv to reduce potential effects of carryover from one test to the next. At least 60 h were allowed for the chamber to equilibrate with each new concentration. At a flow rate of 100 mL min−1, more than 180 times the volume of the test chamber passed through the chamber prior to sampling. The sample port at the mid-point of the test chamber was periodically monitored during the stabilization period using the PID to assess the stability of total ionizable vapor concentrations inside the test chamber and verification testing using pumped sorbent tubes (50 mL min−1 for 20 min with Anasorb 747) and solvent extraction GC/MS analysis until concentrations stabilized. NAPH was slower to equilibrate than the other compounds, presumably because of its tendency to adsorb even to relatively inert surfaces.
For the 1 ppmv test, three replicates of each of the five passive samplers and the 1 L Summa canister samples were collected over 30 minutes in random order (denoted using lower case a, b and c in Table ESI 1A–C†). For the 10 ppmv and 100 ppmv tests, additional Summa canister samples were collected at the beginning and end for a total of five active samples (denoted a through e). For the 1 and 10 ppmv tests, samples were deployed with no delay between them. PID measurements made after the 10 ppmv tests indicated that some of the samplers may have sufficient uptake to influence the concentrations inside the chamber (e.g., 10% lower PID readings after the sample period compared to before for the samplers with higher uptake rates), so a 5 minute interval was allowed for re-equilibration between samples during the 100 ppmv tests. The effect of this change is discussed further in the results section.
Analyses were performed by the laboratories considered by the study team to be most familiar with the respective samplers. Fondazione Salvatore Maugeri in Padova, Italy analyzed the Radiello samplers via solvent extraction GC/MS. The University of Waterloo analyzed the WMS samplers via solvent extraction GC/MS. AirZone One Ltd of Mississauga, Ontario analyzed the OVM 3500 samplers by solvent extraction GC/MS. Columbia Analytical Services of Simi Valley, CA analyzed the SKC Ultra samplers by solvent extraction GC/MS for the Ultra sampler with charcoal and thermal desorption GC/MS for the Ultra II with Carbograph 5 and the Summa canister samples by EPA Method TO-15.24 Air Toxics Ltd. of Folsom, CA analyzed the ATD tube samplers by thermal desorption GC/MS using a modified version of U.S. EPA Method TO-17.25
Low uptake rate sampler tests
Additional tests were performed using available low uptake rate varieties of the passive samplers. Two tests were performed at the midpoint concentration (10 ppmv) with the supply gas flow velocity held at 5 cm min−1 (100 mL min−1) for the first test to maintain consistency with the rest of the experiments. The second was performed with the supply gas shut off to assess the performance of the samplers in a setting with no net gas flow (“stagnant” conditions), which is a worst-case condition for low biases attributable to the starvation effect. No attempt was made to assess whether thermal convection may have contributed to advection within the column, but the temperature was held as constant as possible, so thermal convection was likely negligible. The SKC low-uptake sampler had no detectable concentrations for either of the first two tests, so a third test was performed at 100 ppmv under stagnant conditions (only the SKC and ATD tube samplers were used in this test). The low-uptake varieties of passive samplers used for these tests were:• Radiello – yellow body with charcoal.
• SCK Ultra – 12-hole cap with charcoal.
• WMS-LU – 0.8 mL vial with Anasorb 747.
• ATD tube – low-uptake cap with Tenax TA.
No low-uptake version of the 3M OVM 3500 is available, so it was not included in this set of tests.
Inter-laboratory testing was performed to ensure each analytical laboratory could adequately analyze samplers. Each analytical laboratory adhered to its own QA/QC program (method blanks, surrogate analysis, internal standard analysis, laboratory duplicate analysis, etc.). No significant QA/QC issues were identified.
Results
The concentrations measured using each of the passive samplers and the Summa canisters are presented in Fig. 2(a–c), for the 1, 10 and 100 ppmv tests, respectively. The concentrations were calculated by dividing the mass of each compound adsorbed by each sampler (as determined by the analytical laboratory) by the product of the uptake rate and sample duration (30 min). The passive sampler concentrations were divided by the average of the concentrations measured with the Summa canister samples and EPA Method TO-15 analysis and presented as normalized (C/Co) concentrations. Tables ESI† 1A–C in the ESI present the uptake rates, individual concentrations measurements, the mean, standard deviation and the relative standard deviation (RSD, the standard deviation divided by the mean) for the three replicates for each sampler at each concentration level.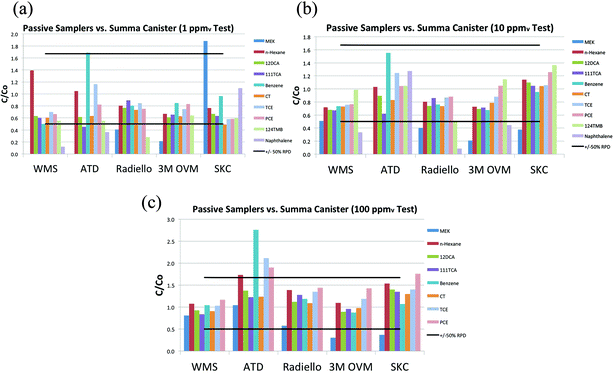 | ||
| Fig. 2 (a) Relative concentrations (C/C0) in the 1 ppmv tests. (b) Relative concentrations (C/C0) in the 10 ppmv tests. (c) Relative concentrations (C/C0) in the 100 ppmv tests. | ||
Most of the samplers provided concentrations within a relative percent difference (RPD) of ±50% of the Summa canister values, with the following exceptions:
1) Naphthalene – Radiello: not detected.
3M OVM 3500: not detected in 0.1 ppmv samples.
SKC Ultra: not detected in the 1 ppmv samples.
WMS: low bias of about 8× in the 0.1 ppmv samples and 3× in 1 ppmv samples.
2) MEK – Radiello: low bias by a factor of about 2 to 3.
ATD tube: not detected in the 1 and 10 ppmv samples.
3M OVM 3500: low bias by a factor of about 3 to 5.
SKC Ultra: high bias with thermal desorption @ 1 ppmv and low bias via solvent extraction at 10 and 100 ppmv.
WMS: not detected in the 1 ppmv samples, low bias by 2× in 10 ppmv samples.
3) 1,2,4-TMB – Radiello: low bias by about 3×.
Naphthalene and 1,2,4-trimethylbenzene were the two compounds with the highest and second highest Koc values (Table 1), and MEK was the compound with the highest solubility. Less soluble and less sorptive compounds yielded better agreement between the passive samplers and Summa canisters.
The accuracy of the passive samplers is summarized in Table 3, which shows the relative concentration (C/C0), where C is the average passive sampler concentration and C0 is the average Summa canister concentration for each compound, sampler and concentration. Overall, the C/C0 values were within the range of 0.5 to 1.67 (corresponding to an RPD of ±50% between the passive and active samplers) in 83% (110 of 133) of sampler/compound pairs with detectable results. The C/C0 values were generally higher for the 100 ppmv tests, which might be attributable to the fact that the chamber was allowed to re-equilibrate for 5 minutes between samples. The compounds that showed the poorest comparison between the passive and active samplers were MEK and naphthalene. These compounds were specifically included in this research because they were expected to be challenging compounds for passive samplers. Note that for the 1 ppmv test, the SKC Ultra sampler was used with Carbograph 5 as the sorbent for better sensitivity and the result showed a high bias for MEK, which demonstrates the importance of sorbent selection.
| MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a NA – not available for SKC because two different sorbents were used. ND – not detected. NT – not tested. | |||||||||||
| C/C 0 for 1 ppm v | |||||||||||
| WMS anasorb 747 | ND | 1.38 | 0.63 | 0.60 | 0.50 | 0.60 | 0.70 | 0.66 | 0.55 | 0.12 | 0.64 |
| ATD tenax TA | ND | 1.04 | 0.61 | 0.45 | 1.68 | 0.63 | 1.16 | 0.82 | 0.55 | 1.10 | 0.89 |
| Radiello charcoal | 0.41 | 0.80 | 0.77 | 0.89 | 0.80 | 0.73 | 0.85 | 0.75 | 0.28 | ND | 0.70 |
| 3M OVM 3500 | 0.21 | 0.65 | 0.60 | 0.64 | 0.83 | 0.62 | 0.73 | 0.82 | 0.63 | ND | 0.64 |
| SKC carbograph 5 | 1.87 | 0.76 | 0.66 | 0.63 | 0.96 | 0.49 | 0.58 | 0.58 | 0.60 | 1.11 | 0.82 |
| C/C 0 for 10 ppm v | |||||||||||
| WMS anasorb 747 | 0.54 | 0.70 | 0.68 | 0.65 | 0.75 | 0.69 | 0.74 | 0.71 | 0.83 | 0.35 | 0.66 |
| ATD tenax TA | ND | 1.00 | 0.89 | 0.60 | 1.59 | 0.79 | 1.21 | 0.96 | 0.88 | 1.33 | 1.03 |
| Radiello charcoal | 0.47 | 0.78 | 0.73 | 0.82 | 0.77 | 0.70 | 0.83 | 0.77 | 0.35 | ND | 0.69 |
| 3M OVM 3500 | 0.22 | 0.70 | 0.68 | 0.68 | 0.68 | 0.74 | 0.85 | 0.96 | 0.95 | 0.46 | 0.69 |
| SKC charcoal | 0.40 | 1.11 | 1.10 | 1.01 | 0.98 | 0.99 | 1.03 | 1.16 | 1.15 | ND | 0.99 |
| C/C 0 for 100 ppm v | |||||||||||
| WMS anasorb 747 | 0.80 | 0.86 | 0.92 | 0.82 | 1.04 | 0.90 | 1.02 | 1.16 | NT | NT | 0.94 |
| ATD tenax TA | 1.04 | 1.39 | 1.36 | 1.21 | 2.74 | 1.23 | 2.10 | 1.89 | NT | NT | 1.62 |
| Radiello charcoal | 0.58 | 1.12 | 1.12 | 1.27 | 1.18 | 1.09 | 1.35 | 1.44 | NT | NT | 1.14 |
| 3M OVM 3500 | 0.30 | 0.86 | 0.88 | 0.94 | 0.86 | 0.96 | 1.16 | 1.40 | NT | NT | 0.92 |
| SKC charcoal | 0.37 | 1.23 | 1.39 | 1.33 | 1.07 | 1.29 | 1.39 | 1.75 | NT | NT | 1.23 |
| Overall average C/C 0 | |||||||||||
| WMS anasorb 747 | 0.67 | 0.98 | 0.74 | 0.69 | 0.76 | 0.73 | 0.82 | 0.84 | 0.69 | 0.24 | 0.72 |
| ATD tenax TA | 1.04 | 1.14 | 0.96 | 0.75 | 2.00 | 0.88 | 1.49 | 1.22 | 0.71 | 1.22 | 1.14 |
| Radiello charcoal | 0.49 | 0.90 | 0.87 | 0.99 | 0.92 | 0.84 | 1.01 | 0.99 | 0.31 | ND | 0.81 |
| 3M OVM 3500 | 0.24 | 0.74 | 0.72 | 0.75 | 0.79 | 0.77 | 0.92 | 1.06 | 0.79 | 0.46 | 0.72 |
| SKC | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
The precision of the passive samplers is summarized in Table 4, which shows the relative standard deviation (RSD, the standard deviation divided by the mean) for all the compound and sampler combinations. The RSD values for the passive samplers were comparable or better than the values for the Summa canister samples. In most cases, the RSD values were less than 15%, which is consistent with passive sampling protocol requirements for occupational monitoring,26 especially at the 10 and 100 ppmv levels where the mass was more readily resolved against reporting limits.
| RSD @ 1 ppmv | MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a ND – not detected. NT – not tested. | |||||||||||
| WMS Anasorb 747 | ND | 0.32 | 0.03 | 0.06 | 0.06 | 0.05 | 0.00 | 0.05 | 0.06 | 0.18 | 0.09 |
| ATD Tenax TA | ND | 0.04 | 0.07 | 0.10 | 0.08 | 0.13 | 0.04 | 0.05 | 0.15 | NA | 0.08 |
| Radiello charcoal | 0.03 | 0.14 | 0.09 | 0.11 | 0.12 | 0.09 | 0.11 | 0.15 | 0.19 | ND | 0.11 |
| 3M OVM 3500 | 0.03 | 0.09 | 0.12 | 0.08 | 0.20 | 0.11 | 0.08 | 0.09 | 0.08 | ND | 0.10 |
| SKC carbograph 5 | 0.05 | 0.13 | 0.16 | 0.18 | 0.05 | 0.21 | 0.18 | 0.18 | 0.19 | 0.14 | 0.15 |
| Summa canister | 0.17 | 0.15 | 0.17 | 0.16 | 0.18 | 0.14 | 0.17 | 0.20 | 0.26 | 0.29 | 0.19 |
| RSD @ 10 ppmv | MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WMS Anasorb 747 | 0.11 | 0.05 | 0.04 | 0.06 | 0.05 | 0.04 | 0.02 | 0.01 | 0.04 | 0.12 | 0.05 |
| ATD Tenax TA | ND | 0.02 | 0.00 | 0.07 | 0.02 | 0.07 | 0.00 | 0.01 | 0.02 | 0.09 | 0.03 |
| Radiello charcoal | 0.17 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | 0.15 | 0.18 | ND | 0.15 |
| 3M OVM 3500 | 0.04 | 0.08 | 0.08 | 0.06 | 0.06 | 0.07 | 0.08 | 0.06 | 0.06 | 0.07 | 0.07 |
| SKC charcoal | 0.18 | 0.14 | 0.02 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 | NA | 0.06 |
| Summa canister | 0.06 | 0.03 | 0.04 | 0.03 | 0.04 | 0.07 | 0.06 | 0.09 | 0.17 | 0.22 | 0.08 |
| RSD @ 100 ppmv | MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WMS Anasorb 747 | 0.10 | 0.09 | 0.06 | 0.05 | 0.09 | 0.07 | 0.06 | 0.06 | NT | NT | 0.07 |
| ATD Tenax TA | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 | NT | NT | 0.04 |
| Radiello charcoal | 0.14 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.10 | NT | NT | 0.05 |
| 3M OVM 3500 | 0.01 | 0.03 | 0.03 | 0.02 | 0.00 | 0.03 | 0.06 | 0.05 | NT | NT | 0.03 |
| SKC charcoal | 0.12 | 0.12 | 0.09 | 0.10 | 0.07 | 0.09 | 0.11 | 0.12 | NT | NT | 0.10 |
| Summa canister | 0.11 | 0.03 | 0.06 | 0.03 | 0.05 | 0.04 | 0.06 | 0.14 | NT | NT | 0.07 |
| Overall Mean RSD | MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Overall Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WMS Anasorb 747 | 0.11 | 0.15 | 0.04 | 0.06 | 0.07 | 0.05 | 0.03 | 0.04 | 0.05 | 0.15 | 0.07 |
| ATD Tenax TA | 0.05 | 0.04 | 0.04 | 0.07 | 0.05 | 0.08 | 0.03 | 0.04 | 0.08 | 0.09 | 0.06 |
| Radiello charcoal | 0.11 | 0.10 | 0.09 | 0.09 | 0.10 | 0.08 | 0.10 | 0.13 | 0.19 | NA | 0.11 |
| 3M OVM 3500 | 0.03 | 0.07 | 0.07 | 0.05 | 0.09 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| SKC | 0.11 | 0.13 | 0.09 | 0.11 | 0.05 | 0.11 | 0.11 | 0.11 | 0.12 | 0.14 | 0.11 |
| Summa canister | 0.11 | 0.07 | 0.09 | 0.08 | 0.09 | 0.08 | 0.09 | 0.14 | 0.22 | 0.26 | 0.12 |
A linear regression analysis was performed to calculate the slope, intercept and correlation coefficient (R2) of the relation between the relative concentration (C/C0) and absolute concentration in the chamber. An ideal correlation would have all C/C0 values equal to 1.0, which would result in a regression with a slope of zero, an intercept of 1.0 and a correlation coefficient (R2) of 100%. Table 5 provides the regression parameters calculated. The intercepts were slightly lower than 1 (0.7 mean for 50 observations), which is attributable to the change in procedure for the 100 ppmv tests where 5 minutes was allowed between samplers for re-equilibration of the chamber concentrations, which resulted in slightly higher concentrations for the 100 ppmv test. Otherwise, the slopes were near zero for all but 124TMB and NAPH in the WMS and 3M OVM 3500 samplers. The R2 values were above 80% for all but:
| Analyte | WMS | ATD | Radiello | 3M OVM | SKC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | R 2 | Slope | Intercept | R 2 | Slope | Intercept | R 2 | Slope | Intercept | R 2 | Slope | Intercept | R 2 | |
| a * - not considered representative because of apparent laboratory blank contamination in 1 ppmv samples. | |||||||||||||||
| 2-Butanone (MEK) | 0.01 | 0.21 | 69% | 0.01 | −0.06 | 99% | 0.00 | 0.40 | 98% | 0.00 | 0.21 | 98% | −0.01 | 1.21 | 33% |
| n-Hexane | 0.00 | 1.07 | * | 0.01 | 1.00 | 99% | 0.01 | 0.77 | 99% | 0.00 | 0.67 | 100% | 0.01 | 0.91 | 83% |
| 1,2-Dichloroethane | 0.00 | 0.64 | 100% | 0.01 | 0.71 | 92% | 0.00 | 1.10 | 36% | 0.00 | 0.64 | 96% | 0.00 | 0.80 | 51% |
| 1,1,1-Trichloroethane | 0.00 | 0.62 | 96% | 0.01 | 0.49 | 98% | 0.00 | 0.85 | 98% | 0.00 | 0.67 | 99% | 0.00 | 0.79 | 39% |
| Benzene | 0.00 | 0.59 | 87% | 0.01 | 1.56 | 97% | 0.00 | 0.76 | 97% | 0.00 | 0.76 | 27% | 0.00 | 0.95 | 97% |
| Carbon tetrachloride | 0.00 | 0.65 | 89% | 0.01 | 0.70 | 94% | 0.00 | 0.71 | 99% | 0.00 | 0.69 | 87% | 0.01 | 0.72 | 63% |
| Trichloroethene | 0.00 | 0.71 | 99% | 0.01 | 1.15 | 100% | 0.01 | 0.83 | 100% | 0.00 | 0.79 | 95% | 0.01 | 0.77 | 74% |
| Tetrachloroethene | 0.00 | 0.69 | 99% | 0.01 | 0.87 | 99% | 0.01 | 0.78 | 99% | 0.01 | 0.91 | 92% | 0.01 | 0.85 | 75% |
| 1,2,4-Trimethylbenzene | 0.05 | 0.50 | 100% | −0.01 | 0.83 | 70% | 0.00 | 0.41 | 72% | 0.06 | 0.58 | 100% | −0.01 | 1.02 | 61% |
| Naphthalene | 0.02 | 0.10 | 100% | −0.01 | 0.84 | 44% | 0.00 | 0.04 | 18% | 0.05 | −0.05 | 100% | −0.01 | 0.61 | 32% |
• MEK and NHEX for the WMS.
• 124TMB for the ATD.
• 12DCA, 124TMB and NAPH for the Radiello.
• BENZ for the 3M OVM 3500 and.
• most of the compounds with the SKC Ultra.
This demonstrates that different compounds pose challenges for each of the samplers, which is an area for further research.
The results for the low-uptake rate samplers are provided in Table 6. The Radiello sampler (yellow body), WMS-LU (0.8 mL vial) and the ATD tube sampler with the low-uptake rate cap (Markes International, Wales) showed average results within a factor of 0.72, 1.08 and 0.72, respectively of the Summa canister results in the 10 ppmv test at a flow rate of 100 mL min−1, which shows the low uptake rate samplers have a comparable accuracy to the regular uptake rate samplers. Under no-flow conditions, the passive samplers showed average C/C0 values of 0.47, 0.73 and 0.1, respectively, which were lower (by a factor of 0.65, 0.68 and 0.71, respectively) compared to the samples collected with 100 mL min−1 flow in the chamber. The low bias under no-flow conditions was similar for all three samplers even though they have considerably different uptake rates (about 25 mL min−1 for the Radiello, about 0.5 mL min−1 for the WMS-LU and about 0.05 mL min−1 for the ATD tube). The low-uptake rate Radiello also showed a low bias of 100× for 124TMB, and a low bias of 5× for tetrachloroethene (PCE) under no flow conditions, which are the compounds with the highest organic carbon partitioning coefficient (Koc) values and lowest free air diffusion coefficients (excepting NAPH which was not detected by the Radiello). The ATD tube sampler showed a high bias of 2× for BENZ and 9× for NAPH and a low bias of about 10× for 1,1,1-trichloroethane (111TCA), carbon tetrachloride (CTET) and 124TMB. The SKC/Charcoal sampler with the low-uptake rate cap showed detectable concentrations for only 3 compounds in the 100 ppmv stagnant test, but the concentrations were quantified within a factor of 2 for all three. The WMS-LU sampler showed concentrations within 2× for all compounds under both flowing and stagnant conditions.
| MEK | NHEX | 12DCA | 111TCA | BENZ | CTET | TCE | PCE | 124TMB | NAPH | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Notably different than other results, so these values were not included in the row averages. ND – not detected. | |||||||||||
| 10 ppm v & 100 mL min −1 | |||||||||||
| Active tube sample 1 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
|
| Active tube sample 2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
884 | |
| Average Active tube concentration | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
953 | |
| Radiello yellow body | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3400 3400 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
230 | ND | |
| Radiello/Active (C/C0) | 0.94 | 0.81 | 0.90 | 1.15 | 0.86 | 0.72 | 0.75 | 0.32 | 0.01 | 0.72 | |
| SKC 12 hole cap | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| WMS 0.8 mL vial 1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1470 | |
| WMS 0.8 mL vial 2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1530 | |
| Average WMS/Active (C/C0) | 1.34 | 0.79 | 1.07 | 1.09 | 0.92 | 1.07 | 1.01 | 1.08 | 0.87 | 1.57 | 1.08 |
| ATD low uptake 1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1870 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2260 | 5600 | |
| ATD low uptake 2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2800 | 6400 | |
| Average ATD/Active (C/C0) | 1.02 | 0.51 | 0.74 | 0.15 | 2.55 | 0.29 | 0.59 | 0.59 | 0.06 | 6.29a | 0.72 |
| 10 ppmv No flow | |||||||||||
| Active tube sample | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
833 | |
| Radiello yellow | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1100 | ND | |
| Radiello/Active (C/C0) | 0.73 | 0.52 | 0.56 | 0.69 | 0.56 | 0.45 | 0.46 | 0.20 | 0.03 | 0.47 | |
| SKC 12 hole cap | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| WMS 0.8 mL vial 1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
733 | |
| WMS 0.8 mL vial 2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
800 | |
| Average WMS/Active (C/C0) | 0.77 | 0.61 | 0.80 | 0.77 | 0.77 | 0.80 | 0.71 | 0.67 | 0.50 | 0.92 | 0.73 |
| ATD low uptake 1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2150 | 9330 | |
| ATD low uptake 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
6200 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2470 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
3130 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
8940 | 2690 | 5130 | |
| Average ATD/Active (C/C0) | 0.69 | 0.30 | 0.45 | 0.11 | 2.32 | 0.12 | 0.28 | 0.24 | 0.06 | 8.68a | 0.51 |
| 100 ppmv No flow | |||||||||||
| Summa | 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| SKC 12 hole cap 1 | ND | 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ND | ND | ND | ND | |||
| SKC 12 hole cap 2 | ND | 321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
442![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
526![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ND | ND | ND | ND | |||
| SKC 12 hole cap 3 | ND | 290![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
403![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
487![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ND | ND | ND | ND | |||
| Average SKC/Summa (C/C0) | 1.28 | 1.71 | 1.50 | 1.50 | |||||||
| ATD low uptake | 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
327![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
429![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
593![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
327![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
610![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| ATD/Summa (C/C0) | 1.86 | 1.08 | 1.31 | 1.41 | 2.38 | 1.35 | 1.09 | 1.60 | 1.51 | ||
Conclusions
The results of this study indicate that passive samplers can provide vapor concentration measurements in settings similar to those expected to be encountered in passive soil vapor sampling and therefore may be a practical alternative for monitoring soil vapor concentrations for many of the volatile organic compounds of interest for human health risk assessment. Most of the concentrations measured with the passive samplers were within a factor of 2 or less of the concentrations measured with Summa canister/EPA Method TO-15 and the precision of the passive samplers was as good or better than the Summa canisters. This is encouraging considering that the passive samplers and analytical methods are all different and the samples were analyzed in different laboratories, and none of the vendor-supplied uptake rates were derived specifically for short (30 minute) duration, high (80%) humidity, and low (5 cm min−1) face velocity settings. Low-uptake rate varieties of four of the samplers yielded similar accuracy to the regular uptake rate samplers, which is encouraging because low uptake rate samplers are expected to minimize the starvation effect in applications of passive soil vapor sampling.12 Highly soluble compounds (like MEK) or highly sorptive compounds (like NAPH) appear to be more challenging to quantify accurately than other compounds.The laboratory testing apparatus cannot simulate field sampling of soil vapor exactly, so further in situ testing is needed. Field conditions could involve a broader range of chemicals, concentrations, sample durations and sampler design modifications (sorbents, uptake rates). Until more is known about these variables, it is prudent to perform inter-method comparisons as a quality assurance procedure (e.g., collect adjacent samples for analysis by conventional methods in a certain percentage of locations to enable calculation of site-specific or field-verified uptake rates).
Acknowledgements
Funding for this work was provided by the Environmental Security Technology Certification Program (ESTCP). Sam Brock of AFCEC and Andrea Leeson of ESTCP were the DOD Liaisons. We gratefully acknowledge Caterina Boaretto of Fondazione Salvatore Maugeri for GC analysis of the Radiello samplers.References
- E. D. Palmes and A. F. Gunnison, Am. Ind. Hyg. Assoc. J., 1973, 34, 78–81 CrossRef CAS PubMed.
- ASTM Standard D4597, Standard Practice for Sampling Workplace Atmospheres to Collect Gases of Vapors with Solid Sorbent Diffusive Samplers, ASTM International, West Conshohocken, PA, 2009, http://www.astm.org Search PubMed.
- E. N. 838, Workplace Atmospheres - Diffusive Samplers for the Determination of Gases and Vapours - Requirements and Test Methods, Comité Européen de Normalisation, Brussels, Belgium, 2010 Search PubMed.
- NIOSH Method 4000, Toluene (diffusive sampler), Issue 2, dated 15 August, 1994 Search PubMed.
- International Standards Organization (ISO), 16017–2, Indoor Ambient and Workplace Air – Sampling and Analysis of Volatile Compounds by Sorbent Tube/Thermal Desorption/Capillary Gas Chromatography – Part 2: Diffusive Sampling, 2003 Search PubMed.
- ASTM Standard D7758, New Practice for Passive Soil Gas Sampling in the Vadose Zone for Source Identification, Spatial Variability Assessment, Monitoring and Vapor Intrusion Evaluations ASTM International, West Conshohocken, PA, 2011, http://www.astm.org Search PubMed.
- USEPA Office of Research and Development, Environmental Technology Verification Report, Soil Gas Sampling Technology, Quadrel Services, Inc., EMFLUX Soil Gas System, EPA Report No. 600/R-98/096, 1998 Search PubMed.
- U.S. EPA Office of Research and Development, Environmental Technology Verification Report, Soil Gas Sampling Technology, W. L. Gore & Associates, Inc. GORE-SORBER Screening Survey, EPA Report No. 600/R-98/095, 1998 Search PubMed.
- California Environmental Protection Agency/Department of Toxic Substances Control (EPA/DTSC), Final Guidance for the Evaluation and Mitigation of Subsurface Vapor Intrusion to Indoor Air (Vapor Intrusion Guidance), October 2011 Search PubMed.
- Interstate Technology and Regulatory Council (ITRC), Vapor Intrusion Pathway: A Practical Guideline, 2007. http://www.itrcweb.org/Documents/VI-1.pdf Search PubMed.
- T. A. McAlary, X. Wang, A. Unger, H. Groenevelt and T. Górecki, Quantitative passive soil vapor sampling for VOCs- part 1: theory, Environ. Sci.: Processes Impacts, 2014 10.1039/c3em00652b.
- T. A. McAlary, H. Groenevelt, P. Nicholson, S. Seethapathy, P. Sacco, D. Crump, M. Tuday, H. Hayes, B. Schumacher, P. Johnson, T. Górecki and I. Rivera-Duarte, Quantitative passive soil vapor sampling for VOCs- part 3: field experiments, Environ. Sci.: Processes Impacts, 2014 10.1039/c3em00653k.
- D. C. Gomes, M. Alarsa, M. C. Salvador and C. Kupferschmid, Water Sci. Technol., 1994, 29, 161 CAS.
- M. Anderson and G. Church, J. Environ. Eng., 1998, 124, 555 CrossRef CAS.
- Beacon, 2013. http://www.beacon-usa.com/services/passive-soil-gas-surveys/, accessed on January 16, 2014.
- ASTM D6246 – 08, Standard Practice for Evaluating the Performance of Diffusive Samplers, ASTM International, West Conshohocken, PA, 2008, http://www.astm.org Search PubMed.
- K. Bergemalm-Rynell, B. Strandberg, E. Andersson and G. Sallsten, Laboratory and field evaluation of a diffusive sampler for measuring halogenated anesthetic compounds, J. Environ. Monit., 2008, 10, 1172–1178 RSC.
- C. Cocheo, C. Boaretto, D. Pagani, F. Quaglio, P. Sacco, L. Zaratin and D. Cottica, J. Environ. Monit., 2009, 11, 297–306 RSC.
- J. T. Purdham, A. M. Sass-Kortsak and P. R. Bozek, Ann. Occup. Hyg., 1994, 38(5), 721–740 CrossRef CAS.
- S. Seethapathy and T. Górecki, J. Chromatogr. A, 2011 Jan 7, 1218(1), 143–155 Search PubMed . Epub 2010 Nov 9.
- S. Seethapathy and T. Górecki, J. Chromatogr. A, 2010 Dec 10, 1217(50), 7907–7913 Search PubMed . Epub 2010 Oct 21.
- S. Batterman, T. Metts and P. Kalliokoski, J. Environ. Monit., 2002, 4, 870–878 RSC.
- V. M. Brown, D. R. Crump and D. Gardiner, Environ. Technol., 1992, 13, 367–375 CrossRef CAS.
- U.S. Environmental Protection Agency (USEPA), Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Compendium Method TO-15-Determination Of Volatile Organic Compounds (VOCs) In Air Collected In Specially-Prepared Canisters And Analyzed By Gas Chromatography/Mass Spectrometry (GC/MS), Center for Environmental Research Information Office of Research and Development Cincinnati, OH, 2nd edn, January 1999, PA/625/R-96/010b Search PubMed.
- USEPA, Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Compendium Method TO-17, Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes, 2nd edn, EPA/625/R-96/010b, 1999 Search PubMed.
- M. Harper and L. V. Guild, Am. Ind. Hyg. Assoc. J., 1996, 57(12), 1115–1123 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3em00128h |
| This journal is © The Royal Society of Chemistry 2014 |