Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures†
Abstract
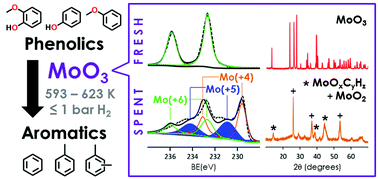
MoO3 is an effective catalyst for the hydrodeoxygenation (HDO) of lignin-derived oxygenates to generate high yields of aromatic hydrocarbons without ring-saturated products. The catalyst is selective for the C–O bond cleavage under low H2 pressures (≤1 bar) and temperatures ranging from 593 to 623 K. A bond-dissociation energy analysis of relevant phenolic C–O bonds indicates that the bond strengths follow an order of Ph–OH > Ph–OMe > Ph–O–Ph > Ph–O–Me. However, for all model compounds investigated, the MoO3 catalyst preferentially cleaves phenolic Ph–OMe bonds over weaker aliphatic Ph–O–Me bonds. Characterisation studies reveal that the catalyst surface undergoes partial carburisation as evidenced by the presence of oxycarbide- and oxycarbohydride-containing phases (i.e., MoOxCyHz). The transformation of bulk phases and the surface modification of MoO3 by carbon–H2 are investigated to understand the role of surface carbon in the stabilisation and enhanced activity of the partially reduced MoO3 surface.

 Please wait while we load your content...
Please wait while we load your content...