Strongly coupled carbon nanofiber–metal oxide coaxial nanocables with enhanced lithium storage properties†
Genqiang
Zhang
ab,
Hao Bin
Wu
b,
Harry E.
Hoster
a and
Xiong Wen (David)
Lou
*ab
aTUM CREATE, 1 CREATE Way, #10-02 CREATE Tower, Singapore 138602. E-mail: xwlou@ntu.edu.sg; Web: http://www.ntu.edu.sg/home/xwlou/
bSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459
First published on 11th November 2013
Abstract
A facile two-step strategy involving a polyol method and subsequent thermal annealing treatment is successfully developed for the general synthesis of metal oxide/carbon coaxial nanocables. Benefitting from the strong coupling effect, these hybrid nanocables exhibit remarkable lithium storage properties with high capacity, long cycle life and excellent rate capability.
Lithium-ion batteries (LIBs) have been considered as one of the most promising electric energy storage systems due to their various merits of high voltage, high capacity, low cost and environmental friendliness.1 However, there are still several challenging issues in LIBs to be overcome in order to fulfil the requirements as high-performance power sources in the future market, including higher capacity, lower cost, longer cycle life and better rate performance.2 Nanostructure engineering has been demonstrated as a powerful and effective strategy in the rational design of new electrode materials with optimized performance through both morphological and compositional solutions.3–10 For example, various nanostructures with controlled morphologies including hollow spheres/cubes, nanowires/rods as well as nanoplates could achieve significantly enhanced lithium storage properties due to the size and shape effects.11–15 However, the intrinsic properties including low electrical conductivity and poor mechanical stability of most oxide electrode materials, leading to unsatisfactory cycling stability and rate performance, still seriously limit their applications. Recently, an emerging concept of hybrid nanostructures has attracted tremendous attention since better performance could be expected in such architectures.16–19 Coaxial nanocables, as one of the most interesting hybrid nanostructures, hold great potential for simultaneously resolving the problems of poor electrical conductivity and weak mechanical stability with rational design and careful choice of core and shell components.20 However, there are not too many such reports showing the attractive properties of nanocable structures for lithium storage, probably due to the lack of appropriate synthesis methods.
Manganese (Mn) based transition metal oxides (TMOs) have recently been extensively investigated as negative electrode materials due to their advantages of low cost, high capacity and low operating voltage and so on. Various nanostructures of Mn-based binary and ternary oxides such as MnO2, ZnMn2O4 and CoMn2O4 have been successfully synthesized and some promising lithium storage properties have been achieved.13,21–25 One of the key issues for Mn-based oxides is the extremely low conductivity, which hinders the electrochemical reactions. In this situation, it would be highly desirable to synthesize coaxial nanocables by integrating high capacity Mn-based oxides with more conductive carbon nanofibers (CNFs).
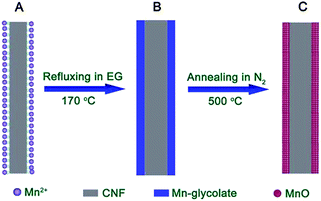
In this work, we have successfully synthesized CNF@MnO and CNF@CoMn2O4 coaxial nanocables with considerably enhanced lithium storage performance through a facile two-step strategy. The detailed synthesis procedures are described in the Experimental section and the formation process is schematically illustrated in Fig. 1. In brief, the CNFs obtained through a Te nanowire directed hydrothermal method26–29 are first dispersed into ethylene glycol dissolved with a certain amount of Mn(CH3COO)2 precursor. Due to the existence of the large amount of functional groups on the surface of the CNFs confirmed by the Fourier transform infrared spectroscopy (FTIR) technique in previous literature,27 the Mn2+ ions will be well coordinated, as illustrated in Fig. 1A. Subsequently, the nucleation and growth of Mn-glycolate will preferentially take place on the surface of the CNFs during refluxing, as shown in Fig. 1B, forming a conformal coating layer. The strongly coupled hybrid structure of the MnO nanocrystal layer on CNFs can be easily obtained through the convenient thermal annealing treatment under the protection of nitrogen gas (Fig. 1C). The strategy can be easily extended to synthesize CNF@CoMn2O4 nanocables by using precursors of Co(CH3COO)2 and Mn(CH3COO)3·4H2O.
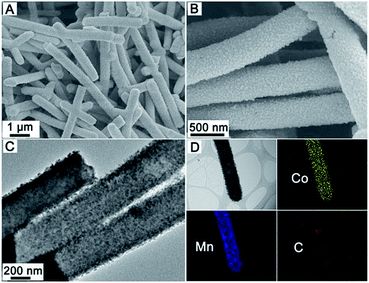
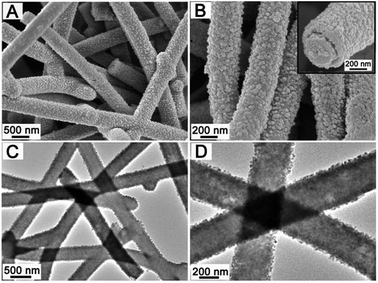
Fig. 2 shows the typical field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) images of CNF@MnO nanocables. As can be seen, large scale and uniform one-dimensional (1D) structures can be obtained (Fig. 2A and B) after annealing the CNF@Mn-glycolate precursor structure (Fig. S1†). The corresponding X-ray diffraction (XRD) pattern (Fig. S2†) can be readily indexed to the cubic MnO phase (JCPDS card no. 78-0424). The cross-sectional view of an individual nanocable (see the inset in Fig. 2B) clearly indicates the coaxial feature between the inner CNF and MnO nanocrystal layer. The coaxial feature can further be elucidated from representative TEM images that there is notable contrast between the inner core and outer shell layer, as shown in Fig. 2C and D. It is worth noting that there is no visible interlayer gap observed between the core and shell layer from the magnified TEM image (Fig. S3†), which implies that the MnO nanocrystals are strongly anchored on the CNF. Moreover, no visible change can be observed when sonicating the CNF@MnO nanocable powder dispersed in ethanol for up to 4 h (Fig. S4†), which undoubtedly further validates the strong coupling between MnO and the CNF surface. This feature might be quite significant for enhancing the lithium storage properties, which will be discussed shortly. As estimated from the TEM images, the thickness of the outer MnO shell is about 100 nm while the diameter of the inner CNF is around 300 nm. The content of CNFs in the CNF@MnO coaxial nanocables is determined by thermogravimetric analysis (TGA) to be about 24 wt% (Fig. S5†). Importantly, the method could be easily extended to grow complex mixed metal oxides on CNFs. As a demonstration, a CNF@CoMn2O4 coaxial nanocable structure is successfully synthesized in this work. Fig. 3A–C give the typical morphological characterizations of the CNF@CoMn2O4 nanocables with high uniformity on a large scale. In addition, the CoMn2O4 nanocrystals are also strongly coupled to the CNF to form a well-defined conformal coating as revealed by the TEM images. This is quite significant since it is more challenging for the formation of a uniform mixed metal oxide nanocrystal layer onto the surface of the CNF considering the higher complexity compared to simple binary metal oxides. The corresponding XRD pattern (Fig. S6†) indicates that the obtained crystalline material is phase-pure and the peaks can be readily indexed to the tetragonal CoMn2O4 phase (JCPDS card no. 77-0471). Fig. 3D shows the elemental mapping results for the distribution of Co, Mn and C elements on an individual CNF@CoMn2O4 nanocable. As can be seen, the Co and Mn signals are much stronger than that of C and the distribution of Co and Mn has a slight difference between the central and edge regions of the individual nanocable, which provides direct evidence for the formation of a coaxial structure. As described above, the present two-step strategy is very efficient and versatile to obtain well-defined coaxial nanocable structures consisting of strongly coupled CNF cores and metal oxide nanocrystal shells.
 | ||
| Fig. 2 Typical FESEM and TEM images of CNF@MnO nanocables: (A and B) FESEM images with different magnifications and (C and D) TEM images with different magnifications. | ||
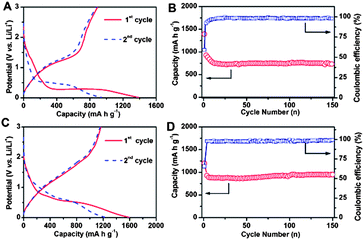
The CNF@MnO and CNF@CoMn2O4 nanocable structures are then evaluated as anode materials for LIBs. In this study, the capacity is calculated based on the total mass of the nanocable structures including the metal oxide and CNF. Fig. 4A shows typical charge–discharge voltage profiles for the first and second cycles of the CNF@MnO nanocable electrode at a current density of 200 mA g−1. As can be seen, there are notable discharge and charge potential plateaus from the charge–discharge curves. The discharge potential plateau appears at around 0.29 V in the first cycle and it shifts to around 0.5 V in the second cycle, while the charge potential plateau starts from around 1 V and almost stays the same for the second cycle. The first discharge capacity is around 1390 mA h g−1, whereas the corresponding charge capacity is around 892.5 mA h g−1, delivering an initial Coulombic efficiency of 64.2%, which is similar to the values reported in previous literature.30,31 The irreversible capacity loss of the first cycle could be attributed to the inevitable formation of a solid electrolyte interface (SEI) film and electrolyte decomposition, which is common to most oxide anode materials and has also been observed in previous literature about MnO electrodes.30–34Fig. 4B shows the cycling performance and the corresponding Coulombic efficiency of the CNF@MnO nanocable electrode under a current density of 200 mA g−1. The discharge capacity of the second cycle is around 936 mA h g−1 and it decreases to around 750 mA h g−1 in the first 10 cycles. After that, the capacity remains quite stable with about 98% retention even when the test is prolonged to 150 cycles, indicating the largely improved cycling performance compared with previous results.31,34–36 The corresponding Coulombic efficiency curve (right axis in Fig. 4B) indicates that the value rapidly increases to around 98% after the relatively low initial Coulombic efficiency of around 59%. The high-rate charging–discharging performance of the CNF@MnO nanocable electrode is further investigated (Fig. S7A†). Impressively, when the current density increases to as high as 1000 mA g−1, it still shows excellent electrochemical performance with both high capacity and good cycling stability. Specifically, the capacity can stabilize at around 510 mA h g−1 with an ultralong cycle life of 300 cycles, which is considered remarkable among oxide-based anode materials. Furthermore, the rate performance is tested with the current densities ranging from 200 to 1600 mA g−1 (Fig. S7B†). As can be seen, the average specific capacities are 750, 585, 472, and 310 mA h g−1 at current densities of 200, 400, 800, and 1600 mA g−1, respectively. After the high-rate charge–discharge cycling, a specific capacity as high as 820 mA h g−1 can be recovered when the current density is reduced stepwise to 200 mA g−1, indicating the good reversibility of the electrode.
The lithium storage properties of the CNF@CoMn2O4 nanocable electrode are also greatly enhanced compared with those in previous literatures, probably benefitting from the well-defined coaxial features.24,25Fig. 4C gives the typical voltage profiles of the first and second cycles, which agree well with those in previous literature.24,25 The cycling performance at a current density of 200 mA g−1 (Fig. 4D) indicates that the capacity exhibits no visible fading from the second cycle with a high specific capacity of 870 mA h g−1 up to a long life span of 150 cycles. More impressively, the CNF@CoMn2O4 nanocable electrode can still show excellent cycling stability for at least 300 cycles with no notable fading and deliver a high capacity of 650 mA g−1 when the current density increases to as high as 1000 mA h g−1 (Fig. S7C†), which is considerably improved compared with other reported results and is superior among various other hybrid nanostrucutures.24,25 The rate performance of the CNF@CoMn2O4 nanocable structure is also outstanding (Fig. S7D†). It can deliver high average specific capacities of 950, 860, 610, 390 and 160 mA h g−1 at current densities of 200, 400, 1200, 2400 and 3600 mA g−1, respectively. After the high-rate charge–discharge cycling, a similarly high specific capacity of 930 mA h g−1 can be recovered when the current density is reduced stepwise to 200 mA g−1.
The remarkable lithium storage properties could be attributed to the advantageous coaxial nanocable structure. Specifically, the morphological characterization indicates that the CNF has a relatively large diameter of about 300 nm and the outer metal oxide shell thickness is around 100 nm. The inner CNF could provide a robust support for the electroactive metal oxide layer to alleviate the strain derived from the volume change during the lithium insertion/extraction process. Importantly, the outer shell layer is strongly anchored onto the CNF core and the outer metal oxide shell consists of small nanocrystals. The core–shell configuration of the nanocables could effectively prohibit the aggregation of the metal oxide nanocrystals during the cycling. In addition, the CNF would be more conducting after annealing in N2 at high temperature compared with the outer metal oxide shell materials, which will facilitate the electrochemical redox reactions. All these features would undoubtedly contribute to the improvement of the electrochemical performance of the active materials, leading to the high capacity, long cycle life and superior rate performance.
In summary, well-defined CNF@MnO and CNF@CoMn2O4 coaxial nanocables can be facilely fabricated through a simple and general two-step strategy involving a polyol process and subsequent thermal annealing treatment. These two nanocable electrodes exhibit remarkable lithium storage properties in terms of high specific capacity, long cycle life and superior rate performance. The enhanced electrochemical performance could be attributed to several advantages of the smart coaxial nanocable configuration, which can effectively alleviate the volume change, prohibit the nanocrystals aggregation and facilitate the electron transport. Such high-performance nanostructures with large-scale production might hold great potential for the fabrication of high energy and power density lithium-ion batteries.
Acknowledgements
This publication is made possible with the financial support from the Singapore National Research Foundation under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.References
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- N. S. Choi, Z. H. Chen, S. A. Freunberger, X. L. Ji, Y. K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- R. Mukherjee, R. Krishnan, T.-M. Lu and N. Koratkar, Nano Energy, 2012, 1, 518–533 CrossRef CAS PubMed.
- A. I. Hochbaum and P. D. Yang, Chem. Rev., 2010, 110, 527–546 CrossRef CAS PubMed.
- P. D. Yang and J. M. Tarascon, Nat. Mater., 2012, 11, 560–563 CrossRef CAS PubMed.
- S. J. Ding, J. S. Chen, G. G. Qi, X. N. Duan, Z. Y. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21–23 CrossRef CAS PubMed.
- L. Zhou, D. Zhao and X. Lou, Angew. Chem., Int. Ed., 2012, 51, 239–241 CrossRef CAS PubMed.
- H.-W. Lee, P. Muralidharan, R. Ruffo, C. M. Mari, Y. Cui and D. K. Kim, Nano Lett., 2010, 10, 3852–3856 CrossRef CAS PubMed.
- S. H. Liu, H. P. Jia, L. Han, J. L. Wang, P. F. Gao, D. D. Xu, J. Yang and S. N. Che, Adv. Mater., 2012, 24, 3201–3204 CrossRef CAS PubMed.
- B. Jang, M. Park, O. B. Chae, S. Park, Y. Kim, S. M. Oh, Y. Piao and T. Hyeon, J. Am. Chem. Soc., 2012, 134, 15010–15015 CrossRef CAS PubMed.
- A. L. M. Reddy, S. R. Gowda, M. M. Shaijumon and P. M. Ajayan, Adv. Mater., 2012, 24, 5045–5064 CrossRef CAS PubMed.
- H. L. Wang, L. F. Cui, Y. A. Yang, H. S. Casalongue, J. T. Robinson, Y. Y. Liang, Y. Cui and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS PubMed.
- G. H. Yu, X. Xie, L. J. Pan, Z. N. Bao and Y. Cui, Nano Energy, 2013, 2, 213–234 CrossRef CAS PubMed.
- C. Z. Wu, X. L. Lu, L. L. Peng, J. L. Huang, G. H. Yu and Y. Xie, Nat. Commun., 2013, 4, 2431 Search PubMed.
- F. F. Cao, Y. G. Guo and L. J. Wan, Energy Environ. Sci., 2011, 4, 1634–1642 CAS.
- S. W. Lee, J. Kim, S. Chen, P. T. Hammond and Y. Shao-Horn, ACS Nano, 2010, 4, 3889–3896 CrossRef CAS PubMed.
- R. Liu, J. Duay and S. B. Lee, ACS Nano, 2010, 4, 4299–4307 CrossRef CAS PubMed.
- G. Q. Zhang, L. Yu, H. Bin Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- L. Zhou, D. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745–748 CrossRef CAS PubMed.
- L. Hu, H. Zhong, X. R. Zheng, Y. M. Huang, P. Zhang and Q. W. Chen, Sci. Rep., 2012, 2, 986 Search PubMed.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101–5105 CrossRef CAS PubMed.
- G. Q. Zhang, L. Yu, H. E. Hoster and X. W. Lou, Nanoscale, 2013, 5, 877–881 RSC.
- G. Q. Zhang and X. W. D. Lou, Sci. Rep., 2013, 3, 1470 CAS.
- G. Q. Zhang, B. Y. Xia, C. Xiao, L. Yu, X. Wang, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 8643–8647 CrossRef CAS PubMed.
- X. Q. Yu, Y. He, J. P. Sun, K. Tang, H. Li, L. Q. Chen and X. J. Huang, Electrochem. Commun., 2009, 11, 791–794 CrossRef CAS PubMed.
- K. Zhong, B. Zhang, S. Luo, W. Wen, H. Li, X. Huang and L. Chen, J. Power Sources, 2011, 196, 6802–6808 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, A1212–A1217 CrossRef CAS PubMed.
- X. P. Fang, X. Lu, X. W. Guo, Y. Mao, Y. S. Hu, J. Z. Wang, Z. X. Wang, F. Wu, H. K. Liu and L. Q. Chen, Electrochem. Commun., 2010, 12, 1520–1523 CrossRef CAS PubMed.
- K. F. Zhong, X. Xia, B. Zhang, H. Li, Z. X. Wang and L. Q. Chen, J. Power Sources, 2010, 195, 3300–3308 CrossRef CAS PubMed.
- B. Sun, Z. X. Chen, H. S. Kim, H. Ahn and G. X. Wang, J. Power Sources, 2011, 196, 3346–3349 CrossRef CAS PubMed.
- X. W. Li, D. Li, L. Qiao, X. H. Wang, X. L. Sun, P. Wang and D. Y. He, J. Mater. Chem., 2012, 22, 9189–9194 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD patterns, TGA curve, more FESEM and TEM images and cycling performance of CNF@MnO and CNF@CoMn2O4 at higher charge–discharge current densities, and detailed discussions on the extra capacity for CNF@MnO. See DOI: 10.1039/c3ee43123a |
| This journal is © The Royal Society of Chemistry 2014 |