Biomineralized α-Fe2O3: texture and electrochemical reaction with Li
J.
Miot†
abc,
N.
Recham
ac,
D.
Larcher
*ac,
F.
Guyot
bd,
J.
Brest
b and
J.-M.
Tarascon
ac
aLaboratoire de Réactivité et Chimie des Solides UMR CNRS 7314, Université de Picardie Jules Verne, 33 rue Saint-Leu, 80039 Amiens Cedex, France. E-mail: dominique.larcher@u-picardie.fr
bInstitut de Minéralogie et de Physique des Milieux Condensés UMR CNRS 7590, Université Pierre et Marie Curie (UPMC), 4 Place Jussieu, 75252 Paris Cedex 05, France
cRéseau sur le Stockage Electrochimique de l'Energie (RS2E), FR CNRS 3459, France
dUniversité Paris Diderot, Sorbonne Paris Cité, Institut de Physique du Globe de Paris, UMR CNRS 7154, F-75013 Paris, France
First published on 7th November 2013
Abstract
Sustainable batteries call for the development of new eco-efficient processes for preparation of electrode materials based on low cost and abundant chemical elements. Here we report a method based on bacterial iron biomineralization for the synthesis of α-Fe2O3 and its subsequent use as a conversion-based electrode material in Li batteries. This high-yield synthesis approach enlists (1) the room temperature formation of γ-FeOOH via the use of an anaerobic Fe(II)-oxidizing bacterium Acidovorax sp. strain BoFeN1 and (2) the transformation of these BoFeN1/γ-FeOOH assemblies into an alveolar bacteria-free α-Fe2O3 material by a short heat treatment under air. As the γ-FeOOH precursor particles are precipitated between the two membranes of the bacterial cell wall (40 nm–thick space), the final material consists of highly monodisperse nanometric (∼40 × 15 nm) and oriented hematite crystals, assembled to form a hollow shell having the same size and shape as the initial bacteria (bacteriomorph). This double level of control (nanometric particle size and particle organization at the micrometric scale) provided powders exhibiting (1) enhanced electrochemical reversibility when fully reacted with Li and (2) an impressive high rate capability when compared to non-textured primary α-Fe2O3 particles of similar size. This bacterially induced eco-efficient and scalable synthesis method opens wide new avenues to be explored at the crossroads of biomineralization and electrochemistry for energy storage.
Broader contextLiving organisms can directly provide concentrated energy sources (cellulose, starch, bio-fuels…) but they can also help us in designing new materials with added value for energy concentration, storage and transportation. Here, we use the ability of the bacterium Acidovorax sp. strain BoFeN1 to precipitate and confine γ-FeOOH in between the two membranes of its cell wall. After a short air-heating treatment, the final material consists of hematite (α-Fe2O3) nano-crystals (40 × 15 nm) assembled in a way to form a hollow and porous shell keeping the bacterial size and shape, and is thus thereafter named a bacteriomorph. Both the nanometric particle size and the micrometric textural organization are inherited from the bacterial origin of the precursor material, and lead to (1) an enhanced electrochemical reversibility of the material when fully reacted with Li (conversion reaction) and (2) an impressive high rate capability in comparison with non-textured α-Fe2O3 particles of similar size. Indeed, hematite bacteriomorph samples only lose 30% of their ability to react with Li when moving from 1 Li per 100 h to 10 Li per h rates. This biomineralization, an eco-efficient and easily scalable synthesis method, provides new expectations and opens wide new avenues to be explored at the crossroads of biomineralization and electrochemical energy storage. |
Introduction
The challenging prospects for electrode improvements for energy storage in Li-ion batteries have led to miscellaneous strategies to revisit the synthesis routes of materials that can react with lithium. Neighboring though still not dethroning pure-insertion materials, electrode compounds reacting through conversion reactions, such as transition metal oxides, have deserved soaring attention in the last few years.1,2 Their attractiveness mainly relies on their high gravimetric capacities compared to insertion compounds, low cost and – for some of them – innocuousness.2 With a theoretical capacity of about 1000 mA h g−1, α-Fe2O3 falls within this area and is of particular interest for Li-ion batteries. Back in the 80's, the study of its full reduction by Li° at room temperature3 and 400 °C4,5 highlighted the existence of a small Li+ insertion domain (x < 0.1, Fe2O3 → LixFe2O3), followed by an irreversible hexagonal → cubic phase transition (LixFe2O3 → Li2Fe2O3) and later on the formation of Fe° + lithia upon further reduction down to 0 volt. Even if deeply lithiated Fe2O3 (4 < x < 6) was early proposed as an anode material for rocking-chair cells,6 acceptable reversibility of the full range of composition was never achieved for simple binder-free carbon–α-Fe2O3 powder mixture electrodes, in contrast to many observations made on CoO, NiO and others.1,2Whereas the use of highly divided grains revealed an unexpected mechanism when reacted with Li, the effect of nanometer-sized particles on the performances of conversion-based electrode compounds is still under debate.2,7 In the case of hematite, downsizing the reacting domains leads to a large apparent increase in the range of the initial solid-solution (x ∼ 1 in LixFe2O3) with very small volume expansion, hence enhancing reversibility when cycled over this domain, but full reaction with Li was still found to be poorly reversible whatever the particle size.8
On the other hand, structural reorganization and volume changes inherent to the conversion reaction (ΔV/V ∼ 100% for Fe2O3 + 6Li → 2Fe + 3Li2O) enhance electrical isolation of the particles and can trigger electrolyte decomposition resulting in the formation of an isolating/passivation layer, which overall strongly hampers capacity retention.2 Aside from thin films or derivatives,9 many alternative strategies have thus been proposed to overcome such limitations, mainly aimed at preserving a texture (particle connectivity) at the micrometer scale, including the use of selected binders7 and/or increased amounts of carbon,10 the deposition of particles onto conducting substrate foams,11 metallic nanopillars,12 foils, the deposition of nanoparticles (rods, horns) at the surface of carbon nanotubes13,14 and electrospining,15,16 the synthesis of oxide nanotubes17 or the preparation of oxide–graphene composites.18 As these approaches enable better conservation of the electronic percolation network, they generally improve cyclability, coulombic efficiencies and rate capabilities, but they generally require long and complex processing.
In the meantime, key challenges in the future of energy storage devices reside in designing innovative eco-compatible approaches lowering energetic and environmental impacts of synthesis routes.19,20 We thus herein explore biomineralization pathways in a combined perspective of improving electrochemical properties and using eco-efficient synthesis routes. Although bacteria-mediated reactions have received a deep attention these last ten years as energy providing tools in microbial fuel cells,21 biomineralization reactions have only been very scarcely explored for applications in the field of Li-ion batteries.22–25 To gain a better insight into the possibilities biomineralization might offer for material applications, the natural environment offers a broad source of inspiration. Indeed, a multitude of minerals exhibiting very specific and controlled morphologies, sizes and compositions are produced by living organisms.26 In particular, a wide variety of metal oxides can precipitate at the contact of organic molecules, due to their high affinity for negatively charged surfaces. This property furthered the use of genetically engineered viruses as templates for the nucleation of minerals such as Co3O4,22,25 V2O5,27 or Si.28 Alternatively to these non-autonomous, protein-rich entities (namely viruses) used as templates, bacteria have the ability to both (1) provide negatively charged surfaces that can potentially adsorb metal cations29 and at the same time (2) catalyze redox reactions that can eventually lead to the biomineralization of metal oxides or phosphates from starting solid or dissolved precursors.30,31 From an application point of view, it is also worth noting that large-scale production is much more easily conceivable with bacteria than with viruses and is mastered for many years/decades at the industrial scale.32 Despite these promising properties, bacteria have received a thinner attention than viruses in the field of Li-ion batteries yet. It is noteworthy that two recent studies have reported the nucleation of abiotically synthesized Co-oxides and Sn-oxides at bacterial surfaces for applications in Li-ion batteries taking advantage of the templating property of bacteria.23,24
Different species of bacteria biomineralize Fe-oxides or phosphates with miscellaneous reported morphologies,33e.g. twisted stalks,34 hollow tubes,35 and hollow shells.30 Here, we use Fe-oxyhydroxides produced by an iron-oxidizing bacteria named BoFeN1 for electrochemical applications in Li-ion batteries. This strain couples the reduction of nitrate to the oxidation of dissolved or solid Fe(II) under strictly anoxic conditions at neutral pH,36 leading to the precipitation of Fe(III)-phosphates30,31 or Fe(III)-oxyhydroxides36 depending on the precursors provided and on the composition of the medium. We explore the textural control of Fe-oxides produced through this biomineralization pathway over the electrochemistry of α-Fe2O3 in Li-ion batteries, while presently conducting a similar survey on Fe-phosphates.
Experimental section
Bacterial culture and biomineralization conditions
For bacterial cultivation, all solutions were prepared with sterile mQ water degassed under Ar and manipulations were conducted in an anaerobic chamber under N2 or N2/H2 (95/5) atmosphere (p(O2) < 5 Pa). The nitrate-reducing Fe(II)-oxidizing bacterium Acidovorax sp. strain BoFeN1 was pre-cultured under strictly anoxic conditions (N2/CO2 80/20) at high cellular densities in freshwater mineral medium devoid of Fe prepared after ref. 37 with acetate as a carbon source and nitrate as a terminal electron acceptor. After 2 days, bacteria were harvested by centrifugation (5000g, 15 min) and twice rinsed in 0.6 g L−1 NaCl solution.For biomineralization experiments, a specific medium was prepared. It was composed of NaCl (11.4 mM), Na-acetate (5 mM), NaNO3 (10 mM) and FeCl2 (10 mM), supplemented with vitamins, trace elements and selenite solutions prepared after ref. 37. The pH was adjusted to 7.0 with NaOH solution before inoculation of the pre-cultured bacteria at 50% (v/v). Cultures prepared in 1 L-flasks, closed under N2 atmosphere with a butyl rubber stopper and crimped, were incubated at 30 °C in the dark. Precipitation of an iron-rich orange phase occurred after 24 h to 3 days. Precipitates were collected after 3 days by centrifugation (5000g, 20 min), twice rinsed with mQ water and dried under vacuum.
These bacteria–inorganic composites were subsequently heated at 700 °C in air for 1 h in an alumina crucible to produce bacteria-free α-Fe2O3 samples (hereafter called α-Fe2O3 bacteriomorph, or α-Fe2O3/BoFeN1). For the sake of comparison, abiotic α-Fe2O3 samples were also prepared by heating commercial γ-FeOOH (Sigma Aldrich) at 700 °C for 1 h in air.
Sample characterization
X-ray powder diffraction patterns were recorded on a Bruker D8 Advance diffractometer with a Co-Kα radiation source (λ1 = 1.78897 Å, λ2 = 1.79285 Å) and a Vantec detector.Thermogravimetric analyses coupled with a mass spectrometer were performed between 30 °C and 700 °C under air (dry N2/O2, 80/20%) using a Simultaneous Thermal Analyzer STA 449C Jupiter from Netzsch, and a heating/cooling rate of 10 K min−1. The isothermal drift and sensitivity values are 0.6 μg h−1 and 0.1 μg, respectively. Alumina crucibles were loaded with 10–20 mg of the sample powder. The mass spectrometer is a quadrupole QMS 403 Aëolos® with a stainless steel capillary and a SEV detector (Channeltron). The counting time for the mass spectrometer is 20 ms per m/z value (scanning window: m/z = 1–100 amu) with a resting time of 1 s. DSC experiments were carried out on a Netzsch DSC 204F1 heat flux differential calorimeter at a heating rate of 10 K min−1 under a constant air flow at 200 mL min−1. The crucibles were loaded with 10–15 mg of sample powder. Samples were weighed in aluminum sample pans covered with a pierced lid. An empty aluminum pan with a pierced lid was used as a reference.
The morphology of the samples was observed with a field emission gun scanning electron microscope (FEI Quanta 200F), operating at 20 kV. In order to optimize electron conduction, samples were covered with a thin layer of Pt by metal pulverization. For Transmission Electron Microscopy (TEM) and Selected Area Diffraction (SAED), two types of samples were prepared. (1) Whole bacteria or bacteriomorphs were deposited on a carbon-coated 200-mesh copper grid. TEM observations were performed with a FEG-Tecnai S-Twin F20 operating at 200 kV. (2) Ultrathin sections were prepared by ultramicrotomy. Cells were fixed for 2 h in 1% glutaraldehyde at 4 °C, centrifuged (5000g, 10 min), rinsed three times in 20 mM HEPES (4-(2-HydroxyEthyl)-1-Piperazine Ethane Sulfonic acid) buffer (pH 7.5) for 18 h at 4 °C. They were then post-fixed for 90 min in 1% OsO4 in the same buffer, rinsed three times in distilled water, dehydrated in graded ethanol and propylene oxide-1,2 and progressively embedded in epoxy resin (Epoxy, Fluka Chemica). Ultrathin sections (40 nm–thick) were cut with a LEICA ultramicrotome (EM-UC6). These thin sections were observed either with a JEOL 2100 FEG-TEM operating at 200 kV or with a JEOL 2100 TEM equipped with a LaB6 source, operating at 200 kV. Alternatively, thin sections were observed with a Zeiss Ultra 55 SEM equipped with a field emission gun in back-scattered electron mode at an operating voltage of 10 kV.
Specific surface areas and pore size/volume distribution were computed from the results of N2 physisorption at 77 K (Micromeritics ASAP 2020, Gemini 2375) using the BET (Brunauer–Emmett–Teller)38 and BJH (Barrett–Joyner–Halenda)39 formalisms, respectively. Prior to porosity analysis, samples were treated at 4 Pa and 100 °C for 4 h.
Electrochemical analyses
Eletrochemical analyses were performed using Swagelok-type cells. The Active Material (AM) consisted of either heat-treated biomineralized products or abiotic α-Fe2O3. Positive electrodes were prepared by mixing AM with Super-P (SP) carbon (10 to 30 wt%) as follows: powders were dispersed in cyclohexane for 40 min (20 min under magnetic stirring + 20 min under ultrasonication) and cyclohexane was then let evaporate at ambient temperature under a fume hood.40,41 This gentle mixed process enables the preservation of the hollow texture. Alternatively, as-prepared AM–SP mixtures were thoroughly hand-milled in order to destroy the oxide hollow shell texture.The cells were assembled in an argon-filled glove box. 6–8 mg of the binder-free AM–SP mixture was separated from the negative electrode (lithium foil) by 2 glass fiber disks, the whole setup being soaked in a LiPF6 (1 M) solution of ethylene carbonate (EC)–dimethylcarbonate (DMC) mixture (1/1 w/w) (LP30, Merck). Two stainless steel current collectors were used at the positive and negative sides. Galvanostatic cycling tests were conducted at room temperature in the 3.2–0.05 V range at discharge/charge rates typically ranging from 1 Li per formula unit in 10 h (noted C/10) to 1 Li per formula unit in 1 h (noted C), with a MacPile (Claix, France) controller. For power rate determination (VMP, Biologic, Claix, France), cells were first slowly discharged (1 Li per 20 h) down to 0 volt and then charged up to 3.0 volts while sequentially decreasing the applied current (10 C to C/100). Specific capacities are all reported per gram of Fe2O3–carbon mixture. All electrochemical tests were at least repeated twice to assess repeatability.
Results and discussion
Biomineralization of α-Fe2O3
BoFeN1 is a rod-shaped bacterium with dimensions ranging from 1 to 2 μm in length and around 200 nm in diameter. Its aspect ratio is thus about 5 to 10. Under strictly anoxic conditions and at neutral pH, it was shown that this bacterium promotes the oxidation of dissolved Fe(II) using nitrate as an electron acceptor, eventually leading to the precipitation of Fe(III) oxides or phosphates depending on the chemical composition of the medium.30,31,36 Moreover, these precipitates are specifically localized within the cell wall of the bacteria.42,43 Being a Gram-negative bacterium, BoFeN1 has a cell wall composed of two lipidic bilayers (outer membrane on the extracellular medium face and plasmic membrane on the cytoplasmic face) enclosing a 40 nm–thick periplasmic space (Fig. 1A). Within this inter-membranous space, a thin layer of peptidoglycan (cross-linked proteins and carbohydrates) plays – among others – a role in maintaining the cell wall structure and rigidity. Additionally, different types of macromolecules, namely lipopolysaccharides, lipoproteins and porins, are included within the cell wall.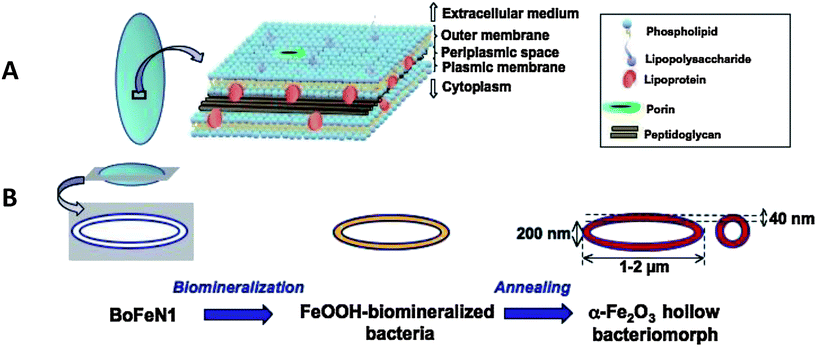 | ||
| Fig. 1 Schematic illustration of the organization of BoFeN1 cell wall (A) and of the synthesis route we used for the production of α-Fe2O3 hollow bacteriomorphs (B). | ||
A recap of the biomineralization process scheme we used is depicted in Fig. 1B. Under the culture conditions applied in the present study, iron biomineralization promoted by BoFeN1 metabolic activity was completed after 3 days, leading to the almost complete consumption of dissolved Fe ([Fe2+] < 2 mmol L−1i.e. yield > 80%) and to the formation of an orange precipitate, consisting of lepidocrocite (γ-FeOOH), as indicated by XRD analysis (Fig. 2). This material was subsequently annealed at 700 °C for 1 h under air to transform into α-Fe2O3 (Fig. 2).
Thermal analyses of BoFeN1/γ-FeOOH assemblies up to 700 °C show an overall weight loss of 24% (Fig. 3). This indicates an initial FeOOH weight content of about 84%, assuming that organic matter is fully transformed into gaseous products (H2O, COx, NOx, SOx…) at this temperature. The initial sloppy weight loss, from RT to ca. 200 °C, is due to the slow departure of adsorbed and physiologic water. Then the sharp loss centered at about 250 °C, accounting for most of the overall loss, can be ascribed to the combination of two phenomena: (1) the combustion of the bacterial organic matter mainly leading to the release of CO2/H2O (see MS data in Fig. 2 and 3) and (2) the dehydration of γ-FeOOH into iron oxide,44 both being irreversible exothermic (see DSC data in Fig. 3) phenomena. The formation of an intermediate magnetic iron oxide (γ-Fe2O3 or Fe3O4) is supported by preliminary observations showing that a BoFeN1/FeOOH assembly air-treated at 400 °C is attracted by a magnetic rod. This intermediate phase most probably consists of nanometric domains given that its XRD pattern looks amorphous (data not shown). The exact nature and properties of this phase will be the topic of a forthcoming study, but preliminary electrochemical tests indicate the signature of magnetite. The subsequent irreversible transformation of maghemite (γ-Fe2O3)/magnetite (Fe3O4) to hematite is known to occur at temperatures below 600 °C, which is consistent with the XRD analysis of the 700 °C-heated sample (Fig. 2), supported by an exothermic DSC signal at 500–550 °C (Fig. 3). As we suspect this hematite sample could result from air-oxidation of FeII/FeIII magnetite, we checked the iron valence state in α-Fe2O3 bacteriomorphs by Mössbauer effect analysis (data not shown). Only ferric species could be detected, ruling out the presence of a large amount of residual ferrous ions in our samples. Consistently, the crystallographic cell parameters of hematite in α-Fe2O3 bacteriomorphs (a = 5.038(3) Å and c = 13.760(1) Å) perfectly match the values reported for a synthetic and stoichiometric hematite reference (a = 5.0356(1) Å and c = 13.7489(7) Å – JCPDS 33-0664). This is an additional clue for a stoichiometric hematite here produced from BoFeN1/γ-FeOOH assemblies.
Fine control of α-Fe2O3 bacteriomorph texture
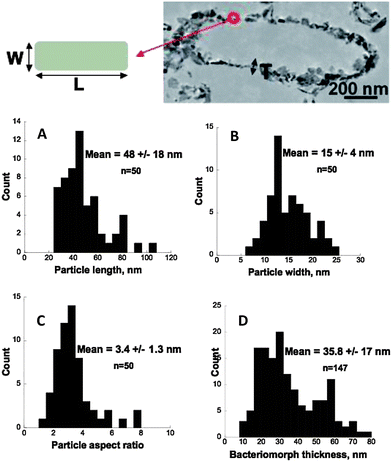
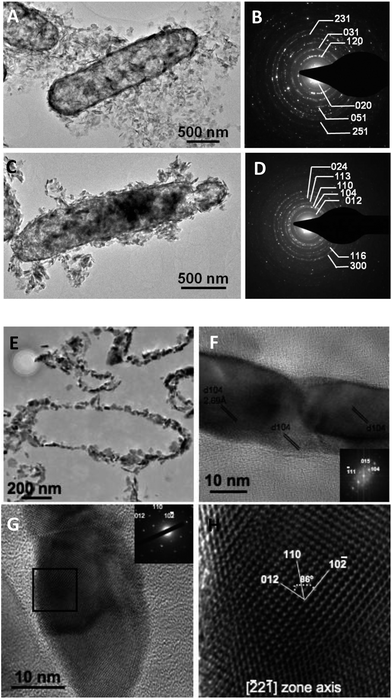
The morphology of the materials formed through this biomineralization process was investigated by electron microscopy (Fig. 4 and 5). SAED analyses confirmed the nature of the mineral phases at the nanometer-scale in both samples (pre- and post-heated mineralized cells) (Fig. 5B and D). γ-FeOOH minerals encrusted the bacteria leading to rod-shaped shells having the same size aspect ratio as bacterial cells. Based on SEM observations (Fig. 4A) of some scarce broken cells and on TEM imaging (Fig. 5A), the minerals are mainly distributed within the bacterial cell wall, i.e. within the periplasmic space, and partly at the cell surface, which is consistent with previous reports.30 Strikingly the texture of these bio-mineralized products, namely the cellular shape and the cell wall thickness, remained preserved after air-heating at 700 °C (Fig. 4B/C and 5C and E) leading to an assemblage of empty shells, providing an alveolar material. These shells consisted of an assemblage of α-Fe2O3 single crystals (Fig. 5F–H). Nitrogen adsorption data (Fig. 6) evidence a mesoporous signature for this powder, with a maximum porous volume detected at around 30 nm in pore diameter, thus corresponding to the in-wall porosity located between the nanometric oxide grains (Fig. 5E). Accordingly, the thickness of the bacteriomorph wall was estimated to be 36 ± 17 nm (n = 147) (Fig. 7D). In addition, the specific surface area of the bacteriomorphs is preserved upon heating, with 33 m2 g−1 before (γ-FeOOH/bacteria assembly) and 31 m2 g−1 after annealing (α-Fe2O3 bacteriomorphs), thus indicating the preservation of particles size and micrometer-scale texture. | ||
| Fig. 6 (A) Nitrogen (77 K) adsorption/desorption isotherm curve for α-Fe2O3 bacteriomorphs and (B) porous volume distribution as a function of pore size computed with the BJH method. | ||
Interestingly, as exemplified by HRTEM analyses (Fig. 7), the α-Fe2O3 grains are strongly anisotropic with a mean size of 48 ± 18 nm (n = 50) in length (L) and 15 ± 4 nm (n = 50) in width (W), i.e. a mean aspect ratio (L/W) of 3.4 ± 1.3 (n = 50). Moreover, the particle width is very constrained (variance = 17) compared to the more largely distributed particle length (variance = 354), suggesting a strong control of the periplasmic space over crystalline growth. Fig. 5F displays three adjacent particles grown in the periplasmic space. Fourier transforms obtained on any of these three adjacent particles exhibit the same pattern, indicating a similar crystallographic orientation. Hence, the dispersed particle length could be related to the fact that adjacent particles having a mean length of around ∼30–40 nm coalesce into ∼60 or ∼90 nm long assemblages exhibiting the same crystallographic orientation (Fig. 5F). Eventually, the particle width deduced from these (HR)TEM observations is consistent with the mean crystallite size estimated to be 20 nm from the FWHM (Full Width at Half Maximum) of the XRD peaks using the Scherrer equation.45
Thus, this biomineralization route provides α-Fe2O3 bacteriomorphs, with a controlled size/texture: 1 to 2 μm long, 200 nm in diameter and ∼40 nm–thick shells composed of 15 to 20 nm wide particles of α-Fe2O3, combining nm-scale (within the bacteriomorph wall) and micrometer-scale (the empty shell) porosities. The present synthesis process notably differs from previously reported bacteria-mediated synthesis routes of metal oxides in that the cell wall of these Gram-negative bacteria intrinsically controls the texture of the α-Fe2O3 assembly. This contrasts with the Gram-positive bacteria Bacillus subtilis, previously used as a template for the synthesis of Co3O423 or SnO2 nanorods,24 which exhibits neither outer membrane nor periplasmic space to confine the mineralization, thus the need in these studies to control the mineral layer thickness through chemical conditions. Moreover, in the present study, BoFeN1 controls and drives the precipitation of iron towards γ-FeOOH, not only serving as a template but also controlling the mineralogy of the end-product of biomineralization through its metabolism. In fine, this bacterium appears as a unique tool to both promote and control Fe-oxide mineralization, size and texture.
Such a specific texture delineating the bacterial shape has been reported for a variety of bacteria and minerals. Given that bacterial cell walls expose negatively charged groups at their surface, interactions with metals leading to their adsorption at the cell surface have been described among Gram-positive46,47 and Gram-negative29 bacteria, even with non-metabolizing cells.48,49 Besides, metals can accumulate within the periplasm50 and a variety of biominerals surrounding or filling the cell wall of Gram-negative bacteria have been reported: e.g. phosphates,30,31,43,51,52 oxides53,54 or sulfides.55 Such minerals might thus produce bacteriomorphs with some similarities to those reported here.
Electrochemical performances of α-Fe2O3 bacteriomorphs
Aside from their outstanding theoretical capacity advantages, conversion reactions were found to suffer among others from large first cycle capacity loss as well as from limited rate capability, both of which were found to be extremely sensitive to the texture of the electrode. Thus, to identify the positive attributes that such hollow rod-type α-Fe2O3 bacteriomorphs could have as electrode materials, Li/α-Fe2O3 Swagelok cells were assembled.To prepare an electro-active material for Li-ion batteries and enhance electronic conductivity, α-Fe2O3 bacteriomorphs were mixed with SP-carbon by magnetic stirring and mild ultrasonication in cyclohexane. This method provided a homogeneous mixture while preserving bacterial texture (Fig. 4D). In contrast, preparation of SP-carbon–α-Fe2O3 bacteriomorph mixtures by grinding in a mortar completely dislocated the initial texture leading to isolated nanometric particles in a carbon matrix (referred to as “untextured α-Fe2O3” in the following, Fig. 4E). An abiotic α-Fe2O3 electrode was also prepared by hand grinding SP carbon with a α-Fe2O3 sample made of micrometric aggregates of 20 nm particles (Fig. 4F).
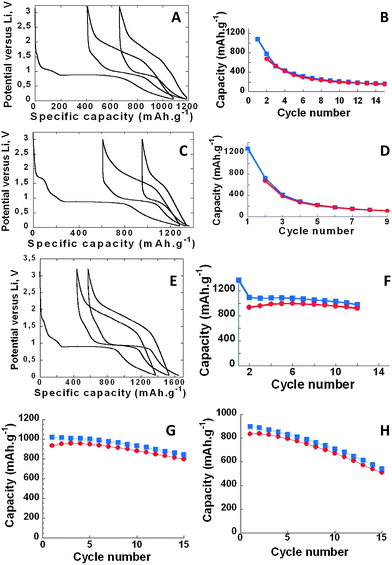
The electrochemical performances of these electrodes are compared in Fig. 8. They all exhibit a large polarization together with large first cycle irreversibility. This polarization was found to be roughly the same for all our samples (0.6 to 0.7 volts), as measured at half (dis)charge on the second cycle. This is not to be unexpected because (i) the hysteresis associated with conversion reactions is now well documented to be intrinsically controlled by the thermodynamics of the reaction itself,56 and thus unaffected by the initial particle organization, and (ii) the large amount of conductive carbon added (30 wt%) mitigates the difference in kinetic limitations between our different samples, hence very similar polarization under current presently observed. The first cycle irreversibility is the greatest for the untextured bacteria electrode material, and initial discharge capacities ranging from 1089 to 1370 mA h g−1, which exceeds the theoretical capacity of 1000 mA h g−1. This extra-capacity, as previously observed, might simply be ascribed to the electrochemical decomposition of the electrolyte during the discharge. Whatever the samples, the first discharge profiles show four different regions which enlist (i) a hint near 1.7 V, (ii) a signature from 1.1 to 0.9 V corresponding to the reaction of Li with SP carbon, (iii) a long plateau located near 0.9 V reminiscent of the aforementioned conversion reaction and finally (iv) a smooth and continuous decay of the voltage from 1 to 0 V whose origin is associated with either faradic or capacitive phenomena.
It is quite obvious that such an aerated textural organization will come with a low volumetric capacity. Assuming a monodisperse population of hematite bacteriomorphs having 1.5 μm in length, 0.2 μm in diameter, and a 40 nm–thick oxide wall, we could compute their apparent density to be around 0.9 g cm−3. If one considers a gravimetric capacity of around 1000 mA h g−1, the corresponding volumetric capacity should be around 900 mA h cm−3, i.e. very close to that of graphite.
The high voltage activity (1.7 V) can be attributed to lithium insertion within the α-Fe2O3 structure.8 It reaches 0.8 Li per formula unit in untextured α-Fe2O3 (isolated nanometric particles, Fig. 8C), which is close to the maximal reported (1 Li per formula unit).8 This number decreases to 0.4 Li per formula unit in α-Fe2O3 bacteriomorphs (micrometric aggregates of nanometric particles, Fig. 8E) and eventually to almost 0 Li in abiotic α-Fe2O3 (pluri-micrometric aggregates of nanometric particles, Fig. 8A). Thus, consistently with previous reports,8 larger insertion ranges (i.e. higher x values in LixFe2O3) are obtained with isolated nanometric particles than with micrometric or massively agglomerated powders.
The most striking differences between the various samples reside in their capacity retention (Fig. 8B, D and F). When cycled at C/10 rate (1 Li in 10 h), only 18% and 8% of the initial capacity are retained after 10 cycles for the abiotic α-Fe2O3 (Fig. 8B) and the untextured bacterial α-Fe2O3 (Fig. 8D), respectively. In contrast, under the same cycling conditions, α-Fe2O3 bacteriomorphs exhibit a largely improved capacity retention (Fig. 8F), with 91% of the initial capacity still maintained after 10 cycles.
The capacity retention of α-Fe2O3 bacteriomorphs was further tested at a higher rate (1 Li per hour, Fig. 8G) and for lower SP contents (10 wt%, Fig. 8H). Both experiments still led to better cycling efficiencies than abiotic (Fig. 8B) and untextured samples (Fig. 8D), even though capacity decayed faster under these modified conditions. So, we conducted additional tests in order to (1) evaluate the rate capability of these α-Fe2O3 bacteriomorph samples and (2) find the possible origin of this decrease in capacity.
Power rate experiments (performed with 10 wt% SP) (Fig. 9) evidence the outstanding behavior of α-Fe2O3 bacteriomorphs (more than 70% of the capacity maintained from C/100 to 10 C), when compared to the untextured sample (less than 20% of the capacity remaining from C/100 to 2 C). This gives evidence of the high rate capability of these α-Fe2O3 bacteriomorphs being supported by their texture. Accordingly, the evolution of this texture was followed by SEM as a function of capacity loss (Fig. 10). After one cycle, the bacteriomorph texture was perfectly preserved (Fig. 10A and B). Then, as the capacity fell towards 40% of the initial capacity, some bacteriomorphs were still recognizable (Fig. 10C), but a large proportion of the electrode was transformed into isolated and divided particles (Fig. 10D). Finally, an almost complete loss of the initial capacity (89%) was associated with an almost complete loss of texture of the bacteriomorphs, which turned into isolated nanometer-scale particles embedded into a matrix most probably consisting of electrolyte decomposition products (Fig. 10E and F). Only very few bacteriomorphs could be barely observed in this sample. These results definitively demonstrate the major impact of the texture on the capacity retention of these electrodes.
 | ||
| Fig. 9 Power rate (1st charge capacity as a function of the C rate) for Li/α-Fe2O3 bacteriomorphs and Li/untextured α-Fe2O3. 20 °C, 10 wt% SP. | ||
Overall, these results fall within the best obtained to date with α-Fe2O3. Remarkably, the active material used in the present study was neither carbon-coated nor mixed with a binder. Enhanced capacity retention and high rate capability can thus be exclusively attributed to the specific texture of α-Fe2O3 bacteriomorphs and this might be explained as follows. Nanoparticles are known to shorten the Li diffusion paths but their assembly at the micrometer-scale, as described in α-Fe2O3 bacteriomorphs, is essential as it enables to self-preserve the electrode texture upon cycling as exemplified in Fig. 10. In contrast to a dense agglomerate of nanoparticles, the present alveolar texture obviously contains much less particles, and thus can more easily accommodate the volume changes inherent to the full Li/hematite reaction, preventing the material from dislocation and electrochemically impairing particle isolation. Aside from this mechanical connectivity, the hollow shape of the bacteriomorphs is also a serious asset for rate performances. Indeed, the micrometer-scale porosity (Fig. 4C and 5E) of this alveolar material combined with its nanometer-scale porosity (Fig. 6) enables and facilitates the electrolyte penetration, hence minimizing the Li+ ion traveling distance between the active material and the electrolyte, but meanwhile also preserving the particles interconnectivity and the conducting network. This differs completely from the micrometric aggregates of nanometric particles in the abiotic sample or the dislocated bacteriomorphs that lack micrometric porosity and stable particle connectivity network and exhibit both poor rate performances and capacity retention.
Biomineralization pathways: alternative promising synthesis routes for electro-active compounds
Biomineralization has received an increasing attention, but was mainly kept aside from the Li-ion battery community with a few exceptions. Apart from the very few studies using bacteria,23,24 most of the previous reports focused on the use of viruses as genetically modifiable templates for controlled material assembly at the nanometer scale (see Belcher's group publications). In contrast to bacteria, viruses are non-autonomous entities that take over the genetic machinery of a host to reproduce. On the one hand, despite the strengths of the viral synthesis methods, this approach suffers from drawbacks (a very small yield combined with a heavy protocol of synthesis) preventing from any hope of industrial application in the near future.25 On the other hand, bacterial biomineralization routes, such as the one proposed in the present study, offer some benefits regarding energetic efficiency, applicability and cost issues: (1) compared to the viral approach, synthesis yields can be maximized. In the present study, 80 to 100% of dissolved Fe(II) precipitated as FeOOH at or within the cell wall and was eventually converted to α-Fe2O3. This yield could, in the future, be optimized by maximizing the extracellular to periplasmic precipitate ratio. (2) Bacterial biomineralization operates at low temperature, and can thus be considered as an eco-efficient route. Here, FeOOH was biomineralized at 30 °C, and only a short annealing step at a high temperature was required to form electroactive α-Fe2O3. By comparison, most of the previously reported methods for the synthesis of α-Fe2O3 with a controlled texture required high temperature or costly methods. In the future, the diversity of bacterial enzymes that might be used as catalysts in reactions not requiring as high temperature as under abiotic conditions opens multiple new avenues to be explored. (3) Bacteria-based routes offer an ease of synthesis compared to abiotic methods (carbon nanotubes, deposition of particles onto substrates, electrospining,…). Moreover, since bacteria can be sub-cultured continuously and their medium at least partly recycled, industrial up-scaling is conceivable. Bacteria are already used for industrial applications in many fields (e.g. materials science, medicine, and food industries). (4) Last but not least, the present study enlightens the potential high electrochemical performances of biomineralized products in relation with their controlled texture that at least equals the performances of abiotically synthesized α-Fe2O3.The wide diversity of bacterial metabolisms, biomineralization pathways and bio-controlled textures existing in nature are thus to be explored for applications in Li-ion batteries and other forthcoming electrochemical storage technologies. Biomineralization pathways have been classified into three different (overlapping) categories,57 which might be assigned to different levels of control: (1) controlled biomineralization, i.e. genetically controlled mineralization providing minerals with a perfectly constrained shape, composition and location, e.g. magnetites produced by magnetotactic bacteria;58 (2) induced biomineralization, i.e. resulting from medium chemistry modification by bacteria and (3) influenced biomineralization, i.e. passive mineralization of and on organic matter acting as a template (for electrochemical applications, see ref. 23 and 24). The present study relies on the last two levels. Within each of these three classes, a wide diversity of minerals with various compositions and morphologies might be formed by a variety of bacteria, under diverse chemical conditions. So, beyond iron oxides, the bacterial synthesis of other metal oxides, phosphates, sulfides, etc. with specific textures remains to be explored for Li-ion battery applications.
Conclusions
In summary, bacterial biomineralization of γ-FeOOH using the Gram-negative iron oxidizing BoFeN1 strain was successfully used to prepare α-Fe2O3 bacteriomorphs. These minerals exhibited a controlled texture, consisting of micrometric (1–2 × 0.2 μm) 40 nm–thick shells, formed by an assembly of nanometer-scale particles. This alveolar texture accounts for an enhanced capacity retention and rate capability (70% of the initial capacity recovered at 10 C), which favorably compared with the best performances achieved so far with 3D-Fe3O4 conversion electrodes.12 Our results suggest a direct control of texture over the performances of this biomineralized material. Although such a biomineralization approach has proved to be successful in preparing binary oxides, we are working towards preparing alkali-based 3d-metal oxides, which are essential for direct use in Li-ion batteries.Acknowledgements
The authors want to thank Matthieu Courty (LRCS) for thermal and mass spectrometry analyses, and Moulay Sougrati (UM2, RS2E) for Mössbauer Effect Analysis. They also thank Philippe Poizot (IMN-CNRS-Nantes), Michel Armand (CNRS), Olivier Wattraint (GEC-CNRS-UPJV) and Isabelle Gosselin (GEC-CNRS-UPJV) for helpful discussions. They are also indebted to Laurent Gutierrez and Stéphanie Guénin for technical assistance and for providing access to the Plateforme de Biologie Moléculaire CRRBM (Amiens, UPJV). This study was funded by RS2E.References
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacin, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS PubMed.
- M. M. Thackeray, W. I. F. David and J. B. Goodenough, Mater. Res. Bull., 1982, 17, 785 CrossRef CAS.
- M. M. Thackeray, W. I. F. David and J. B. Goodenough, J. Solid State Chem., 1984, 55, 280 CrossRef CAS.
- N. A. Godshall, I. D. Raistrick and R. A. Huggins, Mater. Res. Bull., 1980, 15, 561–568 CrossRef CAS.
- B. D. Pietro, M. Patriarca and B. Scrosati, J. Power Sources, 1982, 8, 289–299 CrossRef.
- J. Li, H. M. Dahn, L. J. Krause, D.-B. Le and J. R. Dahn, J. Electrochem. Soc., 2008, 155, A812–A816 CrossRef CAS PubMed.
- D. Larcher, C. Masquelier, D. Bonnin, Y. Chabre, V. Masson, J.-B. Leriche and J.-M. Tarascon, J. Electrochem. Soc., 2003, 150, A133 CrossRef CAS PubMed.
- M. V. Reddy, T. Yu, C.-H. Sow, Z. Xiang Shen, C. Teck Lim, G. V. Subba Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2792–2799 CrossRef CAS.
- P. C. Wang, H. P. Ding, T. Bark and C. H. Chen, Electrochim. Acta, 2007, 52, 6650–6655 CrossRef CAS PubMed.
- T. Yoon, C. Chae, Y. K. Sun, X. Zhao, H. H. Kung and J. K. Lee, J. Mater. Chem., 2011, 21(43), 17325–17330 RSC.
- L. Taberna, S. Mitra, P. Poizot, P. Simon and J.-M. Tarascon, Nat. Mater., 2006, 5(7), 567–573 CrossRef PubMed.
- Z. Liu and S. Tay, Mater. Lett., 2012, 72, 74–77 CrossRef CAS PubMed.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. W. (David) Lou, Energy Environ. Sci., 2012, 5, 5252 CAS.
- C. T. Cherian, J. Sundaramurthy, M. Kalaivani, P. Ragupathy and P. S. Kumar, et al. , J. Mater. Chem., 2012, 22, 12198 RSC.
- L. Ji, O. Topracki, M. Alcoutlabi, Y. Yao, Y. Li, S. Zhang, B. Guo, Z. Lin and X. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 2672–2679 CAS.
- J. Chen, L. Xu, W. Li and X. Gou, Adv. Mater., 2005, 17(5), 582 CrossRef CAS.
- M. Zhang, B. Qu, D. Lei, Y. Chen, X. Yu, L. Chen, Q. Li, Y. Wang and T. Wang, J. Mater. Chem., 2012, 22, 3868 RSC.
- J.-M. Tarascon, Philos. Trans. R. Soc., A, 2011, 368, 3227–3241 CrossRef PubMed.
- N. Recham, M. Armand and J.-M. Tarascon, C. R. Chim., 2010, 13, 106–116 CrossRef CAS PubMed.
- D. Lovley, Curr. Opin. Biotechnol., 2006, 17, 327–332 CrossRef CAS PubMed.
- K. T. Nam, D. Kim, P. J. Yoo, C. Chiang, N. Meethong, P. T. Hammond, Y. Chiang and A. M. Belcher, Science, 2006, 312, 885–888 CrossRef CAS PubMed.
- H. Shim, Y. Jin, S. Seo, S. Lee and S. Kim, ACS Nano, 2011, 5(1), 443–449 CrossRef CAS PubMed.
- A. Lim, H. Shim, S. Seo, G. Lee, K. Park and D. Kim, Nanoscale, 2012, 4, 4694–4701 RSC.
- C. Rosant, B. Avalle, D. Larcher, L. Dupont, A. Friboulet and J.-M. Tarascon, Energy Environ. Sci., 2012, 5(12), 9936–9943 CAS.
- K. Benzerara and J. Miot, in Origins and Evolution of Life, Cambridge University Press, 2010 Search PubMed.
- E. Pomerantseva, K. Gerasopoulos and X. Chen, et al. , J. Power Sources, 2012, 206, 282–287 CrossRef CAS PubMed.
- X. Chen, K. Gerasopoulos and J. Guo, et al. , ACS Nano, 2010, 4(9), 5366–5372 CrossRef CAS PubMed.
- T. J. Beveridge and S. F. Koval, Appl. Environ. Microbiol., 1981, 42, 325–335 CAS.
- J. Miot, K. Benzerara, G. Morin, A. Kappler, S. Bernard, M. Obst, C. Férard, F. Skouri-Panet, J.-M. Guigner, N. Posth, M. Galvez, G.-E. Brown Jr and F. Guyot, Geochim. Cosmochim. Acta, 2009, 73, 696–711 CrossRef CAS PubMed.
- J. Miot, K. Benzerara, G. Morin, S. Bernard, O. Beyssac, E. Larquet, A. Kappler and F. Guyot, Geobiology, 2009, 7, 373–384 CrossRef CAS PubMed.
- C. Sano, Am. J. Clin. Nutr., 2009, 90, 728S–732S CrossRef CAS PubMed.
- K. O. Konhauser, Earth-Sci. Rev., 1998, 43, 91–121 CrossRef CAS.
- C. S. Chan, S. C. Fakra, D. Emerson, E. J. Fleming and K. J. Edwards, ISME J., 2011, 5, 717–727 CrossRef CAS PubMed.
- T. Suzuki, H. Hashimoto, H. Ishihara, T. Kasai, H. Kunoh and J. Takada, Appl. Environ. Microbiol., 2011, 77(21), 7873–7875 CrossRef CAS PubMed.
- A. Kappler, B. Schink and D. K. Newman, Geobiology, 2005, 3, 235–245 CrossRef CAS.
- A. Ehrenreich and F. Widdel, Appl. Environ. Microbiol., 1994, 60, 4517–4526 CAS.
- S. Brunauer, P. H. Emmet and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- E. Barrett, L. Joyner and P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef CAS.
- G. Sudant, E. Baudrin, B. Dunn and J. M. Tarascon, J. Electrochem. Soc., 2004, 151(5), A666–A671 CrossRef CAS PubMed.
- G. Binotto, D. Larcher, A. S. Prakash, R. Herrera Urbina, M. S. Hegde and J.-M. Tarascon, Chem. Mater., 2007, 19, 3032–3040 CrossRef CAS.
- S. Schädler, C. Burkhardt, F. Hegler, K. L. Straub, J. Miot, K. Benzerara and A. Kappler, Geomicrobiol. J., 2009, 26, 93–103 CrossRef.
- J. Miot, K. MacLellan, K. Benzerara and N. Boisset, Geobiology, 2011, 9, 459–470 CrossRef CAS PubMed.
- Y. Cudennec and A. Lecerf, Solid State Sci., 2005, 7, 520–529 CrossRef CAS PubMed.
- P. Scherrer, Nachr. Ges. Wiss. Goettingen, Math.-Phys. Kl., 1919, 2, 98 Search PubMed.
- T. J. Beveridge and R. G. E. Murray, J. Bacteriol., 1976, 127, 1502–1518 CAS.
- J. B. Fein, C. J. Daughney, N. Yee and T. A. Davis, Geochim. Cosmochim. Acta, 1997, 61, 3319–3328 CrossRef CAS.
- D. G. Rancourt, P. J. Thibault, D. Mavrocordatos and G. Lamarche, Geochim. Cosmochim. Acta, 2005, 69, 553–577 CrossRef CAS PubMed.
- M. Fakih, X. Chatellier, M. Davranche and A. Dia, Environ. Sci. Technol., 2008, 42, 3194–3200 CrossRef CAS.
- F. Goulhen, A. Gloter, F. Guyot and M. Bruschi, Appl. Microbiol. Biotechnol., 2006, 71, 892–897 CrossRef CAS PubMed.
- K. Benzerara, N. Menguy, F. Guyot, F. Skouri, G. de Luca, M. Barakat and T. Heulin, Earth Planet. Sci. Lett., 2004, 228, 439–449 CrossRef CAS PubMed.
- S. Dunham-Cheatham, X. Rui, B. Bunker, N. Menguy, R. Hellmann and J. Fein, Geochim. Cosmochim. Acta, 2011, 75, 2828–2847 CrossRef CAS PubMed.
- K. Benzerara, G. Morin, T. H. Yoon, J. Miot, T. Tyliszczak, C. Casiot, O. Bruneel, F. Frages and G.-E. Brown Jr, Geochim. Cosmochim. Acta, 2008, 72, 3949–3963 CrossRef CAS PubMed.
- A. Gloter, M. Zbinden, F. Guyot, F. Gaill and C. Colliex, Earth Planet. Sci. Lett., 2004, 222, 947–957 CrossRef CAS PubMed.
- R. Donald and G. Southam, Geochim. Cosmochim. Acta, 1999, 63, 2019–2023 CrossRef CAS.
- R. Khatib, A.-L. Dalverny, M. Saubanère, M. Gaberscek and M.-L. Doublet, J. Phys. Chem. C, 2013, 117(2), 837 CAS.
- C. Dupraz, R. P. Reid, O. Braissant, A. W. Decho, R. S. Norman and P. T. Visscher, Earth-Sci. Rev., 2009, 96, 141–162 CrossRef CAS PubMed.
- R. P. Blakemore, Science, 1975, 190, 377–379 CrossRef CAS.
Footnote |
| † Now at Laboratoire de Minéralogie et Cosmochimie du Muséum, Muséum National d'Histoire Naturelle, 61 rue Buffon, 75005 Paris, France. |
| This journal is © The Royal Society of Chemistry 2014 |




![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 2
2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] zone axis (H) of the area framed in (G).
] zone axis (H) of the area framed in (G).