Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[Cu(bpy)(P^P)]+ containing light-emitting electrochemical cells: improving performance through simple substitution†
Sarah
Keller
a,
Edwin C.
Constable
*a,
Catherine E.
Housecroft
a,
Markus
Neuburger
a,
Alessandro
Prescimone
a,
Giulia
Longo
b,
Antonio
Pertegás
b,
Michele
Sessolo
b and
Henk J.
Bolink
*bc
aDepartment of Chemistry, University of Basel, Spitalstrasse 51, CH4056 Basel, Switzerland. E-mail: edwin.constable@unibas.ch; catherine.housecroft@unibas.ch; Tel: +41 61 267 1008
bInstituto de Ciencia Molecular, Universidad de Valencia, Catedrático José Beltrán 2, Paterna, E-46980, Spain. E-mail: henk.bolink@uv.es
cFundació General de la Universitat de Valencia (FGUV), PO Box 22085, Valencia, Spain
First published on 8th October 2014
Abstract
Light-emitting electrochemical cells (LECs) containing [Cu(POP)(N^N)][PF6] (POP = bis(2-diphenylphosphinophenyl)ether, N^N = 6-methyl- or 6,6′-dimethyl-2,2′-bipyridine) exhibit luminance and efficiency surpassing previous copper(I)-containing LECs.
Next-generation efficient lighting devices must exhibit reduced thermal energy loss, increased lifetimes and utilize sustainable materials. Light-emitting electrochemical cells (LECs) are an emerging technology and, like organic light-emitting diodes (OLEDs), dissipate only a very limited amount of energy as heat.1 Unlike OLEDs, LECs feature a simple architecture and do not require rigorous sealing to maintain an oxygen-free environment. The emissive component of a LEC is a conjugated light-emitting polymer blended with a salt or an ionic transition metal complex (iTMC), typically an iridium or ruthenium compound.1 We2 and others3,4 have demonstrated the use of Earth-abundant copper with LECs incorporating emissive [Cu(N^N)(P^P)]+ or [Cu(P^P)2]+ complexes (N^N = chelating ligand with a bpy or phen metal-binding domain; P^P = chelating bis(phosphino) ligand). Sterically demanding P^P ligands lead to enhanced emission,1 and the complexes [Cu(N^N)(POP)]+ and [Cu(N^N)(xantphos)]+ (xantphos = 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene) complexes are particularly interesting.5–13 Following from our finding that [Cu(POP)(bpy)]+ shows encouraging LEC performance, we now demonstrate that simple structural modification of the bpy ligand leads to improved luminance and efficiency, and compare the emission behaviours of LECs containing [Cu(POP)(6-Mebpy)][PF6] and [Cu(POP)(6,6′-Me2bpy)][PF6] (6-Mebpy = 6-methyl-2,2′-bipyridine, 6,6′-Me2bpy = 6,6′-dimethyl-2,2′-bipyridine). McMillin, Walton and coworkers have previously demonstrated that increased substitution in the 2,9-positions of 1,10-phenanthroline (phen) results in significantly enhanced emission behaviour of [Cu(POP)(phen)]+-based complexes.14
[Cu(POP)(6-Mebpy)][PF6] (Scheme 1) was prepared† by sequential treatment2 of [Cu(MeCN)4][PF6] with POP and 6-Mebpy. The base peak (m/z 771.5) in the electrospray mass spectrum arose from [Cu(POP)(6-Mebpy)]+. NMR spectra were recorded in CD2Cl2, avoiding MeCN which leads to the formation of [Cu(POP)(MeCN)]+.15 The 31P{1H} NMR spectrum of [Cu(POP)(6-Mebpy)][PF6] shows a septet at ∂ −144.5 ppm ([PF6]−) and a signal at ∂ −12.4 ppm (POP). 1H and 13C NMR spectra were assigned by COSY, HMQC, HMBC and NOESY methods. The 13C NMR spectrum shows one set of signals for the C rings (Scheme 1) confirming that this unit is C2-symmetric in solution. The Me group desymmetrizes each PPh2 unit; one Ph group points towards the Me group, while the other is directed away; this is seen in the 13C NMR spectrum with two signals for each of CD2, CD3 and CD4; CD1 gives one triplet (JPC = 17.1 Hz). Fig. S1† shows the aromatic region of the 1H NMR spectrum. Distinction between the two pairs of D rings is especially well defined for the ortho-protons (labelled HD2 and HD2′ in Fig. S1†).
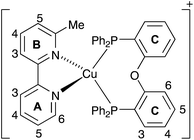 | ||
| Scheme 1 Structure of [Cu(POP)(6-Mebpy)]+ with atom numbering for NMR spectroscopic assignment. Ph rings in PPh2 units are labelled D and D′ (see text). | ||
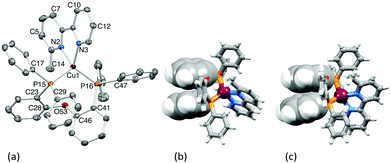
[Cu(POP)(6,6′-Me2bpy)][BF4] has been reported,11 but for comparison with [Cu(POP)(6-Mebpy)][PF6], we report here [Cu(POP)(6,6′-Me2bpy)][PF6].† X-Ray quality crystals of both [PF6]– salts were obtained.† Heavily disordered solvent in the lattice of [Cu(POP)(6-Mebpy)][PF6] necessitated the use of SQUEEZE.16Fig. 1a and S2† show the structures of the [Cu(POP)(6-Mebpy)]+ and [Cu(POP)(6,6′-Me2bpy)]+ cations. In both, atom Cu1 is in a distorted tetrahedral environment. The 6-Mebpy ligand is disordered and modelled over two sites of fractional occupancies 0.925 and 0.075; only the major site is discussed. In [Cu(POP)(6-Mebpy)]+ the bite-angles of the bpy and POP are 80.39(4)° and 112.952(16)°, respectively, compared to 80.2(2) and 113.36(8)° in [Cu(POP)(6,6′-Me2bpy)]+. The P–Cu–P angles in the methyl derivatives are smaller than in [Cu(POP)(bpy)][PF6] (115.01(2)°),2 but the bpy bite angle is unchanged. While the Cu–P distances are similar in [Cu(POP)(6-Mebpy)]+ and [Cu(POP)(bpy)]+, the presence of one Me substituent distorts the coordination sphere: Cu1–N2 = 2.1164(10) and Cu1–N3 = 2.0466(10) Å in [Cu(POP)(6-Mebpy)]+ (Fig. 1) compared to corresponding distances of 2.0480(16) and 2.0738(16) Å in [Cu(POP)(bpy)]+.2 Introducing the Me groups causes significant twisting of the bpy unit coupled with a tilting of the heterocyclic ring plane away from the N–Cu vector (Table S1†). In [Cu(POP)(6-Mebpy)]+, the phenyl ring containing C29 stacks weakly with that containing C41 (Fig. 1b, angle between ring planes = 18.3°, ring centroid⋯centroid = 3.86 Å). Despite the greater steric demand of the N^N ligand in [Cu(POP)(6,6′-Me2bpy)]+, a weak intra-cation π-stacking interaction is observed (Fig. 1c); angle between ring planes = 17.1° and centroid⋯centroid separation = 3.78 Å.
The cyclic voltammogram of [Cu(POP)(6-Mebpy)][PF6] (Fig. S3†) shows a reversible Cu+/Cu2+ process at +0.69 V (vs. Fc+/Fc) compared to +0.72 and +0.82 V in [Cu(POP)(bpy)][BF4] and [Cu(POP)(6,6′-Me2bpy)][BF4].11 No well-defined reduction processes are observed for [Cu(POP)(6-Mebpy)][PF6] within the solvent accessible window.
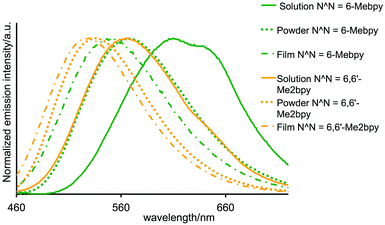
The solution absorption spectrum of [Cu(POP)(6-Mebpy)][PF6] (Fig. S4†) exhibits a broad MLCT band at 380 nm (similar to [Cu(POP)(bpy)]+, [Cu(POP)(6,6′-Me2bpy)]+, [Cu(POP)(phen)]+ and [Cu(POP)(2,9-Me2phen)]+)2,5,11 in addition to intense, high energy absorptions from ligand-centred transitions. Photoluminescence (PL) data for PMMA films doped with [Cu(POP)(6,6′-Me2bpy)][BF4] have been reported (quantum yield (QY) = 14.5%, lifetime (τ) = 15.7 μs).11Table 1 summarizes the emission properties of [Cu(POP)(6-Mebpy)][PF6] and [Cu(POP)(6,6′-Me2bpy)][PF6] in CH2Cl2 solution, PMMA film and solid-state. Excitation of a CH2Cl2 solution of [Cu(POP)(6-Mebpy)][PF6] leads to a broad emission (Fig. 2) with a very low QY. This is rationalized in terms of the tetrahedral copper(I) complex undergoing exciplex formation which can occur even with non-coordinating solvents. Since this favours non-radiative relaxation pathways and results in solvent quenching, the emissive properties are better characterized for powder or thin film samples.17,18 The PL of both complexes is enhanced in PMMA film† or powdered sample (Table 1). The biexponential fit for the luminescence decay of [Cu(POP)(6-Mebpy)][PF6] is consistent with that of related complexes,5,19 and is explained by multiple MLCT transitions at room temperature.19
| [Cu(POP)(6-Mebpy)][PF6] | [Cu(POP)(6,6′-Me2bpy)][PF6] | |||||
|---|---|---|---|---|---|---|
| λ emmax/nm | QY/% | τ ave /μs | λ emmax/nm | QY/% | τb/μs | |
| a A biexponential fit was used, see Table S1. b First order decay. | ||||||
| Solution | 610, 639 | 0.1 | — | 564, 645sh | — | — |
| Film | 550 | 10.7 | 6.0 | 529 | 38.4 | 10.9 |
| Powder | 567 | 9.5 | 2.6 | 535 | 43.2 | 10.5 |
| Device film | 581 | 5.2 | — | 557 | 25.4 | — |
Emissions of the complexes in thin PMMA films are blue-shifted with respect to [Cu(POP)(bpy)][PF6] (610 nm, spin-coated film with the same composition as used in LECs) and the QY increased from 2.0%.2 The blue-shifting (Fig. 2) on going from solution to PMMA film or solid-state is consistent with similar shifts from 590 to 490 nm, or 536 to 465 nm for [Cu(POP)(pypz)]+ or [Cu(POP)(3-Mepypz)]+ (pypz = 2-pyridylpyrazole, 3-Mepypz = 3-methyl-2-pyridylpyrazole).8
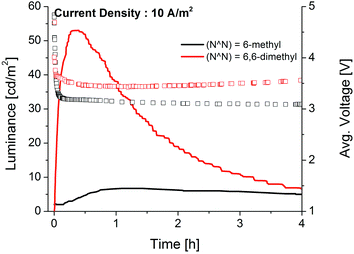
The copper(I) complexes were tested as the active material in LECs. The electroluminescence (EL) of LECs employing [Cu(POP)(6-Mebpy)][PF6] and [Cu(POP)(6,6′-Me2bpy)][PF6] shows λemmax at 574 and 577 nm, respectively (Fig. S5†). These are blue-shifted with respect to the EL of [Cu(POP)(bpy)][PF6] (λemmax ≈ 597 nm).2 The maximum EL emission for [Cu(POP)(6,6′-Me2bpy)][PF6] is red-shifted by 20 nm with respect to its PL (557 nm, Table 1), while [Cu(POP)(6-Mebpy)][PF6] shows a blue shift of 7 nm with respect its PL λmax (581 nm, Table 1). LECs were characterized both applying a constant voltage of 4V or a pulsed current of 10 A m−2 (50% duty cycle, 1 kHz, block wave). These conditions are similar to those reported for LECs with [Cu(POP)(bpy)][PF6] as the iTMC.2 The results from the pulsed current driving are shown in Fig 3. The key parameters of maximum luminance (Lummax), maximum efficacy (Effmax), turn-on time (ton) and lifetime (t1/2) are listed in Table 2; ton is defined as the time to reach the Lummax, and t1/2 is the time to reach half Lummax.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/iTMC
PSS/iTMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Emim][PF6] 1
[Emim][PF6] 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/Al LECs based on the copper(I) complexes at constant voltage (DC 4V) and pulsed current (avg. current density 10 A m−2, 50% duty cycle, 1 kHz, block wave)
1/Al LECs based on the copper(I) complexes at constant voltage (DC 4V) and pulsed current (avg. current density 10 A m−2, 50% duty cycle, 1 kHz, block wave)
| Complex | Driving mode | Lummax/cd m−2 | Effmax/cd A−1 | t on/h | t 1/2/h |
|---|---|---|---|---|---|
| [Cu(POP)(6-Mebpy)][PF6] | DC 4V | 14.4 | 0.1 | 0.15 | 0.58 |
| PC 10 A m−2 | 6.7 | 0.6 | 1.09 | 11.5 | |
| [Cu(POP)(6,6′-Me2bpy)][PF6] | DC 4V | 11.2 | 3.0 | 0.35 | 1.05 |
| PC 10 A m−2 | 53.0 | 5.2 | 0.39 | 1.47 |
As expected, the performances of both copper(I)-LECs vary substantially depending on whether they are driven at constant voltage or under pulsed current. However, the device characteristics show the typical low voltage operation that is the hallmark of LECs (Fig. 3). During operation, the ions in the active layer move towards the electrodes, effectively decreasing the injection barrier and forming p and n doped zones, allowing the LECs to decrease the voltage required to inject electrons and holes independent of the electrode work function.20,21 This implies that the time response is strongly influenced by the ionic mobility of the active layer.22–24 Compared to the non-methylated complex [Cu(POP)(bpy)][PF6], devices employing [Cu(POP)(6,6′-Me2bpy)][PF6] show a slight reduction of ton at the expense of t1/2. This trade-off is typical of LECs, especially when a constant voltage driving mode is used.1,25 On the other hand, the maximum luminance of the methyl-substituted complexes (Table 2) is higher than reported for [Cu(POP)(bpy)][PF6].2 In general, [Cu(POP)(6,6′-Me2bpy)][PF6] showed better performances than [Cu(POP)(6-Mebpy)][PF6], reaching a Lummax of 52 cd m−2 by using a pulsed current, with a corresponding Effmax of 5.2 cd A−1. This value is more than three times higher than for [Cu(POP)(bpy)][PF6]2 (1.64 cd A−1) and is consistent with the higher PLQY of [Cu(POP)(6,6′-Me2bpy)][PF6] (25.4%) versus 2% for [Cu(POP)(bpy)][PF6]. The results demonstrate that a simple modification to the bpy ligand results in noticeably improved LEC luminance and efficiency.
Acknowledgements
We thank the European Research Council (Advanced Grant 267816 LiLo), Swiss National Science Foundation, University of Basel, European Union 7th framework program LUMINET (grant 316906), the Spanish Ministry of Economy and Competitiveness (MINECO) (MAT2011-24594) and the Generalitat Valenciana (Prometeo/2012/053) for support. Ewald Schönhofer prepared 6-Mebpy, and Jonas Schönle assisted with emission measurements. A.P. acknowledges MINECO for an FPI grant.Notes and references
- R. D. Costa, E. Ortí, H. J. Bolink, F. Monti, G. Accorsi and N. Armaroli, Angew. Chem., Int. Ed., 2012, 51, 8178 CrossRef CAS PubMed.
- R. D. Costa, D. Tordera, E. Ortí, H. J. Bolink, J. Schönle, S. Graber, C. E. Housecroft, E. C. Constable and J. A. Zampese, J. Mater. Chem., 2011, 21, 16108 RSC.
- N. Armaroli, G. Accorsi, M. Holler, O. Moudam, J. F. Nierengarten, Z. Zhou, R. T. Wegh and R. Welter, Adv. Mater., 2006, 18, 1313 CrossRef CAS.
- O. Moudam, A. Kaeser, B. Delavaux-Nicot, C. Duhayon, M. Holler, G. Accorsi, N. Armaroli, I. Seguy, J. Navarro, P. Destruel and J. F. Nierengarten, Chem. Commun., 2007, 3077 RSC.
- K. Zhang and D. Zhang, Spectrochim. Acta, Part A, 2013, 124, 341 CrossRef PubMed.
- L. Bergmann, J. Friedrichs, M. Mydlak, T. Baumann, M. Nieger and S. Bräse, Chem. Commun., 2013, 49, 6501 RSC.
- E. Mejía, S.-P. Luo, M. Karnahl, A. Friedrich, S. Tschierlei, A.-E. Surkus, H. Junge, S. Gladiali, S. Lochbrunner and M. Beller, Chem. – Eur. J., 2013, 19, 15972 CrossRef PubMed.
- X.-L. Chen, R. Yu, Q.-K. Zhang, L.-J. Zhou, X.-Y. Wu, Q. Zhang and C.-Z. Lu, Chem. Mater., 2013, 25, 3910 CrossRef CAS.
- A. Kaeser, M. Mohankumar, J. Mohanraj, F. Monti, M. Holler, J.-J. Cid, O. Moudam, I. Nierengarten, L. Karmazin-Brelot, C. Duhayon, B. Delavaux-Nicot, N. Armaroli and J.-F. Nierengarten, Inorg. Chem., 2013, 52, 12140 CrossRef CAS PubMed.
- C. Femoni, S. Muzzioli, A. Palazzi, S. Stagni, S. Zacchini, F. Monti, G. Accorsi, M. Bolognesi, N. Armaroli, M. Massi, G. Valenti and M. Marcaccio, Dalton Trans., 2013, 42, 997 RSC.
- I. Andrés-Tomé, J. Fyson, F. Baiao Dias, A. P. Monkman, G. Iacobellis and P. Coppo, Dalton Trans., 2012, 41, 8669 RSC.
- C. L. Linfoot, M. J. Leitl, P. Richardson, A. F. Rausch, O. Chepelin, F. J. White, H. Yersin and N. Robertson, Inorg. Chem., 2014 DOI:10.1021/ic500889s.
- S.-M. Kuang, D. G. Cuttell, D. R. McMillin, P. E. Fanwick and R. A. Walton, Inorg. Chem., 2002, 41, 3313 CrossRef CAS PubMed.
- D. G. Cuttell, S.-M. Kuang, P. E. Fanwick, D. R. McMillin and R. A. Walton, J. Am. Chem. Soc., 2002, 124, 6 CrossRef CAS PubMed.
- J. Yuasa, M. Dan and T. Kawai, Dalton Trans., 2013, 42, 16096 RSC.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- N. A. Gothard, M. W. Mara, J. Huang, J. M. Szarko, B. Rolczynski, J. V. Lockard and L. X. Chen, J. Phys. Chem. A, 2012, 116, 1984 CrossRef CAS PubMed.
- N. Armaroli, G. Accorsi, F. Cardinali and A. Listorti, Top. Curr. Chem., 2007, 280, 69 CrossRef CAS.
- J.-J. Cid, J. Mohanraj, M. Mohankumar, M. Holler, G. Accorsi, L. Brelot, I. Nierengarten, O. Moudam, A. Kaeser, B. Delavaux-Nicot, N. Armaroli and J.-F. Nierengarten, Chem. Commun., 2013, 49, 859 RSC.
- S. van Reenen, P. Matyba, A. Dzwilewski, R. A. J. Janssen, L. Edman and M. Kemerink, J. Am. Chem. Soc., 2010, 132, 13776 CrossRef CAS PubMed.
- M. Lenes, G. Garcia-Belmonte, D. Tordera, A. Pertegás, J. Bisquert and H. J. Bolink, Adv. Funct. Mater., 2011, 21, 1581 CrossRef CAS.
- S. van Reenen, P. Matyba, A. Dzwilewski, R. A. J. Janssen, L. Edman and M. Kemerink, Adv. Funct. Mater., 2011, 21, 1795 CrossRef CAS.
- R. D. Costa, A. Pertegás, E. Ortí and H. J. Bolink, Chem. Mater., 2010, 22, 1288 CrossRef CAS.
- Y. Shen, D. D. Kuddes, C. A. Naquin, T. W. Hesterberg, C. Kusmierz, B. J. Holliday and J. D. Slinker, Appl. Phys. Lett., 2013, 102, 203305 CrossRef.
- T. Hu, L. He, L. Duan and Y. Qiu, J. Mater. Chem., 2012, 22, 4206 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization and crystallographic data; Tables S1 and S2; Fig. S1–S6. CCDC 996509 and 1009455. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt02847c |
| This journal is © The Royal Society of Chemistry 2014 |