Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ti7-containing, tetrahedral 36-tungsto-4-arsenate(III) [Ti6(TiO6)(AsW9O33)4]20−†
Kai-Yao
Wang
a,
Bassem S.
Bassil
a,
Zheng-Guo
Lin
a,
Ali
Haider
a,
Jie
Cao
b,
Holger
Stephan
c,
Katrin
Viehweger
c and
Ulrich
Kortz
*a
aJacobs University, School of Engineering and Science, P.O. Box 750 561, 28725 Bremen, Germany. E-mail: u.kortz@jacobs-university.de; Fax: +49 421 200 3229; Tel: +49 421 200 3235
bKey Laboratory of Cluster Science, Ministry of Education of China, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry, Beijing Institute of Technology, Beijing 100081, PR China
cHelmholtz-Zentrum Dresden-Rossendorf eV, Institute of Radiopharmaceutical Cancer Research, P.O. Box 510119, 01314 Dresden, Germany
First published on 2nd September 2014
Abstract
We have prepared the Ti7-containing, tetrahedral 36-tungsto-4-arsenate(III) [Ti6(TiO6)(AsW9O33)4]20− (1) by a simple, one pot procedure. Polyanion 1 contains a novel Ti7-core, comprising a central TiO6 octahedron surrounded by six TiO5 square-pyramids, and capped by four {AsIIIW9} trilacunary fragments. The title polyanion is solution-stable, as shown by 183W NMR and mass spectrometry, and exhibits interesting biological properties.
Polyoxometalates (POMs) are a well-known class of discrete, molecular metal oxides comprising early transition metal ions in high oxidation states. POMs are of fundamental importance and technological relevance.1 Lacunary heteropolytungstates can act as inorganic ligands, allowing for incorporation of p-, d-, and f-block metal ions, resulting in products with large structural and compositional versatility and a manifold of properties applicable to catalysis, magnetism, medicine, and materials science.2
The area of Ti4+-containing POMs is well-established nowadays, and many compounds have been isolated. The ionic radius of Ti4+ (0.75 Å) is very similar to that of W6+ (0.74 Å), and hence Ti4+ fits well into the lacunary site of Keggin or Wells–Dawson type POMs, usually adopting octahedral coordination geometry. In terms of Keggin-derivatives, much progress has been made for mono-, di- and tri-Ti4+-substituted species,3 ranging from monomers to oligomers, comprising Ti–O–Ti′ bonds between neighbouring Keggin units, such as dimers, trimers, as well as tetramers. Besides, some work has also been carried out on the Ti4+-containing Keggin-type heteropolytungstates with diverse host/guest features.4
A milestone in recent years was the discovery of the di-Ti4+-containing [Ti2(OH)2As2W19O67(H2O)]8−, exhibiting rare five-coordinated Ti4+ ions.5 The two square-pyramidal TiO4(OH) groups are apparently vital for the unique catalytic properties of this polyanion.6
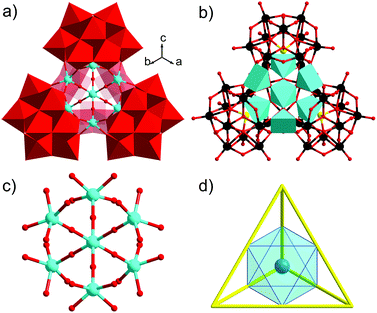
Herein, we report on the synthesis and structure of the novel Ti7-containing, tetrahedral 36-tungsto-4-arsenate(III) [Ti6(TiO6)(AsW9O33)4]20− (1, see Fig. 1).
Polyanion 1 was prepared as the hydrated sodium salt Na20[Ti6(TiO6)(AsW9O33)4]·63H2O (Na-1), by reaction of TiOSO4 and Na9[B-α-AsW9O33]·27H2O7 in NaOAc buffer solution (1 M, pH 4.6) at room temperature (yield 0.105 g, 10%). However, the crystals of Na-1 were not suitable for single crystal X-ray analysis. Therefore, we also isolated the hydrated, mixed sodium–cesium salt Na17.5Cs2.5[Ti6(TiO6)(AsW9O33)4]·72H2O·2NaCH3COO (NaCs-1, yield 0.030 g, 3%), which was suitable for X-ray analysis (see Scheme S1 and ESI† for synthetic details)‡. As expected, the FTIR spectra of Na-1 and NaCs-1 are virtually identical in the POM fingerprint region 400–1000 cm−1, indicating the presence of the same polyanion in both cases (see Fig. S1†). Elemental analysis on Na-1 and NaCs-1 suggested 63 vs. 72 water molecules of hydration, respectively (see ESI† for details). Furthermore, for NaCs-1 the presence of two equivalents of cocrystallized sodium acetate was identified, supported by IR (extra peaks at 1559 and 1411 cm−1) and 13C NMR (two signals at 181.5 and 23.3 ppm, respectively, see also Fig. S4†). Bond valence sum (BVS) calculations confirmed that 1 is not protonated (Table S3†).8 In the present work, all bulk studies were performed on Na-1, due to the higher yield as compared to NaCs-1.
Single crystal X-ray analysis revealed that polyanion 1 contains a novel Ti7-core, comprising a central TiO6 octahedron surrounded by six TiO5 square-pyramids, capped by four {AsIIIW9} trilacunary fragments, leading to an assembly with Td point-group symmetry (see Fig. 1a and b). The structure of 1 somewhat resembles the tetra-Keggin polyanion [Nb4O6(Nb3SiW9O40)4]20−,9 as well as the Wells–Dawson based tetramer {(Ti3P2W15)4},10 which also has an overall tetrahedral shape. However, in 1 the lone pair of electrons on the As hetero atom in {B-α-AsW9O33} does not allow formation of a tri-Ti4+-substituted Keggin unit. As a result, the four {B-α-AsW9O33} units in 1 are linked via six square-pyramidally coordinated TiO5 groups, with the apical oxo ligand O12T (dTi2–O12T = 1.75(2) Å) pointing at the centre of the polyanion, and bridging to the central, unique Ti4+ ion, which is hence octahedrally coordinated. As a result, 1 contains Ti4+ ions in two different coordination geometries, square-pyramidal and octahedral. In the other known polyanions containing square-pyramidal TiO5 groups the apical oxygen is terminal.5,11 The central Ti7 core in 1 has an octahedral shape, with ideal bond angles of 90° and 180°, respectively, around the central Ti1 (see Fig. 1c). For structural clarity, we can simplify the four {B-α-AsW9O33} groups in 1 as the four vertices of a regular tetrahedron, with the six five-coordinated Ti4+ ions being located at the edge centers, thus forming an ideal octahedron, surrounding the central, unique Ti4+ ion (see Fig. 1d).
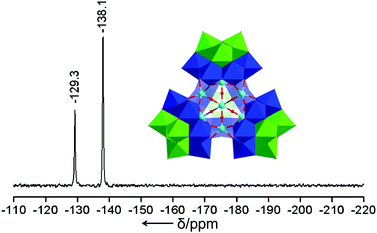
As Na-1 is well-soluble in water, we also performed 183W NMR in D2O/H2O. The resulting spectrum (see Fig. 2) consists of two singlets at −129.3 and −138.1 ppm, respectively, with relative intensities of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, in complete agreement with the solid state structure of 1, indicating that the tetrahedral assembly is maintained in solution.
2, in complete agreement with the solid state structure of 1, indicating that the tetrahedral assembly is maintained in solution.
These observations are further supported by UV-vis spectroscopy. The UV-vis spectrum of Na-1 dissolved in water displays an absorption maximum at 256 nm (see Fig. S5a†), and we demonstrated that polyanion 1 is stable in water and LiOAc solution at pH 4–7 for at least 24 hours (see Fig. S5b–d†).
Electrospray-ionization mass spectrometry (ESI-MS) has proven to be a valuable analytical technique, allowing extraction of structural information of POMs.12 The major peaks observed in the ESI-MS spectrum of Na-1 dissolved in water showed an ensemble of adducts derived from [NaxHyTi7As4W36O138(H2O)z](20−x−y)− with different numbers of sodium ions, protons and water molecules associated with polyanion 1 (see Fig. 3). For instance, the most intense peaks centred at m/z 1375.17 and 1608.02, respectively, can be assigned to the −7 charged [Na2H11Ti7As4W36O138(H2O)6]7− and the −6 charged [Na3H11Ti7As4W36O138(H2O)6]6− adducts. These results indicate that 1 is also stable in the gas phase, as expected based on our solution 183W NMR and UV-vis studies.
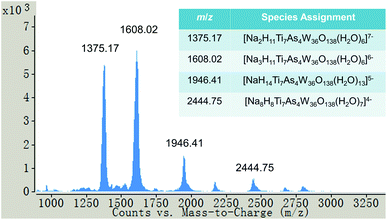 | ||
| Fig. 3 Negative ion mass spectrum of Na-1 in water showing a series of species derived from [NaxHyTi7As4W36O138(H2O)z](20−x−y)−. | ||
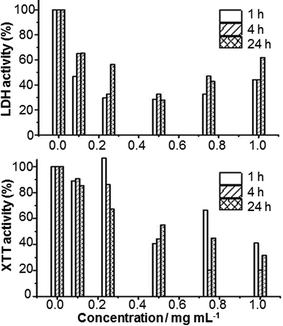
We have also performed biological studies on 1. It has been shown previously that Ti4+-containing POMs can exhibit anticancer activities.13 We investigated the cytotoxicity of 1 in the epidermoid carcinoma cell line A431 (see Fig. 4). These in vitro studies indicated low cytotoxicity of 1 (EC50 ∼ 2.5 mg mL−1 after 24 hours), in agreement with other Ti4+-containing POMs.14 However, there was neither cell growth nor death detectable. A reason for this could be poor cellular accumulation of 1. Hence, it is likely that the main mechanism of the pharmacological effect of 1 is based on inhibiting the binding of native ligands to membrane receptors such as growth factors. Such possible interference in cell signaling and proliferation can be beneficial in cancer treatment.
In summary, we have prepared [Ti6(TiO6)(AsW9O33)4]20− (1), comprising a Ti7 core composed of five- and six-coordinated Ti4+ ions, surrounded by four {B-α-AsW9O33} capping units, resulting in a tetrahedral assembly. The novel polyanion 1 was characterized by various analytical methods including single crystal X-ray diffraction, FTIR, UV-vis, TGA, NMR and ESI-MS. In vitro studies revealed low cytotoxicity in a model cancer cell line, but cell proliferation was inhibited. Currently we investigate these and other properties in more detail.
U. K. thanks the German Research Council (DFG), Jacobs University, and CMST COST Action CM1203 (PoCheMoN) for support. K.-Y. W. thanks the China Scholarship Council (CSC) and A. H. thanks the German Academic Exchange Council (DAAD) for a doctoral fellowship, allowing for Ph.D. studies at Jacobs University. J. C. thanks the National Natural Science Foundation of China (21371025), the 111 Project (B07012) and the Fundamental Research Grant (20121942006) by the Beijing Institute of Technology. K. V. thanks the Helmholtz Virtual Institute NanoTracking (Agreement Number VH-VI-421) for generous financial support. We thank Ms Annika Moje (Jacobs University) for performing the C elemental analysis.
Notes and references
- M. T. Pope and U. Kortz, Polyoxometalates, in Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley, 2012 Search PubMed.
- (a) B. S. Bassil and U. Kortz, Z. Anorg. Allg. Chem., 2010, 636, 2222–2231 CrossRef; (b) O. Oms, A. Dolbecq and P. Mialane, Chem. Soc. Rev., 2012, 41, 7497–7536 RSC; (c) S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623–7646 RSC.
- Recent references include: (a) O. A. Kholdeeva, G. M. Maksimov, R. I. Maksimovskaya, L. A. Kovaleva, M. A. Fedotov, V. A. Grigoriev and C. L. Hill, Inorg. Chem., 2000, 39, 3828–3837 CrossRef CAS; (b) K. Nomiya, M. Takahashi, K. Ohsawa and J. A. Widegren, J. Chem. Soc., Dalton Trans., 2001, 2872–2878 RSC; (c) K. Nomiya, M. Takahashi, J. A. Widegren, T. Aizawa, Y. Sakai and N. C. Kasuga, J. Chem. Soc., Dalton Trans., 2002, 3679–3685 RSC; (d) F. Hussain, B. S. Bassil, L.-H. Bi, M. Reicke and U. Kortz, Angew. Chem., Int. Ed., 2004, 43, 3485–3488 CrossRef CAS PubMed; (e) O. A. Kholdeeva, T. A. Trubitsina, G. M. Maksimov, A. V. Golovin and R. I. Maksimovskaya, Inorg. Chem., 2005, 44, 1635–1642 CrossRef CAS PubMed; (f) Y. Goto, K. Kamata, K. Yamaguchi, K. Uehara, S. Hikichi and N. Mizuno, Inorg. Chem., 2006, 45, 2347–2356 CrossRef CAS PubMed; (g) G. A. Al-Kadamany, F. Hussain, S. S. Mal, M. H. Dickman, N. Leclerc-Laronze, J. Marrot, E. Cadot and U. Kortz, Inorg. Chem., 2008, 47, 8574–8576 CrossRef CAS PubMed; (h) Y.-H. Ren, S.-X. Liu, R.-G. Cao, X.-Y. Zhao, J.-F. Cao and C.-Y. Gao, Inorg. Chem. Commun., 2008, 11, 1320–1322 CrossRef CAS PubMed; (i) R. Tan, D. Li, H. Wu, C. Zhang and X. Wang, Inorg. Chem. Commun., 2008, 11, 835–836 CrossRef CAS PubMed; (j) G. A. Al-Kadamany, B. S. Bassil and U. Kortz, C. R. Chim., 2012, 15, 130–134 CrossRef CAS PubMed.
- (a) K. Hayashi, H. Murakami and K. Nomiya, Inorg. Chem., 2006, 45, 8078–8085 CrossRef CAS PubMed; (b) Y. Mouri, Y. Sakai, Y. Kobayashi, S. Yoshida and K. Nomiya, Materials, 2010, 3, 503–518 CrossRef CAS PubMed; (c) K. Nomiya, Y. Mouri, Y. Sakai and S. Matsunaga, Inorg. Chem. Commun., 2012, 19, 10–14 CrossRef CAS PubMed.
- F. Hussain, B. S. Bassil, U. Kortz, O. A. Kholdeeva, M. N. Timofeeva, P. de Oliveira, B. Keita and L. Nadjo, Chem. –Eur. J., 2007, 13, 4733–4742 CrossRef CAS PubMed.
- (a) O. A. Kholdeeva, B. G. Donoeva, T. A. Trubitsina, G. Al-Kadamany and U. Kortz, Eur. J. Inorg. Chem., 2009, 5134–5141 CrossRef CAS; (b) N. S. Antonova, J. J. Carbó, U. Kortz, O. A. Kholdeeva and J. M. Poblet, J. Am. Chem. Soc., 2010, 132, 7488–7497 CrossRef CAS PubMed; (c) B. G. Donoeva, T. A. Trubitsina, N. S. Antonova, J. J. Carbó, J. M. Poblet, G. Al-Kadamany, U. Kortz and O. A. Kholdeeva, Eur. J. Inorg. Chem., 2010, 5312–5317 CrossRef CAS.
- K.-C. Kim, A. Gaunt and M. Pope, J. Cluster Sci., 2002, 13, 423–436 CrossRef CAS.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244–247 CrossRef.
- G.-S. Kim, H. Zeng, D. VanDerveer and C. L. Hill, Angew. Chem., Int. Ed., 1999, 38, 3205–3207 CrossRef CAS.
- (a) U. Kortz, S. S. Hamzeh and N. A. Nasser, Chem. – Eur. J., 2003, 9, 2945–2952 CrossRef CAS; (b) Y. Sakai, K. Yoza, C. N. Kato and K. Nomiya, Chem. – Eur. J., 2003, 9, 4077–4083 CrossRef CAS PubMed; (c) Y. Sakai, K. Yoza, C. N. Kato and K. Nomiya, Dalton Trans., 2003, 3581–3586 RSC; (d) Y. Sakai, S. Yoshida, T. Hasegawa, H. Murakami and K. Nomiya, Bull. Chem. Soc. Jpn., 2007, 80, 1965–1974 CrossRef CAS.
- T. McGlone, L. Vila-Nadal, H. N. Miras, D.-L. Long, J. M. Poblet and L. Cronin, Dalton Trans., 2010, 39, 11599–11604 RSC.
- (a) T. Ito and T. Yamase, Eur. J. Inorg. Chem., 2009, 5205–5210 CrossRef CAS; (b) J. Yan, J. Gao, D.-L. Long, H. N. Miras and L. Cronin, J. Am. Chem. Soc., 2010, 132, 11410–11411 CrossRef CAS PubMed; (c) Z. Lin, B. Wang, J. Cao, B. Chen, C. Xu, X. Huang, Y. Fan and C. Hu, Eur. J. Inorg. Chem., 2013, 3458–3463 CrossRef CAS; (d) J. Cao, C. Xu, Y. Fan, L. Fan, X. Zhang and C. Hu, J. Am. Soc. Mass Spectrom., 2013, 24, 884–894 CrossRef CAS PubMed; (e) J. Cao, C. Li, Z. Zhang, C. Xu, J. Yan, F. Cui and C. Hu, J. Am. Soc. Mass Spectrom., 2012, 23, 366–374 CrossRef CAS PubMed.
- G. Geisberger, E. B. Gyenge, C. Maake and G. R. Patzke, Carbohydr. Polym., 2013, 91, 58–67 CrossRef CAS PubMed.
- (a) D. L. Barnard, C. L. Hill, T. Gage, J. E. Matheson, J. H. Huffman, R. W. Sidwell, M. I. Otto and R. F. Schinazi, Antiviral Res., 1997, 34, 27–37 CrossRef CAS; (b) T. Meißner, R. Bergmann, J. Oswald, K. Rode, H. Stephan, W. Richter, H. Zänker, W. Kraus, F. Emmerling and G. Reck, Transition Met. Chem., 2006, 31, 603–610 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, XRD data, selected bond lengths and angles, FTIR, TGA, 13C NMR, UV-vis, bond valence sum calculations, and toxicity test results. See DOI: 10.1039/c4dt02494j |
‡ Crystallographic data for NaCs-1: C4H150As4Cs2.5Na19.5O214Ti7W36, λ = 0.71073 Å, M = 11657.39, cubic, space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 38.955(2) Å, V = 59114(9) Å3, Z = 8, T = 100 K, Dcalc = 2.620 g cm−3, μ = 14.985 mm−1, 199 m, a = 38.955(2) Å, V = 59114(9) Å3, Z = 8, T = 100 K, Dcalc = 2.620 g cm−3, μ = 14.985 mm−1, 199![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 total reflections, 2403 unique [Rint = 0.2058], final R1 = 0.0482, wR2 = 0.1217 [I > 2σ(I)]. CSD number: 427928. 037 total reflections, 2403 unique [Rint = 0.2058], final R1 = 0.0482, wR2 = 0.1217 [I > 2σ(I)]. CSD number: 427928. |
| This journal is © The Royal Society of Chemistry 2014 |