Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A luminescent molecular turnstile†
Nicolas
Zigon
,
Patrick
Larpent
,
Abdelaziz
Jouaiti
,
Nathalie
Kyritsakas
and
Mir Wais
Hosseini
*
Molecular Tectonic Laboratory, UMR UDS-CNRS 7140, icFRC, University of Strasbourg, Institut Le Bel, 4, rue Blaise Pascal, F-67000 Strasbourg, France. E-mail: hosseini@unistra.fr
First published on 2nd September 2014
Abstract
A molecular turnstile 1 based on a hydroquinone luminescent hinge bearing two divergently oriented pyridyl units behaving as a rotor and equipped with a handle composed of a tridentate coordinating site considered as the stator was synthesized and its structure was studied in the solid state by X-ray diffraction on single crystal. Its dynamic behaviour in solution was investigated by 1- and 2-D NMR experiments which revealed the free rotation of the rotor around the stator. The rotational movement was locked upon addition of Pd(II) simultaneously complexed by the tridentate moiety of the stator and one of the two monodentate pyridyl sites of the rotor. Interestingly, whereas the open state of the turnstile was luminescent, for its closed state the emission was quenched by the heavy atom effect of Pd(II).
Controlling intramolecular motions in dynamic systems is a topic of current interest. A variety of molecular architectures undergoing translational or rotational movements has been reported.1–20 Among mobile systems designed so far, molecular turnstiles form a particular class of molecules.21 For these compounds, composed of a rotor and a stator covalently interconnected, the free rotation of the rotor around the stator may be blocked by the addition of an external effector. Over the last years, we have reported a series of molecular turnstiles based on the porphyrin backbone22–29 or on Pt(II) organometallic complexes.30–32 Recently, we have described a purely organic turnstile with optical reading between its open and closed states.33 Continuing our efforts in this area, herein, we report on another molecular turnstile 1 (Scheme 1) for which the open and closed states may be differentiated by their emission property.
The design of the turnstile 1 is based on a functionalized hydroquinone backbone known for displaying luminescence.34–36 The latter is equipped with two divergently oriented monodentate pyridyl units and covalently connected to a tridentate coordinating site (Scheme 1).
We arbitrarily consider the hydroquinone moiety bearing the two pyridyl units as the rotor and the tridentate site as the stator. The rational behind the choice of the three components of the turnstile is the following. The hydroquinone core as the hinge was chosen for its luminescence property. The monodentate and tridentate sites are introduced in order to lock the rotational movement of the turnstile, i.e. the rotation of the rotor around the stator, through binding of metal cations in the oxidation state II adopting a square planar coordination geometry such as Pd(II) (1-Pd, Scheme 1). The centric nature of the turnstile, i.e. the presence of two pyridyl units connected to the hinge, is justified by synthetic reasons.
For the turnstile in its open state, the rotor freely rotates around the stator (Fig. 1, O1). This intramolecular dynamic behaviour may be blocked by simultaneous binding of the metal centre behaving as an effector by both coordinating sites of the stator and the rotor and thus leading to the closed state of the turnstile (Fig. 1, C). Based on our previous investigation on an analogous system,33 whereas the open state of the turnstile should luminesce, its closed state, owing to the heavy atom effect of Pd(II), should be far less emissive.
Experimental part
Characterization techniques
1H- and 13C-NMR spectra were recorded at 25 °C on either Bruker AV 300, AV 500 and AV 600 spectrometers in deuterated solvents (CD2Cl2) and residual solvent peak was used as the internal reference.Mass spectrometry was performed by the Service de Spectrométrie de Masse, University of Strasbourg.
Single-crystal analysis
Data were collected at 173(2) K on a Bruker APEX8 CCD Diffractometer equipped with an Oxford Cryosystem liquid N2 device, using graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation. The diffraction data were corrected for absorption. The structure was solved using SHELXS-97 and refined by full matrix least-squares on F2 using SHELXL-97. The hydrogen atoms were introduced at calculated positions and not refined (riding model).37 CCDC 997862.Crystal data for 1: C39H47N5O10·CH2Cl2·H2O, M = 848.76, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.00660(10) Å, b = 13.7975(2) Å, c = 17.5755(2) Å, α = 80.8420(10)°, β = 84.8240(10)°, γ = 79.8220(10)°, V = 2117.97(5) Å3, T = 173(2) K, Z = 2, Dc = 1.331 Mg m−3, μ = 0.218 mm−1, 43
, a = 9.00660(10) Å, b = 13.7975(2) Å, c = 17.5755(2) Å, α = 80.8420(10)°, β = 84.8240(10)°, γ = 79.8220(10)°, V = 2117.97(5) Å3, T = 173(2) K, Z = 2, Dc = 1.331 Mg m−3, μ = 0.218 mm−1, 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643 collected reflections, 11
643 collected reflections, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 independent (Rint = 0.0377), GooF = 1.023, R1 = 0.0470, wR2 = 0.1097 for I > 2σ(I) and R1 = 0.0756, wR2 = 0.1230 for all data.
353 independent (Rint = 0.0377), GooF = 1.023, R1 = 0.0470, wR2 = 0.1097 for I > 2σ(I) and R1 = 0.0756, wR2 = 0.1230 for all data.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 (250 mg, 0.33 mmol, 1 eq.), a MeOH (5 mL) solution of 4-pyridylboronic acid (164 mg, 1.33 mmol, 4 eq.) and an aqueous solution of Na2CO3 (2 M, 6 mL) were successively added. The mixture was degassed before Pd(PPh3)4 (76 mg, 0.07 mmol, 0.2 eq.) was added. The biphasic mixture was heated at 80 °C for 24 h. The solvents were removed under reduced pressure and the resulting solid was partitioned between CH2Cl2 (100 mL) and a 10% NaHCO3 aqueous solution (100 mL). The organic phase was collected and further washed with a 10% NaHCO3 aqueous solution (2 × 100 mL) and dried over MgSO4. After evaporation of the solvent, column chromatography (SiO2, CH2Cl2–MeOH 99/1 to 90/10) afforded the desired compound 1 as a yellow solid (220 mg, 0.29 mmol, 88%). 1H-NMR (CD2Cl2, 600 MHz): δ (ppm) = 2.80 (br, 2H, H2O), 3.42 (m, 4H, He), 3.49 (m, 8H, OCH2), 3.54 (m, 4H, OCH2), 3.58 (m, 4H, OCH2), 3.61 (m, 4H, OCH2), 3.74 (m, 4H, Hk), 4.19 (m, 4H, Hl), 7.23 (s, 2H, Hn), 7.68 (d, 4H, Hq, 3J = 5.7 Hz), 8.00 (t, 1H, Ha, 3J = 7.7 Hz), 8.28 (d, 2H, Hb, 3J = 7.7 Hz), 8.70 (br, 4H, Hr), 9.07 (br t, 2H, NH, 3J = 5.8 Hz). 13C-NMR HSQC-HMBC (CDCl3, 125 MHz): δ (ppm) = 39.5 (Ce), 69.9, 69.9, 70.2, 70.4, 70.7, 70.8, 71.1, 117.1 (Cn), 124.5 (Cq or Cb), 124.8 (Cq or Cb), 129.4 (Cp), 138.7 (Ca), 145.9 (Cc), 149.1 (Co), 149.8 (Cr), 151.0 (Cm), 164.3 (Cd). UV-Vis (CH2Cl2): λmax (log
33 (250 mg, 0.33 mmol, 1 eq.), a MeOH (5 mL) solution of 4-pyridylboronic acid (164 mg, 1.33 mmol, 4 eq.) and an aqueous solution of Na2CO3 (2 M, 6 mL) were successively added. The mixture was degassed before Pd(PPh3)4 (76 mg, 0.07 mmol, 0.2 eq.) was added. The biphasic mixture was heated at 80 °C for 24 h. The solvents were removed under reduced pressure and the resulting solid was partitioned between CH2Cl2 (100 mL) and a 10% NaHCO3 aqueous solution (100 mL). The organic phase was collected and further washed with a 10% NaHCO3 aqueous solution (2 × 100 mL) and dried over MgSO4. After evaporation of the solvent, column chromatography (SiO2, CH2Cl2–MeOH 99/1 to 90/10) afforded the desired compound 1 as a yellow solid (220 mg, 0.29 mmol, 88%). 1H-NMR (CD2Cl2, 600 MHz): δ (ppm) = 2.80 (br, 2H, H2O), 3.42 (m, 4H, He), 3.49 (m, 8H, OCH2), 3.54 (m, 4H, OCH2), 3.58 (m, 4H, OCH2), 3.61 (m, 4H, OCH2), 3.74 (m, 4H, Hk), 4.19 (m, 4H, Hl), 7.23 (s, 2H, Hn), 7.68 (d, 4H, Hq, 3J = 5.7 Hz), 8.00 (t, 1H, Ha, 3J = 7.7 Hz), 8.28 (d, 2H, Hb, 3J = 7.7 Hz), 8.70 (br, 4H, Hr), 9.07 (br t, 2H, NH, 3J = 5.8 Hz). 13C-NMR HSQC-HMBC (CDCl3, 125 MHz): δ (ppm) = 39.5 (Ce), 69.9, 69.9, 70.2, 70.4, 70.7, 70.8, 71.1, 117.1 (Cn), 124.5 (Cq or Cb), 124.8 (Cq or Cb), 129.4 (Cp), 138.7 (Ca), 145.9 (Cc), 149.1 (Co), 149.8 (Cr), 151.0 (Cm), 164.3 (Cd). UV-Vis (CH2Cl2): λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 274 (4.31), 335 (3.90). MS (ESI): m/z calcd for C39H48N5O10+ [M + H]+ 746.34 g mol−1; found 746.36 g mol−1.
ε) = 274 (4.31), 335 (3.90). MS (ESI): m/z calcd for C39H48N5O10+ [M + H]+ 746.34 g mol−1; found 746.36 g mol−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 249 (4.29), 282 (4.42), 348 (4.18). MS (ESI): m/z calcd for C39H46N5O10Pd+ [M + H]+ 850.23 g mol−1; found 850.23 g mol−1; m/z calcd for C117H136N15O30Pd3Na2+ [3M + Na + H]+ 1286.83 g mol−1; found 1286.41 g mol−1; m/z calcd for C39H46N5O10PdNa2+ [M + Na + H]2+ 437.61 g mol−1; found 437.22 g mol−1.
ε) = 249 (4.29), 282 (4.42), 348 (4.18). MS (ESI): m/z calcd for C39H46N5O10Pd+ [M + H]+ 850.23 g mol−1; found 850.23 g mol−1; m/z calcd for C117H136N15O30Pd3Na2+ [3M + Na + H]+ 1286.83 g mol−1; found 1286.41 g mol−1; m/z calcd for C39H46N5O10PdNa2+ [M + Na + H]2+ 437.61 g mol−1; found 437.22 g mol−1.
Results
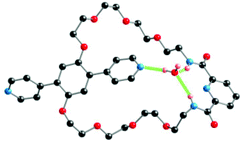
The turnstile 1 was prepared in 8 steps. The precursor macrocyclic compound 2 was obtained in 90% yield as previously described.33 The turnstile 1 was obtained in 88% yield by coupling 4-pyridyl boronic acid with compound 2 in the presence of Pd(PPh3)4. The closed state of turnstile 1-Pd was obtained in 69% yield upon metallation by Pd(OAc)2.The turnstile 1 was characterized both in solution by NMR spectroscopy and in the solid state by X-ray diffraction on single crystal (Fig. 2, see Experimental section). Single crystals of 1 were obtained at 25 °C upon slow diffusion of cyclohexane into a CH2Cl2 solution of 1.
Compound 1 crystallizes (triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) with CH2Cl2 and H2O molecules. The C–C, C–O and C–N distances are within the expected range. The two amide groups (dC
) with CH2Cl2 and H2O molecules. The C–C, C–O and C–N distances are within the expected range. The two amide groups (dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of 1.230 and 1.226 Å) are almost coplanar with the pyridyl unit of the tridentate chelate (tilt angles of −2.162 and 4.373°). The two pyridyl units connected to the hydroquinone moiety are tilted by −39.30 and −50.21° with respect to the central aryl unit. The water molecule is located between the tridentate moiety of the stator and one of the two pyridyl units of the rotor. The H2O molecule behaves both as a H-bond acceptor towards the two NH groups of the amide moieties (NH⋯O distances of 2.362 and 2.220 Å and NHO angles of 150.84 and 148.97°) and as a single H-bond donor towards the pyridyl moiety of the rotor (OH⋯Npy distance of 2.041 Å and OHN angle of 172.60°).
O of 1.230 and 1.226 Å) are almost coplanar with the pyridyl unit of the tridentate chelate (tilt angles of −2.162 and 4.373°). The two pyridyl units connected to the hydroquinone moiety are tilted by −39.30 and −50.21° with respect to the central aryl unit. The water molecule is located between the tridentate moiety of the stator and one of the two pyridyl units of the rotor. The H2O molecule behaves both as a H-bond acceptor towards the two NH groups of the amide moieties (NH⋯O distances of 2.362 and 2.220 Å and NHO angles of 150.84 and 148.97°) and as a single H-bond donor towards the pyridyl moiety of the rotor (OH⋯Npy distance of 2.041 Å and OHN angle of 172.60°).
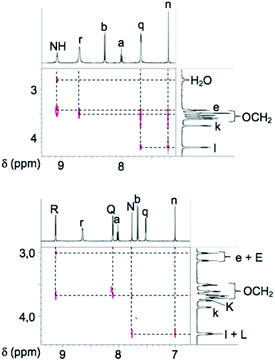
The dynamic behaviour of the turnstile 1 in solution in CD2Cl2 was studied at 25 °C by 1- and 2-D NMR spectroscopy which allowed to assign all H atoms (Scheme 1). In the aromatic region of the 1H-NMR spectrum of 1 (Fig. 3a), the signal at 7.23 ppm corresponding to Hn appears as a unique singlet whereas signals corresponding to Hq and Hr at 7.68 and 8.70 ppm respectively appear as two signals (see Scheme 1 for assignment). These observations imply the equivalence between the two halves of the rotor and therefore the rotation of the rotor inside the stator, defining thus the open state O1 of the turnstile (Fig. 1).
 | ||
| Fig. 3 Portions of 1H-NMR (CD2Cl2, X00 MHz, X = 6 for a and X = 3 for b–e, 298 K): (a) turnstile 1, (b) 1-Pd, (c) 1-Pd + 1 eq. of TBACN, (d) 1-Pd + 3 eq. of TBACN, (e) 1-Pd + excess of TBACN. For signals assignment see Scheme 1. | ||
Addition of Pd(II) leading to 1-Pd (closed state C, Fig. 1) results from the simultaneous binding of the cation by both the tridentate site of the stator and one of the two monodentate pyridyl sites of the rotor (Fig. 3b). As expected, the complexation process leads to the disappearance of the NH signals at 9.07 ppm, the shift of all aromatic signals and the splitting of the signals corresponding to the two pyridyl units belonging to the rotor.
Indeed, upon binding to Pd(II), the two pyridyl units are differentiated leading to Hn, Hq and Hr signals for the unbound and HN, HQ and HR signals corresponding to the coordinated pyridyl moieties. Furthermore, Ha and Hb signals corresponding to the pyridyl moiety of the chelating unit undergo noticeable shifts (Δδ of 0.02 and −0.62 ppm respectively). Both the open and closed states of the turnstile have been investigated by 2D 1H–1H NOESY experiments (Fig. 4).
For the turnstile 1 in its open state, as expected correlations between chemically connected hydrogen atoms such as Hn/Hk-l and HNH/He and between hydrogen atoms of the pyridyl group of the rotor and those of the polyethylene glycol chain of the stator are observed. Interestingly, the 2D spectrum of 1 displays a cross-relaxation peak between the H2O signal at 2.80 ppm and the NH signal at 9.07 ppm (Fig. 4 top). The presence of the water molecule in solution between the stator and the rotor is in agreement with the observation of H bonds in the solid state and further corroborated by the broadening of Hq and Hr signals. However, the interaction between the water molecule and the two parts of the turnstile, although hindering the free rotation of the rotor around the stator, appears to be insufficient at 25 °C to lock the rotational movement leading thus to a sufficiently fast oscillation process with respect to the NMR timescale rendering the two pyridyl unit equivalent.
For the turnstile in its closed position 1-Pd, as in the case of 1 discussed above, the expected through space correlations between chemically connected hydrogen atoms such as Hn-Hl and HN-HL are observed (Fig. 4 bottom). In contrast with 1, owing to the locking of the rotational movement by binding of Pd2+ cation in 1-Pd, only correlations between HQ and HR atoms of the bound pyridyl unit belonging to the rotor and the OCH2 H-atoms of the handle are observed (Fig. 4 bottom).
The switching between the closed state C and open state O2 (Scheme 1) was achieved using CN− anion, a stronger ligand than the pyridyl unit of the rotor. The addition of 1 eq. of CN− anions (Fig. 3c) leads to the open state O2 (1-Pd-CN)− for which the CN− anion replaces the pyridyl unit in 1-Pd. Consequently, as for the open state O1 of the turnstile, the free rotation of the rotor around the stator renders the two pyridyl moieties equivalent thus giving rise to only three sets of signals (singlet for Hn and two doublets for Hq and Hr). Furthermore, the shift observed for Ha and Hb signals and the absence of signal corresponding to NH groups indicate that the Pd(II) cation remains coordinated to the tridentate site of the stator. Further additions of CN− anion (3 eq., Fig. 3d) leads to a mixture of the two open states O1 and O2. Finally, in the presence of an excess of CN− anion, the open state O1 is generated (Fig. 3e).
As stated in the introduction, the turnstile was designed in order to assess its open (1) and closed (1-Pd) states by emission spectroscopy.
The absorption and emission properties of the turnstile in its open (1) and closed (1-Pd) states were studied in solution in CH2Cl2 at 298 K. For compound 1, the UV-visible absorption spectra is composed of three bands at 235 nm, 274 nm (ε = 20.3 × 103 L mol−1 cm−1) and at 335 nm (ε = 7.94 × 103 L mol−1 cm−1) (Fig. 5). Upon metallation by Pd(II) leading to the closed state 1-Pd, again the same three absorption bands are observed however with bathochromic and hyperchromic shifts 249 nm (ε = 19.5 × 103 L mol−1 cm−1); 282 nm (ε = 26.3 × 103 L mol−1 cm−1); 348 nm (ε = 15.1 × 103 L mol−1 cm−1).
Both 1 and 1-Pd emit in the blue part of the visible spectrum at 413 nm when excited at 350 nm. Their emission spectra are almost identical and the emission wavelength is not affected by metallation indicating that their excited states are similar. However, whereas the turnstile 1 in its open state is strongly luminescent when excited at 350 nm, in its closed state 1-Pd, it is far less emissive.
The quantum yields, using quinine sulphate as reference, are 78% for 1 and 0.60% for 1-Pd. The lifetime of the excited state for 1 and 1-Pd is in the 3.1–3.6 ns range. However, in the case of 1-Pd, a second process with a lifetime of ca. 88 ps was observed. A plausible explanation could be that, upon excitation in the π–π* of the 1,4-dipyridylphenyl core, a S1 excited state with a lifetime of ca. 3.5 ns is generated. Owing to the heavy atom effect of Pd(II) located in the proximity of the emitting site, the transition to a T1 excited state is promoted which is subsequently quenched by solvent or O2 molecule.
Interestingly, under the same conditions, for iso-absorbing solutions of 1 or 1-Pd, whereas the open state of the turnstile emits strongly when excited at 350 nm, for the closed state, the emission was found to be below the detection limit of the spectrometer (Fig. 6).
In conclusion, a new molecular turnstile 1 based on a luminescent hydroquinone moiety as a hinge was designed and synthesised. The hinge was equipped both with two monodentate pyridyl units and a tridentate coordinating site. The turnstile 1 may be described as a rotor composed of the two pyridyl moieties and the hinge connected to a stator comprising the hinge and the tridentate unit. For the turnstile in its open state, rotor freely rotates inside the stator. This movement may be blocked upon addition of Pd(II) as an external effector. Indeed, the simultaneous binding of the metal cation by both the tridentate coordinating site of the stator and one of the two monodentate pyridyl groups of the rotor leads to the closed state of the turnstile 1-Pd. The switching between the open and the closed states of the turnstile may be achieved using CN− anion as an auxiliary strong ligand. As a consequence of the design of the turnstile 1 comprising a luminescent hinge, the reading of the open and closed states may be achieved optically. Indeed, whereas the open state of the turnstile is strongly emissive, for its closed state 1-Pd, owing to the heavy atom effect of the cation, the emission process is quenched.
Following the design principle reported here, other turnstiles bearing two different coordinating sites on the rotor are currently under investigation.
Financial supports by the University of Strasbourg, the International Centre for Frontier Research in Chemistry (icFRC), Strasbourg, the Institut Universitaire de France, the CNRS and the Ministry of Education and Research (PhD fellowship to P. L. and N. Z.) are acknowledged.
Notes and references
- J.-P. Sauvage, in Molecular machines and motors, Structure and bonding, ed. J.-P. Sauvage, Springer, Berlin, Heidelberg, 2001, vol. 99, pp. 1–282 Search PubMed; V. Balzani, M. Venturi and A. Credi, in Molecular devices and machines: a journey into the nanoworld, Wiley-VCH, Weinheim, 2003, pp. 1–457 Search PubMed; T. R. Kelly, in Molecular machines, Topics in Current Chemistry, ed. T. R. Kelly, Springer, Berlin, Heidelberg, 2005, vol. 262, pp. 1–227 Search PubMed.
- E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 CrossRef CAS PubMed.
- W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2006, 1, 25 CrossRef CAS PubMed.
- G. S. Kottas, L. I. Clarke, D. Horinek and J. Michl, Chem. Rev., 2005, 105, 1281 CrossRef CAS PubMed.
- G. Vives, H.-P. Jacquot de Rouville, A. Carella, J.-P. Launay and G. Rapenne, Chem. Soc. Rev., 2009, 38, 1551 RSC.
- V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348 CrossRef CAS.
- (a) K. Tashiro, K. Konishi and T. Aida, J. Am. Chem. Soc., 2000, 122, 7921 CrossRef CAS; (b) K. Kinbara, T. Muraoka and T. Aida, Org. Biomol. Chem., 2008, 6, 1871 RSC.
- M. Takeuchi, T. Imada and S. Shinkai, Angew. Chem., Int. Ed., 1998, 37, 2096 CrossRef CAS.
- (a) V. Balzani, M. Gomez-Lopez and J. F. Stoddart, Acc. Chem. Res., 1998, 31, 405 CrossRef CAS; (b) J. F. Stoddart, Acc. Chem. Res., 2001, 34, 410 CrossRef CAS; (c) V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348 CrossRef CAS; (d) A. H. Flood, R. J. A. Ramirez, W.-Q. Deng, R. P. Muller, W. A. Goddard III and J. F. Stoddart, Aust. J. Chem., 2004, 57, 301 CrossRef CAS; (e) C. A. Schalley, K. Beizai and F. Vögtle, Acc. Chem. Res., 2001, 34, 465 CrossRef CAS PubMed; (f) A. M. Fuller, D. A. Leigh, P. J. Lusby, I. D. H. Oswald, S. Parsons and D. B. Walker, Angew. Chem., Int. Ed., 2004, 43, 3914 CrossRef CAS PubMed; (g) D. A. Leigh, P. J. Lusby, A. M. Z. Slawin and D. B. Walker, Angew. Chem., Int. Ed., 2005, 44, 4557 CrossRef CAS PubMed; (h) F. Coutrot and E. Busseron, Chem. – Eur. J., 2009, 15, 5186 CrossRef CAS PubMed; (i) E. Busseron, C. Romuald and F. Coutrot, Chem. – Eur. J., 2010, 16, 10062 CrossRef CAS PubMed; (j) C. Clavel, C. Romuald, E. Brabet and F. Coutrot, Chem. – Eur. J., 2013, 19, 2982 CrossRef CAS PubMed.
- (a) J.-P. Sauvage, Science, 2001, 291, 2105 CrossRef CAS; (b) J.-P. Sauvage, Acc. Chem. Res., 1998, 31, 611 CrossRef CAS; (c) J.-P. Collin, C. Dietrich-Buchecker, P. Gavina, M. C. Jimenez-Molero and J.-P. Sauvage, Acc. Chem. Res., 2001, 34, 477 CrossRef CAS PubMed.
- (a) T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150 CrossRef CAS PubMed; (b) T. R. Kelly, Acc. Chem. Res., 2001, 34, 514 CrossRef CAS PubMed; (c) T. R. Kelly, X. Cai, F. Damkaci, S. B. Panicker, B. Tu, S. M. Bushell, I. Cornella, M. J. Piggott, R. Salives, M. Cavero, Y. Zhao and S. Jasmin, J. Am. Chem. Soc., 2007, 129, 376 CrossRef CAS PubMed.
- (a) N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS PubMed; (b) M. K. J. ter Wiel, R. A. van Delden, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2003, 125, 15076 CrossRef CAS PubMed; (c) R. A. van Delden, M. K. J. ter Wiel, N. Koumura and B. L. Feringa, in Molecular Motors, ed. M. Schliwa, Wiley-VCH, Weinheim, 2003, pp. 559–577 Search PubMed.
- (a) J. F. Morin, Y. Shirai and J. M. Tour, Org. Lett., 2006, 8, 1713 CrossRef CAS PubMed; (b) Y. Shirai, J.-F. Morin, T. Sasaki, J. M. Guerrero and J. M. Tour, Chem. Soc. Rev., 2006, 35, 1043 RSC; (c) G. Vives and J. M. Tour, Acc. Chem. Res., 2009, 42, 473 CrossRef CAS PubMed.
- (a) L. Grill, K.-H. Rieder, F. Moresco, G. Jimenez-Bueno, C. Wang, G. Rapenne and C. Joachim, Surf. Sci., 2005, 584, L153 CrossRef CAS PubMed; (b) U. G. E. Perera, F. Ample, H. Kersell, Y. Zhang, G. Vives, J. Echeverria, M. Grisolia, G. Rapenne, C. Joachim and S.-W. Hla, Nat. Nanotechnol., 2013, 8, 46 CrossRef CAS PubMed.
- I. Pochorovski, M. O. Ebert, J. P. Gisselbrecht, C. Boudon, W. B. Schweizer and F. Diederich, J. Am. Chem. Soc., 2012, 134, 14702 CrossRef CAS PubMed.
- S. Sengupta, M. E. Ibele and A. Sen, Angew. Chem., Int. Ed., 2012, 51, 8434 CrossRef CAS PubMed.
- K. C.-F. Leung, C.-P. Chak, C.-M. Lo, W.-Y. Wong, S. Xuan and C. H. K. Cheng, Chem. – Asian J., 2009, 4, 364 CrossRef CAS PubMed.
- W. Zhou, Y. J. Guo and D. H. Qu, J. Org. Chem., 2013, 78, 590 CrossRef CAS PubMed.
- S. Sengupta, M. E. Ibele and A. Sen, Angew. Chem., Int. Ed., 2012, 51, 8434 CrossRef CAS PubMed.
- C. S. Vogelsberg and M. A. Garcia-Garibay, Chem. Soc. Rev., 2012, 41, 1892–1910 RSC.
- (a) T. C. Bedard and J. S. Moore, J. Am. Chem. Soc., 1995, 117, 10662 CrossRef CAS; (b) A. Carella, J. Jaud, G. Rapenne and J.-P. Launay, Chem. Commun., 2003, 2434 RSC; (c) N. Weibel, A. Mishchenko, T. Wandlowski, M. Neuburger, Y. Leroux and M. Mayor, Eur. J. Org. Chem., 2009, 6140 CrossRef CAS PubMed; (d) K. Skopek, M. C. Hershberger and J. A. Gladysz, Coord. Chem. Rev., 2007, 251, 1723 CrossRef CAS PubMed.
- A. Guenet, E. Graf, N. Kyritsakas, L. Allouche and M. W. Hosseini, Chem. Commun., 2007, 2935 RSC.
- A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Inorg. Chem., 2010, 49, 1872 CrossRef CAS PubMed.
- T. Lang, A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. Commun., 2010, 46, 3508 RSC.
- A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. – Eur. J., 2011, 17, 6443 CrossRef CAS PubMed.
- T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2011, 40, 3517 RSC.
- T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2011, 40, 5244 RSC.
- T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, Chem. – Eur. J., 2012, 18, 10419 CrossRef CAS PubMed.
- T. Lang, E. Graf, N. Kyritsakas and M. W. Hosseini, New J. Chem., 2013, 37, 112 RSC.
- N. Zigon, A. Guenet, E. Graf and M. W. Hosseini, Chem. Commun., 2013, 49, 3637 RSC.
- N. Zigon, A. Guenet, E. Graf, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2013, 42, 9740 RSC.
- N. Zigon, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2013, 43, 152 RSC.
- N. Zigon, P. Larpent, A. Jouaiti, N. Kyritsakas and M. W. Hosseini, Chem. Commun., 2014, 50, 5040 RSC.
- J. K. Kallitsis, K. G. Gravalos, A. Hilberer and G. Hadziioannou, Macromolecules, 1997, 30, 2989 CrossRef.
- H.-R. Liao, Y.-J. Lin, Y.-M. Chou, F.-T. Luo and B.-C. Wang, J. Lumin., 2008, 128, 1373 CrossRef CAS PubMed.
- L. Larios-López, D. Navarro-Rodríguez, E. M. Arias-Marín, I. Moggio, C. V. Reyes-Castañeda, B. Donnio, J. LeMoigne and D. Guillon, Liq. Cryst., 2003, 30, 423 CrossRef.
- G. M. Sheldrick, Program for Crystal Structure Solution,University of Göttingen, Germany, 1997.
Footnote |
| † Electronic supplementary information (ESI) available: Characterisation of new compounds, additional excitation and emission spectra and experimental crystallographic data. CCDC 997862. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt02258k |
| This journal is © The Royal Society of Chemistry 2014 |