Fragmentation pathways analysis for the gas phase dissociation of protonated carnosine-oxaliplatin complexes†
Abstract
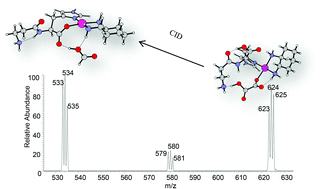
Collision-induced dissociation (CID) experiments on the protonated carnosine-oxaliplatin complex, [Carnosine + OxPt + H]+ using several collision energies were shown to yield nine different fragment ions. Energy-resolved CID experiments on [Carnosine + OxPt + H]+ showed that the generation of the product ion [Carnosine − H + Pt(dach)]+ (where dach is 1,2-diaminocyclohexane) is the lowest energy process. At slightly higher collision energies, the loss of neutral carnosine from [Carnosine + OxPt + H]+ to produce [OxPt + H]+ was observed, followed by the loss of oxaliplatin from the same precursor ion to produce [Carnosine + H]+. At significantly higher energies, the ion [OxPt − CO2 + H]+ was shown to be formed, while the last two investigated ions [Carnosine + OxPt − CO2 + H]+ and [Carnosine − NH3 − H + Pt(dach)]+ did not attain any significant relative abundance. Density functional calculations at the B3LYP/LANL2DZ level were employed to probe the fragmentation mechanisms that account for all experimental data. The lowest free energy barriers for the generation of each of the ions [Carnosine − H + Pt(dach)]+, [OxPt + H]+, [Carnosine + H]+, [Carnosine + OxPt − CO2 + H]+ and [Carnosine − NH3 − H + Pt(dach)]+ from [Carnosine + OxPt + H]+ according to the fragmentation mechanisms offered here were calculated to be 31.9, 38.8, 49.3, 75.2, and 85.6 kcal mol−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...