Nickel oxide thin film from electrodeposited nickel sulfide thin film: peroxide sensing and photo-decomposition of phenol†
Abstract
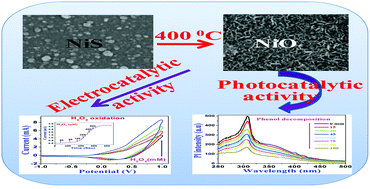
A novel non-enzymatic peroxide sensor has been constructed by using nickel oxide (NiO) thin films as sensing material, which were prepared by a two-step process: (i) electrodeposition of nickel sulfide (NiS) and (ii) thermal air oxidation of as-deposited NiS to NiO. The resultant material is highly porous and comprises interconnected nanofibers. UV-Vis spectroscopy, FTIR spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and field-emission scanning electron microscopy (FESEM) were used for a complete characterization of nanostructured NiO thin films. Cyclic voltammetry study shows that NiO/ITO electrode facilitates the oxidation of hydrogen peroxide and exhibits excellent catalytic activity towards its sensing. The amperometric study of NiO/ITO was carried out to determine the sensitivity, linear range, detection limit of the proposed sensor. The sensor exhibits prominent electrocatalytic activity toward the oxidation of H2O2 with a wide linear range and a low detection limit. The possible use of the synthesized NiO thin films as an effective photocatalyst for the decomposition of phenol is also discussed.

 Please wait while we load your content...
Please wait while we load your content...