Photophysical properties of an unusual bichromophoric species constructed from a cyclometalated Pt(ii) chromophore and a blue Bodipy-acetylacetonate species†
Abstract
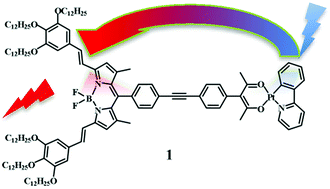
A Bodipy species bearing an acetyl-acetonate (acac) group, 3, has been prepared from a blue absorbing borondipyrromethene core bearing gallate substituted paraffin chains. Compound 3 chelates a Pt(II) center having an orthometalated 2-phenyl-pyridine anion (ppy) as an additional ligand, giving rise to a new bichromophoric Pt(II)-Bodipy species, 1. The absorption spectra, redox behavior and photophysical properties of 1, 3 and of the neutral Pt(II) compound 2, containing ppy and an acac derivative as ligands, have been studied. Compounds 3 and 2 are used as models for the Bodipy-based and the metal-based subunits of 1, respectively. The 3LC emission of 2 is fully quenched in 1, whereas the Bodipy fluorescence is only weakly reduced in 1 compared to 3, indicating weak interaction between the subunits. Two different charge-separated (CS) states have a role in the intercomponent excited state decays of 1. Notably, whereas in all the previously investigated bichromophoric metal(polypyridine)-Bodipy compounds, the light absorbed by the metal-based unit leads to population of the lowest-energy triplet Bodipy-based level, in 1 it contributes with high efficiency (>99%) to the Bodipy fluorescence. An efficient and formally forbidden 3LC to 1Bodipy energy transfer occurring by Förster mechanism is, unprecedently, the dominant 3LC decay process in 1.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...