Open Access Article
Open Access ArticleStructural and thermodynamic similarities of phases in the Li–Tt (Tt = Si, Ge) systems: redetermination of the lithium-rich side of the Li–Ge phase diagram and crystal structures of Li17Si4.0−xGex for x = 2.3, 3.1, 3.5, and 4 as well as Li4.1Ge†
Michael
Zeilinger
and
Thomas F.
Fässler
*
Department Chemie, Technische Universität München, Lichtenbergstraße 4, 85747 Garching b. München, Germany. E-mail: Thomas.Faessler@lrz.tum.de; michael.zeilinger@mytum.de; Tel: (+49) 89 289 13131
First published on 13th May 2014
Abstract
A reinvestigation of the lithium-rich section of the Li–Ge phase diagram reveals the existence of two new phases, Li17Ge4 and Li4.10Ge (Li16.38Ge4). Their structures are determined by X-ray diffraction experiments of large single crystals obtained from equilibrated melts with compositions Li95Ge5 and Li85Ge15. Excess melt is subsequently removed through isothermal centrifugation at 400 °C and 530 °C, respectively. Li17Ge4 crystallizes in the space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (a = 18.8521(3) Å, V = 6700.1(2) Å3, Z = 20, T = 298 K) and Li4.10Ge (Li16.38Ge4) in Cmcm (a = 4.5511(2) Å, b = 22.0862(7) Å, c = 13.2751(4) Å, V = 1334.37(8) Å3, Z = 16, T = 123 K). Both phases are isotypic with their Si counterparts and are further representative of the Li17Pb4 and Li4.11Si structure types. Additionally, the solid solutions Li17Si4−xGex follows Vegard's law. A comparison of the GeLin coordination polyhedra shows that isolated Ge atoms are 13- and 14-coordinated in Li17Ge4, whereas in Li16.38Ge4 the Ge atoms possess coordination numbers 12 and 13. Regarding the thermodynamic stability, Li16.38Ge4 is assigned a high-temperature phase existing between ∼400 °C and 627 °C, whereas Li17Ge4 decomposes peritectically at 520–522 °C. Additionally, the decomposition of Li16.38Ge4 below ∼400 °C was found to be very sluggish. These findings are manifested by differential scanning calorimetry, long-term annealing experiments and the results from melt equilibration experiments. Interestingly, the thermodynamic properties of the lithium-rich tetrelides Li17Tt4 and Li4.1Tt (Li16.4Tt4) are very similar (Tt = Si, Ge). Besides Li15Tt4, Li14Tt6, Li12Tt7, and LiTt, the title compounds are further examples of isotypic tetrelides in the systems Li–Tt.
3m (a = 18.8521(3) Å, V = 6700.1(2) Å3, Z = 20, T = 298 K) and Li4.10Ge (Li16.38Ge4) in Cmcm (a = 4.5511(2) Å, b = 22.0862(7) Å, c = 13.2751(4) Å, V = 1334.37(8) Å3, Z = 16, T = 123 K). Both phases are isotypic with their Si counterparts and are further representative of the Li17Pb4 and Li4.11Si structure types. Additionally, the solid solutions Li17Si4−xGex follows Vegard's law. A comparison of the GeLin coordination polyhedra shows that isolated Ge atoms are 13- and 14-coordinated in Li17Ge4, whereas in Li16.38Ge4 the Ge atoms possess coordination numbers 12 and 13. Regarding the thermodynamic stability, Li16.38Ge4 is assigned a high-temperature phase existing between ∼400 °C and 627 °C, whereas Li17Ge4 decomposes peritectically at 520–522 °C. Additionally, the decomposition of Li16.38Ge4 below ∼400 °C was found to be very sluggish. These findings are manifested by differential scanning calorimetry, long-term annealing experiments and the results from melt equilibration experiments. Interestingly, the thermodynamic properties of the lithium-rich tetrelides Li17Tt4 and Li4.1Tt (Li16.4Tt4) are very similar (Tt = Si, Ge). Besides Li15Tt4, Li14Tt6, Li12Tt7, and LiTt, the title compounds are further examples of isotypic tetrelides in the systems Li–Tt.
Introduction
In the last decade, the demand for high capacity lithium-ion batteries (LIBs) decisively influenced numerous fields of research; in particular, the chemistry of group 14 elements (tetrel = Tt) plays an important role in the development of more efficient anode materials. Since Si theoretically offers a specific capacity of 3579 mA h g−1 (based on the formation of Li15Si4) and thus massively exceeds the capacity of the commonly used graphite anode (372 mA h g−1, LiC6),1,2 research on Li–Si materials has been in focus for many years. As the high capacity of Si is associated with several problems such as a large volume expansion of up to 300% upon lithiation accompanied by contact loss of electrodes and poor cycle life, a large number of these studies target these issues.3 Further, Li–Si phases predominantly occur amorphously during charging and discharging, and only at low discharge voltages, crystalline Li15Si4 is observed.1,2,4,5 However, the processes in working LIBs can be nicely monitored by in situ/ex situ NMR investigations.6,7 Moreover, a fundamental understanding of thermodynamic properties and an unambiguous structural characterization of Li–Si phases are of considerable importance. Just recently, we reported on detailed investigations on lithium-rich silicides, including the metastable phase Li15Si4,8 Li17Si49 and the high-temperature phase Li4.11Si (Li16.42Si4).10 Further examples are given in ref. 11–17.The heavier tetrel element Ge has received less attention regarding its use as an anode material due to its low natural abundance connected with a lower theoretical specific capacity compared to silicon (1564 mA h g−1vs. 4056 mA h g−1, based on the formation of Li16.95Ge418 and Li17Si4,9 respectively). However, the diffusivity of lithium in Ge is approximately 400 times larger than in Si at room temperature,19,20 which intrinsically puts germanium to the foreground. Since some Si analogues are not known we put emphasis on the structural variety of lithium germanides and their thermodynamic relation.
Li–Ge phases were first postulated in the 1950s and 1960s (Li3Ge, Li4Ge, LiGe),21–23 fueling numerous investigations in this field later on. Before 2001, the ascertained Li–Ge representatives included Li21Ge524 (formerly described as Li20Ge525,26 and Li22Ge527,28), Li15Ge4,28,29 Li13Ge430 (formerly reported as Li7Ge231), Li14Ge6,25,26 Li9Ge4,32 Li12Ge7,25,26,33 LiGe (space group I41/a, ambient pressure),22,34 LiGe (space group I41/amd, high pressure),35 and Li7Ge12.26,36 A previously described phase Li11Ge637 could not be reproduced in the course of the redetermination of the Li–Ge phase diagram by Grüttner,25 and due to the “striking similarity” to Li8MgSi6,38 it might have been ternary Li8MgGe6. Until then, solely Li21Tt5, Li14Tt6, Li12Tt7, and LiTt (LiSi39 is obtainable from high-pressure synthesis) were known for both Si and Ge. We note that Li13Si4 and Li13Ge4 are not isotypic and crystallize with their own structure types. In the following years new Li–Tt phases were found and others were revised, e.g. a hexagonal high-pressure form of LiGe (space group P63/mmc),40 metastable Li15Si41,2 being isotypic with the congruently melting phase Li15Ge425 (Li15Tt4), Li∼17Ge4 (Li21+3/16Si5 = Li16.95Ge4)18 revised from Li21Ge5, and the revision of the Li7Ge12 structure.41 The synthesis of solid solutions Li15Si4−xGex by mechanical ball-milling was also reported.42 An experimental determination of the Li–Ge phase diagram involving most of the aforementioned phases is given by Grüttner in his dissertation.25
We recently reported on the single crystal structures and thermodynamic properties of Li17Si4,9 the high-temperature phase Li4.11Si (Li16.42Si4),10 and metastable Li15Si4.8 The Li–Si phase diagram was revised accordingly. Consequently, we extended our studies on the Li–Ge system. Herein, we present the single crystal X-ray structure determination of Li17Ge4 and Li4.10Ge which crystallize isotypically with their Si counterparts. Earlier structure reports on Li∼17Ge4 (Li21+3/16Si5 = Li16.95Ge4)18 involving partially occupied Li sites could not be confirmed. Solid solutions of the isotypic phases Li17Si4 and Li17Ge4 follow Vegard's law.43 Due to clarity and a better comparability, the phase Li4.10Ge is referred to as Li16.38Ge4. Furthermore, the lithium-rich section of the Li–Ge phase diagram (Li concentrations >79 at%) is reinvestigated by differential scanning calorimetry and long-term annealing experiments, manifesting Li16.38Ge4 as a high-temperature phase which possesses a very sluggish decomposition behavior below ∼400 °C. Li17Ge4 is peritectically formed at 520–522 °C from cooling an according melt.
Results and discussion
Single crystal X-ray structure determination of Li17Ge4 and Li16.38Ge4
Large single crystals of Li17Ge4 and Li16.38Ge4 were grown in Li–Ge melts Li95Ge5 and Li85Ge15 at 400 °C and 530 °C, respectively. For Li16.38Ge4, bar-shaped crystals with a size of up to 0.6 × 0.25 × 0.25 cm3 could be obtained. A representative specimen is depicted in Fig. 1. Generally, crystals of Li17Ge4 grew much smaller in a block-like shape with diameters of 0.1 × 0.1 × 0.1 cm3. Those crystals allowed acquisition of high quality single crystal X-ray diffraction data. | ||
| Fig. 1 Example of a bar-shaped single crystal of Li16.38Ge4 obtained from a melt Li85Ge15 at 530 °C. | ||
The phase Li17Ge4 crystallizes in the space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m with a = 18.8521(3) Å (V = 6700.1(2) Å3) and Z = 20. The asymmetric unit consists of 13 Li and four Ge atoms each being located on a special position (Table 1). As already reported for Li17Si4, Li17Sn4, and Li17Pb4,9,18,44 the Wyckoff position 4a with symmetry
3m with a = 18.8521(3) Å (V = 6700.1(2) Å3) and Z = 20. The asymmetric unit consists of 13 Li and four Ge atoms each being located on a special position (Table 1). As already reported for Li17Si4, Li17Sn4, and Li17Pb4,9,18,44 the Wyckoff position 4a with symmetry ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m was found to be fully occupied. We note that the refinement of occupancy factors did not indicate significantly large deviations from full occupancy and thus they were regarded as being fully occupied. Accordingly, the cubic unit cell of Li17Ge4 contains 340 Li and 80 Ge atoms (cF420). Further, atomic displacement parameters were refined anisotropically with meaningful results for all atoms, revealing excellent reliability factors of R1 = 0.022 and wR2 = 0.038 (all data) for the final model (Table 6). The structure of Li17Ge4 is isotypic with Li17Si4, Li17Sn4, and Li17Pb4.9,18,44
3m was found to be fully occupied. We note that the refinement of occupancy factors did not indicate significantly large deviations from full occupancy and thus they were regarded as being fully occupied. Accordingly, the cubic unit cell of Li17Ge4 contains 340 Li and 80 Ge atoms (cF420). Further, atomic displacement parameters were refined anisotropically with meaningful results for all atoms, revealing excellent reliability factors of R1 = 0.022 and wR2 = 0.038 (all data) for the final model (Table 6). The structure of Li17Ge4 is isotypic with Li17Si4, Li17Sn4, and Li17Pb4.9,18,44
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m, Z = 20, T = 298 K, estimated standard deviations in parentheses)
3m, Z = 20, T = 298 K, estimated standard deviations in parentheses)
| Atom | Wyckoff position | x | y | z | U eq/Å2·103 |
|---|---|---|---|---|---|
| Ge1 | 16e | 0.159545(8) | x | x | 13.03(4) |
| Ge2 | 16e | 0.916568(8) | x | x | 11.37(4) |
| Ge3 | 24f | 0.32102(1) | 0 | 0 | 13.27(5) |
| Ge4 | 24g | 0.57015(1) | 1/4 | 1/4 | 13.06(4) |
| Li1 | 16e | 0.0734(2) | x | x | 31(1) |
| Li2 | 16e | 0.3031(2) | x | x | 21.6(8) |
| Li3 | 16e | 0.4175(2) | x | x | 22.3(9) |
| Li4 | 16e | 0.5575(2) | x | x | 23(1) |
| Li5 | 16e | 0.6877(2) | x | x | 28(1) |
| Li6 | 16e | 0.8314(2) | x | x | 27(1) |
| Li7 | 24f | 0.1677(3) | 0 | 0 | 25(1) |
| Li8 | 24g | 0.0743(3) | 1/4 | 1/4 | 23.1(9) |
| Li9 | 48h | 0.0913(2) | x | 0.2624(2) | 32.0(9) |
| Li10 | 48h | 0.0896(2) | x | 0.7612(2) | 31.1(8) |
| Li11 | 48h | 0.1547(1) | x | 0.5205(2) | 32.5(8) |
| Li12 | 48h | 0.1637(1) | x | 0.0027(2) | 22.5(8) |
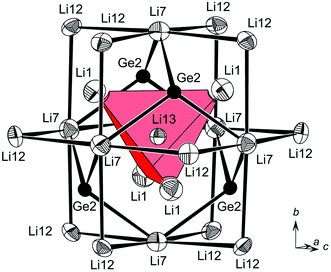
| Li13 | 4a | 0 | 0 | 0 | 17(2) |
Li16.38(2)Ge4 crystallizes in the space group Cmcm with a = 4.5511(2) Å, b = 22.0862(7) Å, c = 13.2751(4) Å (V = 1334.37(8) Å3) and Z = 16 referring to Li4.096(4)Ge as one formula unit. The unit cell contains 10 Li and three Ge atom positions (Table 2) where Li4 and Li5 are disordered. A careful analysis of difference Fourier maps after assigning all Ge and nine Li atom positions (Li1–Li3 and Li5–Li10) revealed occupational disorder along the crystallographic a-axis like that reported for Li16.42Si4.10 As can be seen in Fig. 2a and 2b, the difference Fourier maps showing the strand-like residual electron density with peak-maxima at Wyckoff positions 4c and 8g are almost identical for Li16.42(1)Si4 and Li16.38(2)Ge4. Applying the disorder model as reported for Li16.42(1)Si4,10 we subsequently obtained a very similar occupancy ratio of 0.616(8)/0.384(8) for Li4A on 4c (1/2, y, 1/4) and Li4B on 8g (x, y, 1/4), respectively, compared with 0.575(3)/0.425(3) found in Li16.42(1)Si4. Analogously to Li16.42(1)Si4,10 a split position for Li5 on 8f (0, x, y) was introduced. The split fractions converged to 0.75(4) for Li5A and 0.25(4) for Li5B (0.848(7)/0.152(7) in Li16.42(1)Si4;10 refinement details and the geometric relevance of the atom split are given in the ESI†). In case of atoms being uninvolved in disorder, site occupancy factors were refined to values close to full occupancy and therefore those positions were constrained to full occupancy. Thus, the unit cell contains 65.54(7) Li atoms as a consequence of the disorder (65.70(3) in Li16.42(1)Si410) and 16 Ge atoms, resulting in a crystallographic density of 2.011 g cm−3. The structure was finally solved with reliability factors of R1 = 0.023 and wR2 = 0.027 for all data (Table 6). Li16.38(2)Ge4 crystallizes isotypically with Li16.42(1)Si4.10
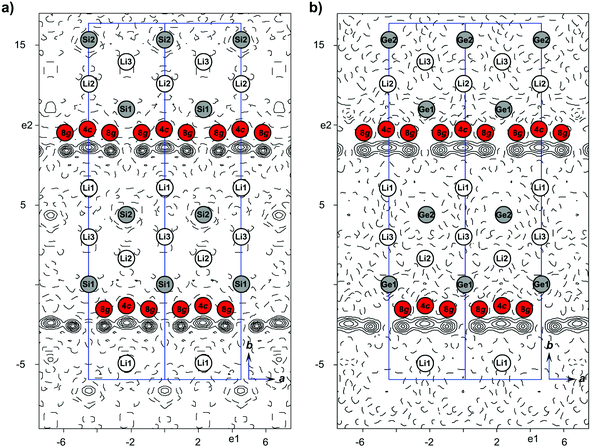 | ||
| Fig. 2 Difference Fourier map (Fo − Fc) shown for the layer defined by Tt1, Tt2 and Li2 in (a) Li16.42Si410 and (b) Li16.38Ge4 (parallel to the ab-plane, calculated from single crystal data at 100 K and 123 K for Li16.42Si4 (contour lines ±0.6 e Å−3) and Li16.38Ge4 (contour lines ±0.5 e Å−3), respectively; cell edges are shown in blue). | ||
| Atom | Wyckoff position | x | y | z | s.o.f. | U eq./Å2·103 |
|---|---|---|---|---|---|---|
| Ge1 | 4c | 0 | 0.256372(6) | 1/4 | 1 | 7.83(3) |
| Ge2 | 4c | 1/2 | 0.454314(5) | 1/4 | 1 | 6.35(3) |
| Ge3 | 8f | 1/2 | 0.105220(4) | 0.067609(6) | 1 | 6.83(2) |
| Li1 | 4c | 1/2 | 0.0333(1) | 1/4 | 1 | 14.4(4) |
| Li2 | 4c | 1/2 | 0.3297(1) | 1/4 | 1 | 13.5(4) |
| Li3 | 4c | 0 | 0.3922(1) | 1/4 | 1 | 17.5(5) |
| Li4A | 4c | 1/2 | 0.1476(2) | 1/4 | 0.616(8) | 52(3) |
| Li4B | 8g | 0.210(1) | 0.1385(2) | 1/4 | 0.384(8) | 21(2) |
| Li5A | 8f | 0 | 0.1741(3) | 0.0842(7) | 0.75(4) | 14(1) |
| Li5B | 8f | 0 | 0.164(1) | 0.123(4) | 0.25(4) | 24(6) |
| Li6 | 8f | 0 | 0.04650(7) | 0.1225(1) | 1 | 13.9(3) |
| Li7 | 8f | 0 | 0.31636(9) | 0.0788(1) | 1 | 16.5(3) |
| Li8 | 8f | 0 | 0.47089(9) | 0.0908(1) | 1 | 18.7(3) |
| Li9 | 8f | 1/2 | 0.23155(8) | 0.1356(1) | 1 | 19.1(3) |
| Li10 | 8f | 1/2 | 0.40425(8) | 0.0639(1) | 1 | 14.5(3) |
Structure description of Li17Ge4 and Li16.38Ge4
In our previous work we reported on the structures of Li17Si4 and Li16.42Si4 in detail.9,10 Both phases were comparatively highlighted on the basis of SiLin coordination polyhedra and the disorder in Li16.42Si4 was illustrated with various structure models. Hence, we analogously elaborate on the structures of the isotypic phases Li17Ge4 and Li16.38Ge4 herein.The structure of Li17Ge4 is closely related to the previously reported phase Li21Ge524 (Li16.8Ge4) only differing in the occupation of one fourfold special position. In detail, their common space group F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m possesses four positions with site symmetry
3m possesses four positions with site symmetry ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (4a–d). Whereas Wyckoff positions 4a–d were claimed to be void in Li21Ge5, we found a fully occupied 4a site in Li17Ge4 with short but reasonable next nearest neighbor distances of 2.397(7) Å for Li1–Li13 (cf. Fig. 3 and Table 4). We note that since Li22Si527,45 (its composition corresponds to a full occupancy of sites 4a–d) was revised to Li21Si5,46 heavier analogues such as Li22Ge5 were supposed to crystallize with the Li21Si5 structure type as well (Li21Ge5).24 We have already shown by computational methods that the fully relaxed structures of Li17Si4 and Li21Si5 decisively differ regarding the coordination environment around the 4a site.9 If this position is unoccupied, the first coordination shell, which is a (Li1)4 tetrahedron (cf. Fig. 3), is markedly contracted, which was not observed in experimental data of Li21Si5 (Table S5 in the ESI†). Therefore it could be concluded that Li might have been overseen in the previous structure refinement (most likely a partial occupancy). Turning to Li17Ge4, the positional parameters are almost identical with Li17Si4 (Table S6 in the ESI†) and therefore an equal conclusion is reasonable.
3m (4a–d). Whereas Wyckoff positions 4a–d were claimed to be void in Li21Ge5, we found a fully occupied 4a site in Li17Ge4 with short but reasonable next nearest neighbor distances of 2.397(7) Å for Li1–Li13 (cf. Fig. 3 and Table 4). We note that since Li22Si527,45 (its composition corresponds to a full occupancy of sites 4a–d) was revised to Li21Si5,46 heavier analogues such as Li22Ge5 were supposed to crystallize with the Li21Si5 structure type as well (Li21Ge5).24 We have already shown by computational methods that the fully relaxed structures of Li17Si4 and Li21Si5 decisively differ regarding the coordination environment around the 4a site.9 If this position is unoccupied, the first coordination shell, which is a (Li1)4 tetrahedron (cf. Fig. 3), is markedly contracted, which was not observed in experimental data of Li21Si5 (Table S5 in the ESI†). Therefore it could be concluded that Li might have been overseen in the previous structure refinement (most likely a partial occupancy). Turning to Li17Ge4, the positional parameters are almost identical with Li17Si4 (Table S6 in the ESI†) and therefore an equal conclusion is reasonable.
In 2001, Goward et al.18 have already reported on the revision of Li22M5 to Li17M4 (M = Ge, Sn, Pb). However, their model for “Li17Ge4” involved partially occupied Li sites (Table 3), namely Li1A on 16e (3/4 occ.), Li1B on 16e (1/4 occ.), and Li13 on 4a (3/4 occ.) resulting in the composition Li16.95Ge4 (note that for a better comparability the fractional atomic coordinates and labels were adapted to Li17Ge4). In the case of a void 4a site, the surrounding (Li1A)4 tetrahedron (comparable with Li1 in Fig. 3) is slightly contracted to (Li1B)4 whose vertices are markedly closer to the 4a center (2.52 Å vs. 1.91 Å).18 Interestingly, this scenario is in close agreement with the computationally relaxed structure of Li21Si59 (Table S7 in the ESI†) where all fourfold positions are void. Consequently, a partial occupancy of the 4a position as reported for Li16.95Ge4 and hence the existence of a small homogeneity region Li17−xTt4 (0 < x < 0.2) are indeed meaningful. More recently, Lacroix-Orio et al.47 presented a Zn-doped derivative of the Li17Ge4 compound. They could not find any evidence for partially occupied Li positions and instead found small concentrations of Zn being incorporated at the 4a position (Li17−εZnεGe4, ε = 0.005(1)).
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3 m (for all phases), Z = 20, estimated standard deviations in parentheses). Listed are x values for the special positions 16e (x, x, x), 24f (x, 0, 0), 24g (x, 1/4, 1/4) and x, z pairs for 48h (x, x, z)
3 m (for all phases), Z = 20, estimated standard deviations in parentheses). Listed are x values for the special positions 16e (x, x, x), 24f (x, 0, 0), 24g (x, 1/4, 1/4) and x, z pairs for 48h (x, x, z)
| At. | Wyck. pos. | Li17Ge4 | Li17−εZnεGe447 | Li16.95Ge418 | At. | Wyck. pos. | Li17Ge4 | Li17−εZnεGe447 | Li16.95Ge418 |
|---|---|---|---|---|---|---|---|---|---|
| Ge1 | 16e | 0.15955(1) | 0.15958(2) | 0.15952(5) | Li8 | 24g | 0.0743(3) | 0.0740(6) | 0.075(1) |
| Ge2 | 16e | 0.91657(1) | 0.91667(3) | 0.91684(5) | Li9 | 48h | 0.0913(2) | 0.0906(3) | 0.0907(7) |
| Ge3 | 24f | 0.32102(1) | 0.32118(4) | 0.32112(7) | 0.2624(2) | 0.2631(4) | 0.2660(8) | ||
| Ge4 | 24g | 0.57015(1) | 0.57020(4) | 0.56965(7) | Li10 | 48h | 0.0896(2) | 0.0904(4) | 0.0914(7) |
| Li1A | 16e | 0.0734(2) | 0.0747(6) | 0.0775 | 0.7612(2) | 0.7613(4) | 0.7597(9) | ||
| Li1B | 16e | — | — | 0.0587 | Li11 | 48h | 0.1547(1) | 0.1554(3) | 0.1540(5) |
| Li2 | 16e | 0.3031(2) | 0.3033(3) | 0.3032(6) | 0.5205(2) | 0.5216(4) | 0.5216(9) | ||
| Li3 | 16e | 0.4175(2) | 0.4179(5) | 0.4169(9) | Li12 | 48h | 0.1637(1) | 0.1632(3) | 0.1625(5) |
| Li4 | 16e | 0.5575(2) | 0.5584(4) | 0.5579(6) | 0.0027(2) | 0.0025(5) | 0.005(1) | ||
| Li5 | 16e | 0.6877(2) | 0.6864(4) | 0.6876(7) | Li13 | 4a | 0 | 0 | 0 |
| Li6 | 16e | 0.8314(2) | 0.8331(4) | 0.8329(7) | Zn1 | 4a | — | 0 | — |
| Li7 | 24f | 0.1677(3) | 0.1678(6) | 0.170(1) |
Concluding, the fractional atomic coordinates of all Li17Ge4 related phases listed in Table 3 are very similar and the scenario of an unoccupied 4a position in Li16.95Ge4 fits the calculated data of the corresponding Li21Si5 structure very well. This strengthens the existence of a homogeneity region Li17−xTt4 (0 < x < 0.2) which is deduced from the flexibility of the (Li1)4(Tt2)4 tetrahedral star around the Wyckoff position 4a.9 Accordingly, the herein reported phase Li17Ge4 is regarded as the lithium-richest representative in the Li–Ge system.
The structure of Li17Ge4 is closely related to a 6 × 6 × 6 superstructure of the body centered cubic (bcc) structure.46 As already shown by von Schnering and Nesper,46 it can be easily interpreted by 26 atom clusters (M26 with M = Tt, Li) centered at the special positions 4a–d whereas Ge4 tetrahedra and Ge6 octahedra (note that Ge atoms are isolated by distances larger than 4.44 Å) are situated around 4a, 4c and 4b, 4d, respectively, corresponding to the arrangement of Na and Tl atoms in the NaTl structure.46
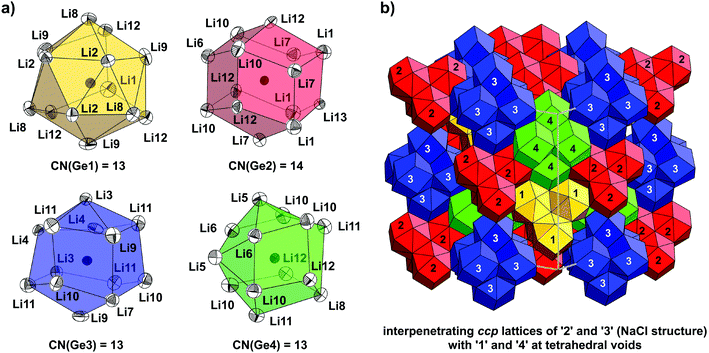
Yet another possible way of looking at the structure of Li17Ge4 is the comparison of GeLin coordination polyhedra and their arrangement in the unit cell. This has proven to be a neat method for the comparison of lithium-rich Li–Si phases solely bearing isolated Si atoms in their structures (Li17Tt4, Li16.4Tt4 and Li15Tt4).10 As shown in Fig. 4a, Ge1, Ge3 and Ge4 are 13-coordinated by Li atoms, whereas Ge2 attains a coordination number (CN) of 14. Corresponding to the first coordination shell of Ge atoms, Li–Ge distances range from 2.659(4) Å to 3.053(3) Å (Table 4). The second Li shell is clearly separated with distances larger than 4.2564(3) Å. Interestingly, the polyhedra around Wyckoff positions 4a, 4c and 4b, 4d either form supratetrahedra ([Ge1Li13]4 and [Ge2Li14]4 denoted as (1) and (2)) or supraoctahedra ([Ge3Li13]6 and [Ge4Li13]6 denoted as (3) and (4)), respectively (Fig. 4b). Accordingly, the structure of Li17Ge4 can be considered as two interpenetrating ccp lattices of (2) and (3), i.e. the NaCl structure, where tetrahedral voids are filled with (1) and (4).
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m, Z = 20, T = 298 K, estimated standard deviations in parentheses)
3m, Z = 20, T = 298 K, estimated standard deviations in parentheses)
| Atom pair | d(Å) | Atom pair | d(Å) | ||||
|---|---|---|---|---|---|---|---|
| Ge1 | Li9 | ×3 | 2.659(4) | Ge3 | Li10 | ×2 | 2.849(4) |
| Li1 | 2.813(7) | Li3 | ×2 | 2.855(2) | |||
| Li2 | ×3 | 2.884(1) | Li7 | 2.890(5) | |||
| Li8 | ×3 | 2.898(3) | Li11 | ×4 | 2.977(2) | ||
| Li12 | ×3 | 2.959(4) | Ge4 | Li12 | ×2 | 2.680(3) | |
| Ge2 | Li12 | ×3 | 2.686(3) | Li11 | ×2 | 2.709(3) | |
| Li13 | 2.7244(3) | Li8 | 2.723(5) | ||||
| Li7 | ×3 | 2.734(3) | Li5 | ×2 | 2.769(4) | ||
| Li6 | 2.780(6) | Li6 | ×2 | 2.856(2) | |||
| Li10 | ×3 | 2.933(4) | Li10 | ×4 | 3.053(3) | ||
| Li1 | ×3 | 2.969(4) | Li13 | Li1 | ×4 | 2.397(7) | |
| Ge3 | Li9 | ×2 | 2.673(4) | Ge2 | ×4 | 2.7244(3) | |
| Li4 | ×2 | 2.760(3) | Li7 | ×6 | 3.162(5) | ||
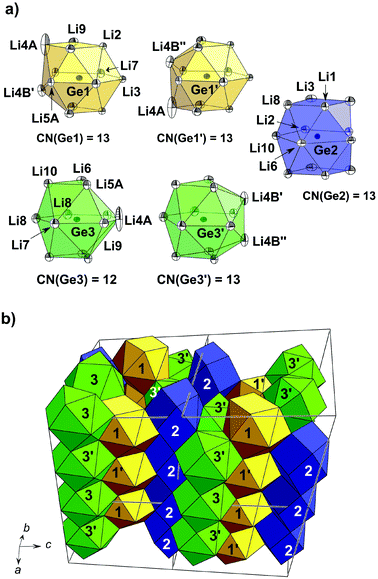
Besides Li17Ge4 and Li15Ge4, Li16.38Ge4 is yet another representative exclusively bearing isolated Ge atoms. It is isotypic with Li16.42Si410 which possesses a peculiar structure involving occupational and positional disorder along the crystallographic a-axes (cf. Fig. 2). A convenient way of illustrating the disordered structure of Li16.38Ge4 is the assumption of a simplified ordered model which has already been applied for the description and band structure calculations of Li16.42Si4.10 In detail, the atom positions Li4A, Li4B and Li5A (cf. Table 2) affected by disorder corresponding to the Wyckoff positions 4c (0.616(8) occ.), 8g (0.384(8) occ.) and 8f (0.75(4) occ.), respectively, are either regarded as half (Li4A, Li4B) or fully occupied (Li5A) resulting in the composition Li16.5Ge4. An accordingly ordered model with fully occupied atom positions and crystallographically independent sites for Li4B′ and Li4B′′ (translate to Li4B in Cmcm) can be achieved by symmetry reduction and a cell enlargement (space group P21/m, a = 9.1016(4) Å, b = 13.2751(4) Å, c = 11.2744(4) Å, β = 101.643(1)°; for crystallographic details, see ref. 10). The GeLin polyhedra occurring in the respective model “Li16.5Ge4” are depicted in Fig. 5a.
Similar to Li17Ge4, the first shell of Li atoms surrounding each Ge atom is clearly separated from the second one with distances between 2.596(2) Å to 3.309(3) Å and distances above 4.103(4) Å (Table 5). Whereas Ge1 and Ge2 are permanently 13-coordinated (Ge1Li13/Ge1′Li13 and Ge2Li13 denoted as (1)/(1′) and (2)), Ge3 attains coordination numbers of either 12 or 13 (Ge3Li12 and Ge3′Li13 denoted as (3) and (3′)). The different coordination of Ge3 is attributed to the varying occupation of atom positions Li4A, Li4B′ and Li4B′′. The arrangement of GeLin polyhedra in the unit cell of Li16.38Ge4 is achieved by stacking them into strands which proceed along the crystallographic a-axis (Fig. 5b). For the ordered model “Li16.5Ge4”, the stacking sequence is indicated by numbers corresponding to various GeLin polyhedra highlighted in Fig. 5a.
| Atom pair | d(Å) | Atom pair | d(Å) | ||||
|---|---|---|---|---|---|---|---|
| Ge1 | Li7 | ×2 | 2.631(2) | Ge2 | Li8 | ×4 | 3.127(1) |
| Li5B | ×2 | 2.64(2) | Ge3 | Li4A | 2.596(2) | ||
| Li4B | ×2 | 2.772(4) | Li7 | 2.603(2) | |||
| Li9 | ×4 | 2.790(1) | Li8 | 2.693(2) | |||
| Li2 | ×2 | 2.792(1) | Li6 | ×2 | 2.7188(9) | ||
| Li5A | ×2 | 2.854(8) | Li5B | ×2 | 2.723(8) | ||
| Li3 | 3.001(2) | Li5A | ×2 | 2.747(3) | |||
| Li4A | ×2 | 3.309(3) | Li4B | ×2 | 2.854(3) | ||
| Ge2 | Li6 | ×2 | 2.647(2) | Li10 | ×2 | 2.875(1) | |
| Li3 | ×2 | 2.657(1) | Li1 | 2.896(1) | |||
| Li10 | ×2 | 2.707(2) | Li9 | 2.932(2) | |||
| Li2 | 2.753(2) | Li8 | 2.983(2) | ||||
| Li1 | ×2 | 2.867(2) | |||||
Comparing the TtLin coordination polyhedra of all lithium-rich Li–Tt phases, which exclusively comprise isolated Tt atoms in their structures, the coordination numbers consistently increase from CN = 12 (Li15Tt4), over CN = 12–13 (Li16.4Tt4) to CN = 13–14 (Li17Ge4).
Solid solutions Li17Si4−xGex [x = 2.30(2), 3.08(4), 3.53(3)]
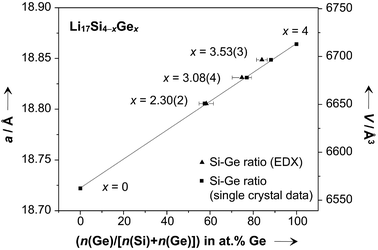
Li17Si4 and Li17Ge4 are isotypic phases that form solid solutions. Single crystalline samples of Li17Si4−xGex were obtained from melt equilibration experiments and analyzed by single crystal and powder X-ray diffraction as well as energy dispersive X-ray spectroscopy. The Si–Ge ratios for Li17Si4−xGex samples obtained from single crystal X-ray diffraction data were x = 2.30(2), 3.08(4), and 3.53(3). We note that these values deviate from the initial ones x = 1.0, 2.0, and 3.0 corresponding to the employed melts “Li90Si7.5Ge2.5”, “Li90Si5Ge5” and “Li90Si2.5Ge7.5” as the Si amount is reduced due to a partial reaction of Si with the stainless steel ampules; thus the Si–Ge ratios in the products are shifted toward higher Ge contents. As can be seen in Fig. 6, the cell axes and volumes (determined from PXRD patterns of the respective samples by Rietveld refinement, Fig. S2†) linearly increase with increasing Ge concentrations in Li17Si4−xGex revealing a perfect behavior obeying Vegard's law.43 Additionally, results from EDX measurements agree well with the crystallographically determined Si–Ge ratios (impurities originating from the stainless steel ampule were not detected).Analyzing the distribution of Si and Ge on atom positions Tt1–4, the Si–Ge ratios are very similar for Tt1, Tt3, and Tt4, whereas Tt2 on the Wyckoff position 16e (x, x, x) features a slight preference for Si (Table S1–4†). Comparing the coordination environment of Tt2 and Tt1, Tt3, Tt4, the former is 14- and the latter are 13-coordinated by Li atoms with similar Ge–Li distances (2.68 Å–2.95 Å vs. 2.65–3.04 Å). The phenomenon that different crystallographic sites are substituted differently is also known as the “coloring problem”.48 In some cases the site preferences cannot be deduced on the basis of simple chemical reasoning (e.g. differences in electronegativities) and quantum chemical calculations may give a reasonable explanation.49 Here, the focus is set on experimental work and we note that small differences in the distribution of Si and Ge on atom positions Tt2 and Tt1, Tt3, Tt4 were traced.
Thermodynamic stability of Li17Ge4 and Li16.38Ge4
The Li–Ge phase diagram was determined by Federov & Molochka23 in 1966 and later revised by Grüttner25 in his dissertation. However, a current compilation of the Li–Ge system50 did not include Grüttner's results and, hence, significant information is missing. Therefore and due to the recent improvements of the Li–Si phase diagram (>76 at% Li),8–10 a detailed redetermination of the respective portion of the Li–Ge system is essential.The lithium-rich section of the Li–Ge phase diagram (>79 at% Li) was studied by DSC investigations of samples with systematically different compositions Li17Ge4, “Li16.5Ge4” and “Li16Ge4”. Accordingly, PXRD patterns correspond to pure-phase Li17Ge4, a mixture of Li17Ge4 and Li16.38Ge4, and a mixture of Li16.38Ge4 and Li15Ge4, respectively (Fig. 7).
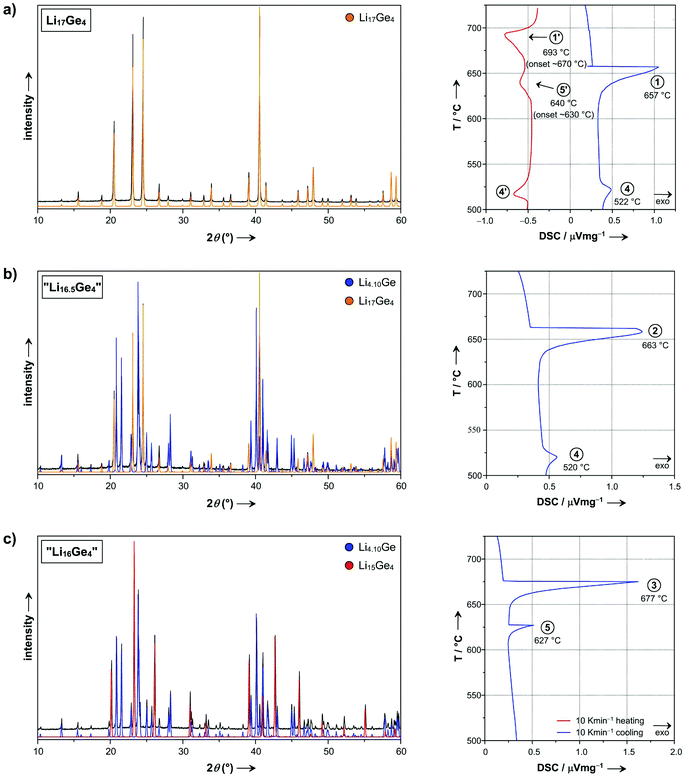 | ||
| Fig. 7 PXRD patterns and corresponding DSC thermograms of bulk samples (a) Li17Ge4, (b) Li16.5Ge4, and (c) Li16Ge4 (PXRD patterns: experimental = black, Li17Ge4 (calc.) = yellow, Li4.10Ge (calc.) = blue, Li15Ge4 (calc.) = red; DSC thermograms: heating and cooling traces are shown in red and blue, respectively, a signal assignment is given in the Li–Ge phase diagram in Fig. 8b). | ||
The thermograms of these samples are depicted in Fig. 7. The first thermal events in these cooling traces (signal 1–3) are assigned to the crossing of the liquidus boundary. Analogously to the Li–Si system,10 signals (4) and (5) at 520–522 °C and 627 °C are attributed to the peritectic formation temperatures of Li17Ge4 (481–486 °C for Li17Si4) and Li16.38Ge4 (618 °C for Li16.42Si4), respectively. This is additionally strengthened by our results from melt equilibration experiments (see above) where crystals of Li16.38Ge4 are only afforded above temperatures of 520 °C. Note that signal (5) corresponding to the peritectic formation of Li16.38Ge4 from melt and Li15Ge4 is superimposed by signals (1) and (2) in the cooling traces of Li17Ge4 and “Li16.5Ge4” (Fig. 7), respectively, but clearly visible for the “Li16Ge4” sample. Instead, it can be recognized at around 630 °C in the respective heating trace, exemplarily shown for Li17Ge4 (effect 5′). Furthermore, long term annealing experiments of “Li16Ge4” samples (Fig. S1 in the ESI†) established Li16.38Ge4 as a high-temperature phase being stable above ∼400 °C until 627 °C, just like Li16.42Si4 existing between 470 and 618 °C.10 We note that the decomposition behavior of Li16.38Ge4 is very sluggish and even harder to trace than in the case of its Si counterpart.
Finally, the recent results from DSC investigations are compiled in an updated Li–Ge phase diagram (Fig. 8b) which was revised from Grüttner's previously determined one shown in Fig. 8a. A comparison with the Li–Si phase diagram (Fig. 8c) reveals similarities to the Li–Ge system. An interesting difference is the stability of Li15Tt4. Whereas Li15Si4 is metastable and decomposes above ∼170 °C,8 Li15Ge4 is thermodynamically stable and melts congruently at 720 °C.25 Furthermore, uncertainties regarding the isotherms at 610 °C and 618 °C in the Li–Si phase diagram10 could not be found for the Li–Ge system.
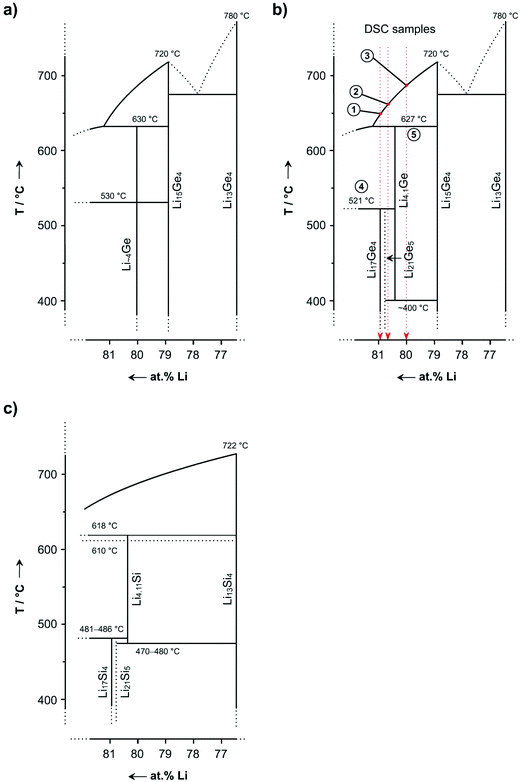 | ||
| Fig. 8 (a) Excerpt of the Li–Ge phase diagram as reported from Grüttner25 (the composition of the eutectic between Li15Ge4 and Li13Ge4 was not determined), (b) its revision based on DSC investigations and annealing experiments of “Li16Ge4” samples reported herein, and (c) the most recent Li–Si phase diagram for Li concentrations >76%.10 | ||
Conclusion
The germanides Li17Ge4 and Li16.38Ge4 were established as further representatives of the Li–Ge system. The latter is assigned a high-temperature phase which exists between ∼400 and 627 °C, the former, the lithium-richest Li–Ge phase, decomposes peritectically at 521 °C into melt and Li16.38Ge4. Li16.38Ge4 can be retained at room temperature if according melts are cooled to the respective temperature region and subsequently quenched. Both germanides are isotypic to the silicides Li17Si4 and Li16.42Si4 extending the family of isotypic lithium tetrelides to Li17Tt4, Li16.4Tt4, Li15Tt4, Li14Tt6, Li12Tt7, and LiTt (Tt = Si, Ge). The previously reported Li16.95Ge4 can be regarded as representative of a homogeneity region Li17−xTt4 (0 < x < 0.2) with Li17Ge4 and Li16.8Ge4 (Li21Ge5) as border phases only differing in the occupation of the Wyckoff position 4a. Moreover, the validity of Vegard's law for the solid solutions Li17Si4−xGex was confirmed and small differences in the distribution of Si and Ge to the four crystallographically independent atom positions were observed. Regarding the thermodynamic properties, the regions of stability for Li17Tt4 and Li16.42Tt4 are very similar. Interesting are also the thermodynamic and structural differences of lithium silicides and germanides. For instance, whereas Li15Si4 is a metastable phase, Li15Ge4 melts congruently at 720 °C. Further, the phases Li9Ge4 and Li7Ge12 are not known in the Li–Si system. In particular, the synthesis of a hypothetical phase Li7Si12 would be an intriguing field of research since it may serve as a precursor for a new allotrope of Si, just as was reported for Li7Ge12 and its mild oxidation to allo-Ge.36,51Experimental section
Synthesis
Starting materials were Li rods (99%, Rockwood-Lithium), Si powder (99.999%, Alfa Aesar) and Ge pieces (99.999%, Chempur). All steps of synthesis and sample preparation were carried out in a glove box (MBraun, Ar atmosphere, H2O and O2 levels <0.1 ppm). Ta and stainless steel ampules were thoroughly cleaned, heated to 1000 °C (Ta) or 800 °C (stainless steel) under dynamic vacuum (p < 1 × 10−3 mbar) for at least 2 h and transferred to the glove box. An all-glass Schlenk line supplied with Ar, which is dried over P2O5, molecular sieve and heated titanium sponge (T = 750 °C), was used for heating and handling under inert conditions.Large single crystals of Li17Ge4 and Li4.10Ge (Li16.38Ge4) (cf. Fig. 1) were obtained from equilibrating melts with compositions “Li95Ge5” and “Li85Ge15” at temperatures of 400 and 530 °C in Ta ampules (slow cooling from 700 °C at a rate of 5 K h−1 followed by 48 hours dwelling at specified temperatures) and subsequent isothermal melt-centrifugation. Details of this procedure have already been described in ref. 9 and 52. Crystals of Li17Si4−xGex [x = 0, 2.30(2), 3.08(4), 3.53(3)] were grown analogously in stainless steel ampules from melts with compositions “Li90Si10”, “Li90Si7.5Ge2.5”, “Li90Si5Ge5” and “Li90Si2.5Ge7.5” equilibrated at 450 °C.
Furthermore, elemental mixtures with compositions Li17Ge4, “Li16.5Ge4” and “Li16Ge4” with a total amount of 2.5 g each were loaded into Ta ampules which were sealed by arc welding inside the glove box. For achieving targeted compositions with sufficient precision, a batch size of 2.5 g was deemed appropriate to keep weighing errors minimal.9,10 Subsequently, ampules were sealed in silica jackets under vacuum and annealed in a muffle furnace. The temperature was raised to 750 °C at a rate of 10 K min−1 and held for 0.5 h followed by cooling to 500–550 °C at a rate of 10 K min−1. After a dwell time of one hour, ampules were quenched in water and transferred back to the glove box. Obtained products were ground in agate mortars and characterized by powder X-ray diffraction (cf. Fig. 7).
Differential scanning calorimetry (DSC)
Differential scanning calorimetry was carried out with a Netzsch DSC 404 Pegasus apparatus. Cylindrical Nb crucibles (L = 15.0 mm, OD = 6.5 mm, ID = 5.0 mm) were thoroughly cleaned, heated to 1000 °C under dynamic vacuum (p < 1 × 10−3 mbar) for 2 h and transferred to an Ar-filled glove box. Crucibles were loaded with 30–50 mg of the sample (Li17Ge4, “Li16.5Ge4” and “Li16Ge4”). Subsequently, the open end was roughly closed by crimping and then sealed by arc-welding inside the glove box under cooling. A sealed Nb crucible without the sample served as a reference. For all measurements an Ar-flow of 60–70 mL min−1 and a heating/cooling rate of 10 K min−1 were used. Samples were recovered after the measurement inside an Ar-filled glove box. Data were handled with the program Proteus Thermal Analysis.53Annealing experiments
In order to further investigate the thermodynamic stability of Li4.10Ge (Li16.38Ge4), batches of 100–150 mg of “Li16Ge4” bulk material were sealed in Ta ampules and annealed in a muffle furnace at 200, 400 and 510 °C (10 K min−1 heating rate) for three days. Thereafter, ampules were quenched in water and transferred inside an Ar-filled glove box. Products were ground in agate mortars and subsequently characterized by powder X-ray diffraction (Fig. S1†).Single crystal X-ray diffraction and structure determination
Crystals of Li17Ge4 and Li4.096(4)Ge (Li16.38(2)Ge4) were handled in an Ar-filled glove box, selected under a microscope and sealed inside glass capillaries. For the best specimen, intensity data were collected at room temperature (Li17Ge4) and 123 K (Li16.38(2)Ge4) on a Bruker X-ray diffractometer equipped with a CCD detector (Apex II, κ-CCD), a fine-focused sealed tube with MoKα radiation (λ = 0.71073 Å) and a graphite monochromator using the Bruker Apex2 software.54 Integration, data reduction and absorption correction were done using SAINT and SADABS.55,56 The space groups F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (Li17Ge4) and Cmcm (Li16.38(2)Ge4) were assigned on the basis of the systematic absence and the statistical analysis of the intensity distributions. For Li17Ge4, Friedel pairs were not merged since the assigned space group is non-centrosymmetric. The Flack57 parameter was determined as 0.50(3). The structures were solved by direct methods (Shelxs-9758) and refined with full-matrix least squares on F2 (Shelxl-9759). Difference Fourier maps Fo − Fc were calculated with Jana2006.60 All refinement results are compiled in Table 6.
3m (Li17Ge4) and Cmcm (Li16.38(2)Ge4) were assigned on the basis of the systematic absence and the statistical analysis of the intensity distributions. For Li17Ge4, Friedel pairs were not merged since the assigned space group is non-centrosymmetric. The Flack57 parameter was determined as 0.50(3). The structures were solved by direct methods (Shelxs-9758) and refined with full-matrix least squares on F2 (Shelxl-9759). Difference Fourier maps Fo − Fc were calculated with Jana2006.60 All refinement results are compiled in Table 6.
| a R 1 = ∑||Fo|−|Fc||/∑|Fo|. b wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | ||
|---|---|---|
| Empirical formula | Li17Ge4 | Li4.096(4)Ge |
| T/K | 298(2) | 123(2) |
| Formula weight/g·mol−1 | 408.34 | 101.01 |
| Crystal size/mm3 | 0.40 × 0.40 × 0.35 | 0.37 × 0.24 × 0.23 |
| Crystal color | Metallic silver | Metallic silver |
| Crystal shape | Block | Bar |
| Space group |
F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m 3m |
Cmcm |
| Structure type | Li17Pb4 | Li4.11Si |
| Unit cell dimension/Å | a = 18.8521(3) | a = 4.5511(2) |
| b = 22.0862(7) | ||
| c = 13.2751(4) | ||
| V/Å3 | 6700.1(2) | 1334.37(8) |
| Z | 20 | 16 |
| ρ (calc.)/g cm−3 | 2.024 | 2.011 |
| μ/mm−1 | 8.832 | 8.860 |
| F (000) | 3580 | 709 |
| θ Range/° | 1.87–45.26 | 1.84–40.25 |
| Index range hkl | −37 ≤ h ≤ +23 | −6 ≤ h ≤ +8 |
| −37 ≤ k ≤ +30 | −32 ≤ k ≤ +40 | |
| −37 ≤ l ≤ +37 | −24 ≤ l ≤ +19 | |
| Reflections collected | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241 241 |
| Independent reflections | 2757 (Rint = 0.047) | 2369 (Rint = 0.023) |
| Reflections with I > 2σ(I) | 2547 (Rσ = 0.017) | 1920 (Rσ = 0.017) |
| Data/restraints/parameter | 2757/0/68 | 2369/0/82 |
| Absorption correction | Multi-scan | Multi-scan |
| Goodness-of-fit on F2 | 1.106 | 1.042 |
| Final R indices [I > 2σ(I)]a,b | R 1 = 0.018 | R 1 = 0.015 |
| wR2 = 0.037 | wR2 = 0.026 | |
| R indices (all data)a,b | R 1 = 0.022 | R 1 = 0.023 |
| wR2 = 0.038 | wR2 = 0.027 | |
| Extinction coefficient | 1.59(9) × 10−4 | 1.52(7) × 10−3 |
| Flack parameter | 0.50(3) | — |
| Largest diff. peak and hole/e Å−3 | 0.46 and −0.69 | 0.69 and −0.52 |
Details of the single crystal X-ray structure determination, refinement data, fractional atomic coordinates and isotropic equivalent atomic displacement parameters for Li17Si4−xGex [(x = 2.30(2), 3.08(4), 3.53(3)] are given in Table S1–4 in the ESI.† Further data may be obtained from Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen, Germany (fax: (+49)7247-808-666; e-mail: crysdata@fiz-karlsruhe.de) on quoting the depository numbers CSD-427231 (Li4.10Ge (Li16.38Ge4)), CSD-427232 (Li17Ge4), CSD-427233 (Li17Si0.47Ge3.53), CSD-427234 (Li17Si0.92Ge3.08), and CSD-427235 (Li17Si1.70Ge2.30).
Powder X-ray diffraction (PXRD)
PXRD patterns were recorded on a Stoe Stadi P diffractometer (Ge(111) monochromator for CuKα1 radiation, λ = 1.54056 Å) equipped with a Dectris Mythen DCS 1 K solid state detector. Investigated samples were (i) single crystals of Li17Si4−xGex (x = 0, 2.30(2), 3.08(4), 3.53(3), 4), (ii) bulk samples of Li17Ge4, “Li16.5Ge4” and “Li16Ge4”, and (iii) samples of “Li16Ge4” annealed at different temperatures. These were thoroughly ground in agate mortars, sealed inside 0.3 mm glass capillaries and measured in a 2θ-range of 5–90° (PSD steps: 0.06–1.00°; time per step: 20–40 s).Energy dispersive X-ray spectroscopy (EDX)
A Jeol-JSM 7500F scanning electron microscope equipped with an Oxford X-Max EDX analyzer with Mn as an internal standard was used for determining the Si–Ge ratios in Li17Si4−xGex [x = 2.30(2), 3.08(4), 3.53(3)]. Samples were handled inside an Ar-filled glove box and fixed on a graphite platelet which was mounted on an aluminum stub.Acknowledgements
M.Z. thanks the Fonds der Chemischen Industrie for his fellowship, the authors thank K. Rodewald for EDX measurements.References
- M. N. Obrovac and L. Christensen, Electrochem. Solid-State Lett., 2004, 7, A93–A96 CrossRef CAS PubMed.
- T. D. Hatchard and J. R. Dahn, J. Electrochem. Soc., 2004, 151, A838–A842 CrossRef CAS PubMed.
- W. J. Zhang, J. Power Sources, 2011, 196, 13–24 CrossRef CAS PubMed.
- P. Limthongkul, Y. I. Jang, N. J. Dudney and Y. M. Chiang, Acta Mater., 2003, 51, 1103–1113 CrossRef CAS.
- P. Limthongkul, Y. I. Jang, N. J. Dudney and Y. M. Chiang, J. Power Sources, 2003, 119–121, 604–609 CrossRef CAS.
- B. Key, M. Morcrette, J. M. Tarascon and C. P. Grey, J. Am. Chem. Soc., 2011, 133, 503–512 CrossRef CAS PubMed.
- B. Key, R. Bhattacharyya, M. Morcrette, V. Seznec, J. M. Tarascon and C. P. Grey, J. Am. Chem. Soc., 2009, 131, 9239–9249 CrossRef CAS PubMed.
- M. Zeilinger, V. Baran, L. van Wüllen, U. Häussermann and T. F. Fässler, Chem. Mater., 2013, 25, 4113–4121 CrossRef CAS.
- M. Zeilinger, D. Benson, U. Häussermann and T. F. Fässler, Chem. Mater., 2013, 25, 1960–1967 CrossRef CAS.
- M. Zeilinger, I. M. Kurylyshyn, U. Häussermann and T. F. Fässler, Chem. Mater., 2013, 25, 4623–4632 CrossRef CAS.
- M. Zeilinger and T. F. Fässler, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, 69, i81–i82 CAS.
- D. Thomas, M. Abdel-Hafiez, T. Gruber, R. Hüttl, J. Seidel, A. U. B. Wolter, B. Büchner, J. Kortus and F. Mertens, J. Chem. Thermodyn., 2013, 64, 205–225 CrossRef CAS PubMed.
- P. Wang, A. Kozlov, D. Thomas, F. Mertens and R. Schmid-Fetzer, Intermetallics, 2013, 42, 137–145 CrossRef CAS PubMed.
- A. Debski, W. Gasior and A. Goral, Intermetallics, 2012, 26, 157–161 CrossRef CAS PubMed.
- S. Dupke, T. Langer, R. Pöttgen, M. Winter, S. Passerini and H. Eckert, Phys. Chem. Chem. Phys., 2012, 14, 6496–6508 RSC.
- S. Dupke, T. Langer, R. Pöttgen, M. Winter and H. Eckert, Solid State Nucl. Magn. Reson., 2012, 42, 17–25 CrossRef CAS PubMed.
- A. Kuhn, P. Sreeraj, R. Pöttgen, H. D. Wiemhöfer, M. Wilkening and P. Heitjans, J. Am. Chem. Soc., 2011, 133, 11018–11021 CrossRef CAS PubMed.
- G. R. Goward, N. J. Taylor, D. C. S. Souza and L. F. Nazar, J. Alloys Compd., 2001, 329, 82–91 CrossRef CAS.
- C. S. Fuller and J. C. Severiens, Phys. Rev., 1954, 96, 21–24 CrossRef CAS.
- J. Graetz, C. C. Ahn, R. Yazami and B. Fultz, J. Electrochem. Soc., 2004, 151, A698–A702 CrossRef CAS PubMed.
- E. M. Pell, J. Phys. Chem. Solids, 1957, 3, 74–76 CrossRef CAS.
- G. I. Oleksiv, Probl. Rozvitku Prirodn. i Tochn. Nauk, Sb., 1964, 76–77 CAS.
- P. I. Federov and V. A. Molochka, Izv. Akad. Nauk. SSSR, Neorg. Mater., 1966, 2, 1870–1871 Search PubMed.
- R. Nesper, Prog. Solid State Chem., 1990, 20, 1–45 CrossRef CAS.
- A. Grüttner, PhD thesis, University of Stuttgart, 1982.
- A. Grüttner, R. Nesper and H. G. von Schnering, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1981, 37, C161 Search PubMed.
- E. I. Gladyshevskii, G. I. Oleksiv and P. I. Kripyakevich, Kristallografiya, 1964, 9, 338–341 CAS.
- Q. Johnson, G. S. Smith and D. Wood, Acta Crystallogr., 1965, 18, 131–132 CrossRef CAS.
- E. I. Gladyshevskii and P. I. Kripyakevich, Kristallografiya, 1960, 5, 574–576 CAS.
- R. Nesper, Habilitation, University of Stuttgart, 1988 Search PubMed.
- V. Hopf, W. Müller and H. Schäfer, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1972, 27, 1157–1160 CAS.
- V. Hopf, H. Schäfer and A. Weiss, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1970, 25, 653 CAS.
- H. G. von Schnering, R. Nesper, J. Curda and K. F. Tebbe, Angew. Chem., 1980, 92, 1070 CrossRef.
- E. Menges, V. Hopf, H. Schäfer and A. Weiss, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1969, 21, 1351–1352 Search PubMed . According to ref. 25, the structures of LiGe reported in ref. 22 and 34 are identical (only two different cell settings).
- J. Evers, G. Oehlinger, G. Sextl and H. O. Becker, Angew. Chem., Int. Ed. Engl., 1987, 26, 76–78 CrossRef.
- A. Grüttner, R. Nesper and H. G. von Schnering, Angew. Chem., Int. Ed. Engl., 1982, 21, 912–913 CrossRef.
- U. Frank and W. Müller, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1975, 30, 313–315 Search PubMed.
- R. Nesper, J. Curda and H. G. von Schnering, J. Solid State Chem., 1986, 62, 199–206 CrossRef CAS.
- J. Evers, G. Oehlinger and G. Sextl, Angew. Chem., Int. Ed. Engl., 1993, 32, 1442–1444 CrossRef.
- J. Evers and G. Oehlinger, Angew. Chem., Int. Ed., 2001, 40, 1050–1053 CrossRef CAS.
- F. Kiefer and T. F. Fässler, Solid State Sci., 2011, 13, 636–640 CrossRef CAS PubMed.
- Y. Hashimoto, N. Machida and T. Shigematsu, Solid State Ionics, 2004, 175, 177–180 CrossRef CAS PubMed.
- L. Vegard, Z. Phys., 1921, 5, 17–26 CrossRef CAS.
- C. Lupu, J. G. Mao, J. W. Rabalais, A. M. Guloy and J. W. Richardson, Inorg. Chem., 2003, 42, 3765–3771 CrossRef CAS PubMed.
- H. Axel, H. Schäfer and A. Weiss, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1966, 21, 115–117 Search PubMed.
- R. Nesper and H. G. von Schnering, J. Solid State Chem., 1987, 70, 48–57 CrossRef CAS.
- L. Lacroix-Orio, M. Tillard and C. Belin, J. Alloys Compd., 2008, 465, 47–50 CrossRef CAS PubMed.
- G. J. Miller, Eur. J. Inorg. Chem., 1998, 523–536 CrossRef CAS.
- N. Kazem, W. Xie, S. Ohno, A. Zevalkink, G. J. Miller, G. J. Snyder and S. M. Kauzlarich, Chem. Mater., 2014, 26, 1393–1403 CrossRef CAS.
- J. Sangster and A. D. Pelton, J. Phase Equilib., 1997, 18, 289–294 CrossRef CAS and references therein.
- F. Kiefer, A. J. Karttunen, M. Döblinger and T. F. Fässler, Chem. Mater., 2011, 23, 4578–4586 CrossRef CAS.
- K. Puhakainen, M. Boström, T. L. Groy and U. Häussermann, J. Solid State Chem., 2010, 183, 2528–2533 CrossRef CAS PubMed.
- Netzsch Proteus Thermal Analysis, Version 4.8.2, Netzsch-Gerätebau GmbH, Selb, Germany, 2006 Search PubMed.
- APEX suite of crystallographic software, APEX 2 Version 2008.4, Bruker AXS Inc., Madison, WI, USA, 2008 Search PubMed.
- SAINT, Version 7.56a, Bruker AXS Inc., Madison, WI, USA, 2008 Search PubMed.
- SADABS, Version 2008/1, Bruker AXS Inc., Madison, WI, USA, 2008 Search PubMed.
- H. D. Flack, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 1983, 39, 876 CrossRef.
- G. M. Sheldrick, Shelxs-97 – Program for the Determination of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, Shelxl-97 – Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- V. Petricek, M. Dusek and L. Palatinus, Jana 2006: The Crystallographic Computing System, Version 03/15/2013, Institute of Physics, Praha, Czech Rupublic, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, refinement results, fractional atomic coordinates, and isotropic equivalent atomic displacement parameters for Li17Si4−xGex [x = 2.30(2), 3.08(4), 3.53(3)] (Table S1–4), comparison of experimental and computational relaxed fractional atomic coordinates for both Li21Si5 and Li17Si4 (Table S5), experimental fractional atomic coordinates for Li17Si4, Li17Ge4, Li17−εZnεGe4, and Li16.95Ge4 (Table S6), comparison of experimental fractional atomic coordinates for Li16.95Ge4 and computational relaxed ones for Li21Si5 (Table S7), PXRD patterns of “Li16Ge4” samples annealed at various temperatures (Fig. S1), Rietveld refinement results for Li17Si4−xGex (x = 0, 2.30(2), 3.08(4), 3.53(3), 4) (Fig. S2). See DOI: 10.1039/c4dt00743c |
| This journal is © The Royal Society of Chemistry 2014 |