The reductive supercritical hydrothermal process, a novel synthesis method for cobalt nanoparticles: synthesis and investigation on the reaction mechanism
Abstract
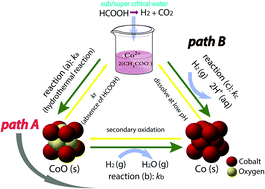
Highly crystalline cobalt nanoparticles with low surface oxidation were synthesized by the reductive supercritical hydrothermal process in the temperature range from 340 to 420 °C. Under these reaction conditions, hydrogen generated from formic acid decomposition is maximally soluble in water, enabling the effective reduction of cobalt ions and cobalt oxide. The reaction mechanism was investigated by kinetic analysis on the formation of cobalt nanoparticles. This analysis assumed the first order irreversible reaction and two different types of shrinking core models (chemical reaction and inter-diffusion dominated). According to the proposed reaction mechanism, cobalt monoxide is probably formed at the early reaction stage, where insufficient H2 is available, or under high temperature conditions. Moreover, cobalt monoxide influences the entire reaction rate. Thus, suppressing the formation and growth of cobalt monoxide is of primary importance in the optimal synthesis of cobalt nanoparticles by the reductive supercritical hydrothermal process.

 Please wait while we load your content...
Please wait while we load your content...