 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pentadienyl chemistry of the heavy alkaline-earth metals revisited†
Matthias
Reiners
,
Ann Christin
Fecker
,
Matthias
Freytag
,
Peter G.
Jones
and
Marc D.
Walter
*
Institut für Anorganische und Analytische Chemie, Technische Universität, Braunschweig, Hagenring 30, 38106 Braunschweig, Germany. E-mail: mwalter@tu-bs.de; Fax: +49 531 391 5387; Tel: +49 531 391 5312
First published on 20th March 2014
Abstract
Open-metallocenes of the heavy alkaline-earth metals [(η5-Pdl′)2M(thf)n] (M = Ca (1), Sr (2), n = 1; M = Ba (3), n = 2; Pdl′ = 2,4-tBu2C5H5) are readily prepared by salt-metathesis between MI2 and KPdl′ and characterized by NMR spectroscopy and X-ray diffraction studies.
Over the last decade organometallic calcium, strontium and barium complexes have attracted some interest,1–3e.g. as precursors for metalorganic vapour deposition (MOCVD)4,5 or as catalyst systems in polymerisation and hydrofunctionalization reactions.6–9 Nevertheless, the isolation and characterization of these highly reactive compounds required the development of suitable ligands and synthetic techniques. In this context, the η5-coordinate cyclopentadienyl ligand has played a prominent role in stabilizing half-sandwich and metallocene complexes.10–16 More recently, these investigations have been extended to allyl systems, which can adopt both π-(η3) and σ-(κC) bonding modes.17–25 Pentadienyls, with their ability to adopt κC-, η3- and η5-coordination modes, occupy an intermediate position between the cyclopentadienyl and allyl systems, and their coordination chemistry with transition metals has been well established.26–31 Surprisingly, reports on group 2 complexes (Be,32 Mg,33 and Ca34) show only two of them are structurally characterized, [(κC-2,4-Me2C5H5)2Mg(tmeda)]33 and [(η5-Pdl′)2Ca(thf)] (Pdl′ = 2,4-tBu2C5H5; 1).34 Overby and Hanusa report that the Pdl′ ligand provides only limited solubility, thus precluding the synthesis of the open metallocenes of the heavier homologues, Sr and Ba,34 but this is in contrast to the general observation that sterically demanding substituents such as tBu and SiMe3 enhance solubility and stabilize the corresponding metal complexes. For instance, several heavy alkaline-earth metal complexes have been successfully prepared with tBu-substituted cyclopentadienyl,5,35,36 pyrrolyl37 and P-heterocyclic38,39 ligands, and all exhibit good solubility in aliphatic and aromatic hydrocarbon solvents. Within our research program on pentadienyl ligands,40 we therefore decided to revisit the heavy alkaline-earth metals.
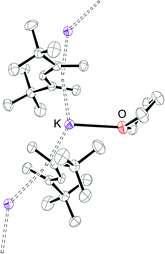
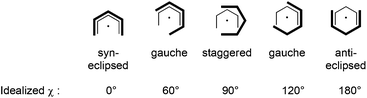
The potassium salt KPdl′41 was prepared according to a modified literature procedure† and can be crystallized as its thf-adduct from saturated THF solutions at −30 °C.‡ A snapshot of the molecular structure of [(thf)K(μ–η5:η5-Pdl′)]∞ is shown in Fig. 1 and reveals that the U-shaped η5-Pdl′ anion is coordinated in the solid state by two potassium cations to form a zigzag polymeric chain via a 21 screw axis. Selected bond distances and angles are listed in Table 1. The C–C bond distances within the pentadienyl moiety (C1–C5) follow the typical short–long–long–short pattern. The conformation angle χ is defined as the angle between the two planes, [centroid(C1–C5)–C3–M] and [centroid(C1′–C5′)–C3′–M], and allows the mutual orientation of the two pentadienyl moieties to be quantified. In [(thf)K(μ-Pdl′)]∞ the pentadienyl ligands adopt a nearly anti-ecliptic conformation with χ = 178.5°, thereby minimizing repulsive interactions between the sterically demanding tBu-groups.
| [(thf)K(μ-Pdl′)]x | 1 | 2 | 3 | |
|---|---|---|---|---|
| a Two independent molecules in the asymmetric unit. b Pdlcentroid is the centroid of the pentadienyl ligand. c α is defined as the angle formed by the two dienyl planes. | ||||
| M–C (pdl) (Å, range) | 3.0166(19)–3.235(3) | 2.7908(10)–2.6681(10) | 2.786(3)–2.978(3)/2.800(3)–3.010(3) | 2.9627(14)–3.1937(17) |
| M–C1/5 (pdl) (Å, average) | 3.159(65) | 2.722(54) | 2.902(59)/2.907(78) | 3.147(33) |
| M–C2/4 (pdl) (Å, average) | 3.056(16) | 2.768(31) | 2.888(20)/2.896(27) | 3.113(10) |
| M–C3 (pdl) (Å, average) | 2.982(50) | 2.728(28) | 2.805(27)/2.817(23) | 2.981(26) |
| M–C (pdl) (Å, average) | 3.082 ± 0.083 | 2.738 ± 0.040 | 2.887 ± 0.053/2.884 ± 0.060 | 3.100 ± 0.068 |
| M–Pdlcentroidb (Å, average) | 2.70 | 2.28 | 2.45/2.46 | 2.70 |
| C1–C5 (Å, mean) | 3.20 | 3.18 | 3.19/3.18 | 3.21 |
| M–O (Å) | 2.7577(18) | 2.4074(7) | 2.5383(17)/2.5224(17) | 2.7488(11)/2.7943(12) |
| Pdlcentroid–M–Pdlcentroidb (°) | 165.5 | 145.6 | 143.5/143.6 | 130.9 |
| α | 29.9 | 28.8 | 30.3/33.4 | 53.1 |
| χ | 178.5 | 175.0 | 176.1/176.0 | 124.8 |
In contrast to the previous report,34 the salt metatheses of two equivalents of KPdl′ with MI2 (M = Ca, Sr, Ba) proceed smoothly at ambient temperature and yield the corresponding open-metallocenes [(η5-Pdl′)2M(thf)n] (M = Ca (1), Sr (2), n = 1; M = Ba (3), n = 2) in crystalline form and moderate yields (eqn (1)).†
 | (1) |
Nevertheless, we also found that the anionic Pdl′ ligand behaves as a strong base and readily undergoes radical coupling to 2,4,7,9-tetra-tert-butyl-1,3,7,9-decatetraene.41,42 Therefore, it is imperative for the synthesis of these complexes to use metal iodides MI2 of high purity. Trace amounts of NH3 or I2 as a result of the MI2 preparation must be removed, otherwise the yield of the salt metathesis reaction is dramatically reduced and insoluble material is obtained. However, pure open-metallocenes 1–3 are readily soluble in aliphatic hydrocarbons (pentane, hexane), aromatic solvents (benzene, toluene) and ethers (tetrahydrofuran, diethyl ether), and can readily be crystallized as their thf adducts from concentrated pentane solutions at −30 °C.‡ Since our synthetic protocol yields very soluble products, we also recorded 1H and 13C{1H} NMR spectra in C6D6 of these complexes and as expected, the NMR spectra of these complexes are very similar. In addition, no dynamic behaviour of the Pdl′ ligands is observed at ambient temperature in solution.†
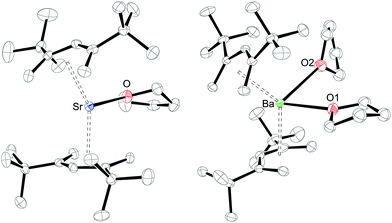
The molecular structures of 2 and 3 are shown in Fig. 2, and relevant bond distances and angles are listed in Table 1. In addition significantly improved X-ray diffraction data for the open-calcocene 1 were obtained, so we decided, for a better comparison, to include our data in Table 1. In all cases, the Pdl′ ligand adopts a η5-U coordination mode and the structural features of these complexes resemble those of 1, except that two THF ligands are coordinated in the case of Ba. Whereas one THF ligand resides in the usual position at the open edge of one Pdl′ ligand, the second THF molecule is positioned at the backside of the second pentadienyl ligand. In [(η5-2,4-Me2C5H5)2Zr(CO)2] the two CO ligands feature a similar orientation with respect to the dienyl ligands.43 The conformational angles χ range from 175° for 1 to 124.8° for 3 and the predominant driving force for these arrangements is the minimization of repulsive interactions between the Pdl′ and thf ligands. Furthermore, the C–C distances within the pentadienyl system show a distinct short–long–long–short-pattern. Previous studies on [(η5-2,4-Me2C5H5)3Nd], which also shows ionic bonding between the dienyl ligand and the metal cation, established that the M–C bonds to the formally charged C1-, C3-, and C5-positions are shorter than those to the uncharged C2- and C4-positions.44 For the complexes 1–3 the M–C distances vary significantly and the shortest is found to be the central carbon atom of the Pdl′ ligand (C3-position) consistent with the observation on [(η5-2,4-Me2C5H5)3Nd]. However, the average Ca–C1/5 distances (2.722(54) Å) in 1 are essentially identical to the average Ca–C3 distances (2.728(28) Å), a different trend is observed for the heavier homologues 2 and 3, for which the average M–C2/4 and M–C1/5 bonds become progressively longer (Table 1). The increase in the average M–C distances of 2.738(40) Å in 1 to 3.100(68) Å in 3 nicely mirrors the increase in ionic radii for heptacoordinate Ca2+ (1.06 Å) and Sr2+ (1.21 Å) and octacoordinate Ba2+ (1.40 Å).45 These observations can be compared to those in metal–allyl complexes, for which average M–C distances in [(η3-1,3-(Me3Si)2C3H3)2Ca(thf)2] (2.654(4) Å),17 [(η3-C3H5)2(trigylme-κ4)] (2.727(72) Å),46 and [(η3-1,3-(Me3Si)2C3H3)2Sr(thf)2] (2.801 Å)21 are found. All attempts to prepare a momomeric allyl Ba analogue resulted in the formation of a heterometallic barium/potassium complex, [K(thf)Ba2(1,3-(Me3Si)2C3H3)5].21 This further supports the important features of the pentadienyl fragment in the stabilization of the heavy-alkaline earth metals by adopting an intermediate position between the allyl and cyclopentadienyl fragments. Furthermore, the average M–C (M = Ca, Sr, Ba) bond distances in 1–3 are longer than those in the related metallocenes, e.g. [(η5-1,3-(Me3Si)2C5H3)2M(thf)] (M = Ca (2.678(14) Å),47 Sr (2.82 Å)48) and [(η5-1,2,4-(Me3C)3C5H2)2Sr(thf)] (2.87 Å).35 This presumably indicates a more flexible and therefore weaker metal–ligand bonding in the pentadienyl systems, and therefore a hapticity switch should be relatively facile in the open group 2 metallocenes.
Conclusions
We have extended the series of group 2 open-metallocenes to Sr and Ba and established a reliable synthetic protocol that allows the preparation of synthetically useful quantities. In all cases the Pdl′ ligand adopts a η5-U coordination mode in the solid state and in solution. It can be expected that the other group 2 open-metallocenes are also accessible and that they exhibit broad reactivity towards unsaturated molecules. These and related studies are ongoing and will be reported in due course.Acknowledgements
MDW acknowledges the financial support from the TU Braunschweig through the “Zukunftsfonds” and from the Deutsche Forschungsgemeinschaft (DFG) through the Emmy Noether program (WA 2513/2).Notes and references
- M. Westerhausen, Z. Anorg. Allg. Chem., 2009, 635, 13 CrossRef CAS PubMed.
- S. Krieck, L. Yu, M. Reiher and M. Westerhausen, Eur. J. Inorg. Chem., 2010, 197 CrossRef CAS PubMed.
- M. Westerhausen, J. Langer, S. Krieck, R. Fischer, H. Goerls and M. Koehler, Top. Organomet. Chem., 2013, 45, 29 CrossRef CAS.
- W. A. Wojtczak, P. F. Fleig and M. J. Hampden-Smith, Adv. Organomet. Chem., 1996, 40, 215 CrossRef CAS.
- T. Hatanpää, M. Vehkamaeki, I. Mutikainen, J. Kansikas, M. Ritala and M. Leskelae, Dalton Trans., 2004, 1181 RSC.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484 RSC.
- C. A. Wheaton, P. G. Hayes and B. J. Ireland, Dalton Trans., 2009, 4832 RSC.
- S. Harder, Chem. Rev., 2010, 110, 3852 CrossRef CAS PubMed.
- A. G. M. Barrett, M. R. Crimmin, M. S. Hill and P. A. Procopiou, Proc. R. Soc. London, Ser. A, 2010, 466, 927 CrossRef CAS.
- T. P. Hanusa, Chem. Rev., 1993, 93, 1023 CrossRef CAS.
- D. J. Burkey and T. P. Hanusa, Comments Inorg. Chem., 1995, 17, 41 CrossRef CAS.
- H. Sitzmann, T. Dezember and M. Ruck, Angew. Chem., Int. Ed., 1998, 37, 3114 CrossRef CAS.
- P. Jutzi and N. Burford, Chem. Rev., 1999, 99, 969 CrossRef CAS PubMed.
- M. J. Harvey, K. T. Quisenberry, T. P. Hanusa and V. G. Young Jr., Eur. J. Inorg. Chem., 2003, 3383 CrossRef CAS PubMed.
- M. D. Walter, G. Wolmershäuser and H. Sitzmann, J. Am. Chem. Soc., 2005, 127, 17494 CrossRef CAS PubMed.
- L. Orzechowski, D. F. J. Piesik, C. Ruspic and S. Harder, Dalton Trans., 2008, 4742 RSC.
- M. J. Harvey, T. P. Hanusa and V. G. Young Jr., Angew. Chem., Int. Ed., 1999, 38, 217 CrossRef CAS.
- K. T. Quisenberry, C. K. Gren, R. E. White, T. P. Hanusa and W. W. Brennessel, Organometallics, 2007, 26, 4354 CrossRef CAS.
- S. C. Chmely, C. N. Carlson, T. P. Hanusa and A. L. Rheingold, J. Am. Chem. Soc., 2009, 131, 6344 CrossRef CAS PubMed.
- S. C. Chmely, T. P. Hanusa and W. W. Brennessel, Angew. Chem., Int. Ed., 2010, 49, 5870 CrossRef CAS PubMed.
- K. T. Quisenberry, R. E. White, T. P. Hanusa and W. W. Brennessel, New J. Chem., 2010, 34, 1579 RSC.
- P. Jochmann, T. S. Dols, T. P. Spaniol, L. Perrin, L. Maron and J. Okuda, Angew. Chem., Int. Ed., 2010, 49, 7795 CrossRef CAS PubMed.
- C. Lichtenberg, P. Jochmann, T. P. Spaniol and J. Okuda, Angew. Chem., Int. Ed., 2011, 50, 5753 CrossRef CAS PubMed.
- P. Jochmann, S. Maslek, T. P. Spaniol and J. Okuda, Organometallics, 2011, 30, 1991 CrossRef CAS.
- P. Jochmann, J. P. Davin, S. Maslek, T. P. Spaniol, Y. Sarazin, J.-F. Carpentier and J. Okuda, Dalton Trans., 2012, 41, 9176 RSC.
- R. D. Ernst, Struct. Bonding, 1984, 57, 1 CrossRef CAS.
- R. D. Ernst, Acc. Chem. Res., 1985, 18, 56 CrossRef CAS.
- P. Powell, Adv. Organomet. Chem., 1986, 26, 125 CrossRef CAS.
- R. D. Ernst, Chem. Rev., 1988, 88, 1255 CrossRef CAS.
- R. D. Ernst, Comments Inorg. Chem., 1999, 21, 285 CrossRef CAS.
- L. Stahl and R. D. Ernst, Adv. Organomet. Chem., 2007, 55, 137 CrossRef.
- H. Yasuda, Y. Ohnuma, A. Nakamura, Y. Kai, N. Yasuoka and N. Kasai, Bull. Chem. Soc. Jpn., 1980, 53, 1101 CrossRef CAS.
- H. Yasuda, M. Yamauchi, A. Nakamura, T. Sei, Y. Kai, N. Yasuoka and N. Kasai, Bull. Chem. Soc. Jpn., 1980, 53, 1089 CrossRef CAS.
- J. S. Overby and T. P. Hanusa, Angew. Chem., Int. Ed. Engl., 1994, 33, 2191 CrossRef PubMed.
- F. Weber, H. Sitzmann, M. Schultz, C. D. Sofield and R. A. Andersen, Organometallics, 2002, 21, 3139 CrossRef CAS.
- T. Hatanpää, M. Ritala and M. Leskela, J. Organomet. Chem., 2007, 692, 5256 CrossRef PubMed.
- H. Schumann, J. Gottfriedsen and J. Demtschuk, Chem. Commun., 1999, 2091 RSC.
- M. Westerhausen, M. H. Digeser, H. Noeth, W. Ponikwar, T. Seifert and K. Polborn, Inorg. Chem., 1999, 38, 3207 CrossRef CAS.
- M. D. Francis, P. B. Hitchcock and J. F. Nixon, Chem. Commun., 2000, 2027 RSC.
- A. C. Fecker, A. Glöckner, C. G. Daniliuc, M. Freytag, P. G. Jones and M. D. Walter, Organometallics, 2013, 32, 874 CrossRef CAS.
- R. D. Ernst, J. W. Freeman, P. N. Swepston and D. R. Wilson, J. Organomet. Chem., 1991, 402, 17 CrossRef CAS.
- J. S. Overby and T. P. Hanusa, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 51, 313 CrossRef.
- T. E. Waldman, L. Stahl, D. R. Wilson, A. M. Arif, J. P. Hutchinson and R. D. Ernst, Organometallics, 1993, 12, 1543 CrossRef CAS.
- R. D. Ernst and T. H. Cymbaluk, Organometallics, 1982, 1, 708 CrossRef CAS.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751 CrossRef.
- P. Jochmann, T. S. Dols, T. P. Spaniol, L. Perrin, L. Maron and J. Okuda, Angew. Chem., Int. Ed., 2009, 48, 5715 CrossRef CAS PubMed.
- P. Jutzi, W. Leffers, G. Müller and B. Huber, Chem. Ber., 1989, 122, 879 CrossRef CAS PubMed.
- L. M. Engelhardt, P. C. Junk, C. L. Raston and A. H. White, J. Chem. Soc., Chem. Commun., 1988, 1500 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, NMR, crystallographic and computational data. CCDC 982693–982696. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt00545g |
‡ Crystal data for [(thf)K(μ–η5:η5-Pdl′)]∞: C17H31KO, M = 290.52, orthorhombic, a = 10.1190(2) Å, b = 10.7148(6) Å, c = 16.4367(9) Å, V = 1782.11(14) Å3, T = 130(2) K, space group P212121, Z = 4 (monomers), μ(CuKα) = 2.5 mm−1, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 reflections measured, 3697 independent reflections (Rint = 0.108). The final R1 values were 0.0498 (I > 2σ(I)) and 0.0572 (all data). The final wR(F2) values were 0.1245 (I > 2σ(I)) and 0.1304 (all data). The goodness of fit on F2 was 1.02. 651 reflections measured, 3697 independent reflections (Rint = 0.108). The final R1 values were 0.0498 (I > 2σ(I)) and 0.0572 (all data). The final wR(F2) values were 0.1245 (I > 2σ(I)) and 0.1304 (all data). The goodness of fit on F2 was 1.02.Crystal data for 1: C30H54OCa, M = 470.81, monoclinic, a = 9.9014(2) Å, b = 21.7075(4) Å, c = 14.4003(3) Å, β = 105.501(2)°, V = 2982.56(10) Å3, T = 100(2) K, space group P21/n, Z = 4, μ(MoKα) = 1.9 mm−1, 78 Crystal data for 2: C30H54OSr, M = 518.35, triclinic, a = 9.7948(5) Å, b = 14.1834(5) Å, c = 23.3962(10) Å, α = 88.549(3)°, β = 79.821(2)°, γ = 71.131(2)°, V = 3025.4(2) Å3, T = 100(2) K, space group P( Crystal data for 3: C34H62BaO2, M = 640.18, triclinic, a = 9.5411(3) Å, b = 12.0829(3) Å, c = 15.6171(5) Å, α = 96.320(2)°, β = 97.530(3)°, γ = 98.661(3)°, V = 1748.97(9) Å3, T = 100(2) K, space group P( |
| This journal is © The Royal Society of Chemistry 2014 |

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ),
),