Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ratiometric detection of enzyme turnover and flavin reduction using rare-earth upconverting phosphors†
Peter
Harvey
abc,
Chloë
Oakland
abc,
Max D.
Driscoll
b,
Sam
Hay
*bc and
Louise S.
Natrajan
*ac
aSchool of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: louise.natrajan@manchester.ac.uk; Tel: +44 (0)161 275 1426
bManchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN, UK. E-mail: Sam.Hay@manchester.ac.uk; Tel: +44 (0)161 306 5141
cThe Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK
First published on 7th February 2014
Abstract
Gd4O2S:Yb:Tm rare-earth upconversion phosphors have been utilised to monitor the redox behaviour of flavin mononucleotide and report on the turnover of a flavo-protein, (pentaerythritol tetranitrate reductase). The presence of two bands separated by over 300 nm in the UCP emission spectra allows ratiometric signalling of these processes with high sensitivity.
Rare-earth upconverting phosphors (UCPs) are a rapidly emerging class of (nano)particles with potential application in sensing and imaging, optical memory, security, and photodynamic therapy.1 Upon excitation with near-infrared (NIR) light, UCPs exhibit efficient photoluminescence in the visible spectrum due to photon upconversion (UC). The type and efficiency of the UC process, in addition to the size and phase of the UCPs, can be tuned by doping the UCPs with varying levels of certain lanthanides2 and involves a non-linear multi-photon absorption process. This mechanism is based on sequential absorption and energy transfer of NIR photons, leading to the population of real, metastable excited states (here of μs order), differing to the virtual excited state involved in two-photon absorption (Fig. S1†).3 Due to this UC behaviour, probes based on these UCPs exhibit no auto-fluorescence (i.e. noise) in biological media and negligible photobleaching.4 These advantages over conventional fluorophores, combined with relatively low associated toxicities,5 has led to a particular focus on bioimaging, with relevant applications in in vitro studies.6 UCPs have also be applied as optical chemosensors, with systems developed for pH, O2, Hg(II), Cu(II), and Fe(III) recognition,7 amongst others. The synthesis, characterisation, and properties of these UCPs have been well summarised in a number of key reviews.8
The unparalleled substrate selectivity and sub-nM substrate affinity of enzymes offers a sensitive and selective method of sensing biomolecules. For enzymes that contain chromophoric substrates/intrinsic cofactors, it should be feasible to induce Förster resonance energy transfer (FRET)9 between suitable UCPs (donor) and enzyme/substrate chromophores (acceptor). As the spectral absorption properties of enzyme chromophores typically change during redox enzyme turnover, the FRET efficiency will be sensitive to the presence of enzyme substrates. This is the proposed basis for a new class of luminescent enzyme-based biosensor, with proof-of-principle studies described herein.
Pentaerythritol tetranitrate reductase (PETNR) belongs to the old yellow enzyme (OYE) family of NAD(P)H (reduced Nicotinamide Adenine Dinucleotide (Phosphate)) dependant enzymes.10 This family of flavoproteins, which are capable of accommodating a wide-range of substrates,11 have played a significant role in the development of enzymology. PETNR was first isolated from explosives-contaminated soil12 and catalyses the reduction of unsaturated activated alkenes (e.g. 2-cyclohexen-1-one), aromatic and aliphatic nitro-containing compounds (e.g. PETN), and aromatic ring systems (e.g. TNT).13 Despite the considerable amount of work carried out on OYEs, their physiological role is still a matter of contention.
In this study, we present a novel application of rare-earth UCPs to probe the redox behaviour of PETNR, utilising FRET between the emission bands of the UCP and the enzyme absorbance band. Due to a variation in the absorbance profile of the flavin core of the enzyme upon reduction/oxidation, the FRET between the two can effectively be turned ‘on’ or ‘off’ by changing the redox state of PETNR (Fig. 1). To the best of our knowledge, this is the first time such upconversion particles have been used to probe protein function.
 | ||
| Fig. 1 Schematic illustration of ‘on–off’ FRET process based on UCPs and PETNR. Reduction of the enzyme inhibits FRET and alters the intensity of the 475 nm band in the UCP emission spectrum. | ||
The UCPs used in this study (PTIR 475) were obtained from Phosphor Technology Ltd and directly suspended in aqueous TRIS buffer solution (pH 7). Following continuous wave (CW) 980 nm excitation, these UCPs give dual-emission bands in the blue and near IR regions of the electromagnetic spectrum.
The PTIR 475 emission bands centred at 475 nm and 800 nm can be assigned to 1G4→3H6 and 3H4→3H6 transitions of Tm3+, respectively (Fig. S1†).14 The separation of over 300 nm between these transitions can be utilised to great effect for determining relative concentrations by normalising the band at 800 nm (also known as ratiometric analysis), a technique that has been previously applied for related lanthanide-based systems.15 As the intensity of the NIR emission at 800 nm is independent of added flavin/enzyme concentration, measuring responses as a variation in the ratio between these two emission bands negates any probe concentration dependence.16 This ratiometric analysis is particularly important for such systems that exist as a dispersion in solution with variable concentration per scan. All experiments were carried out in 100 mM TRIS buffer, 10 mM NaCl, pH 7.
Initial studies focussed on the energy transfer between the UCPs and flavin mononucleotide (FMN) free in solution. The flavin absorption band centred at ∼460 nm has good spectral overlap with the 475 nm UNCP emission band (Fig. 2), allowing FRET to occur from the UNCP to FMN. Upon two-electron reduction, the 460 nm absorption of FMN is lost and the solution becomes colourless (Fig. S8†), thus preventing such FRET. This variable FRET behaviour should result in a decrease in the 475 nm emission band of the UCP upon FMN addition, followed by full return of this emission upon reduction of the FMN. As the UCPs are excited with NIR laser at 980 nm, direct FMN excitation is avoided and background noise is eliminated.
 | ||
| Fig. 2 Overlay of FMN absorption (solid red line), PETNR absorption (dashed red line), and PTIR 475 emission (black line) spectra with relative intensities scaled for comparison. | ||
In order to further test this FRET process, an aqueous solution of UCPs (0.01 wt%) was irradiated by CW 980 nm diode laser excitation and the emission spectrum recorded. To this solution was added FMN (2 mM aqueous solution) in incremental additions, from 10 to 500 μM, resulting in a gradual decrease of the 475 nm UCP emission band (Fig. 3). While the absorption of the FMN may cause significant inner filter effects during visible excitation of these samples, this is negated by the NIR excitation of the UCPs. Also, by analysing the total area of the 475 nm band as a ratio of the area of the 800 nm emission band, which is unaffected by the addition of FMN, slight fluctuations in emission intensity per measurement due to the UCPs existing as a dispersion in solution can be avoided. A Stern–Volmer analysis (see ESI†) of this ratio as a function of FMN concentration displays multiple linear regions, suggesting the occurrence of both static and dynamic quenching mechanisms, the relative contributions of which change as a function of increasing concentration (Fig. S2†). The existence of additional non-radiative quenching pathways that serve to decrease the emission intensity of the Tm3+ based emission by the introduction of proximal O–H and C–H oscillators may also affect the Stern–Volmer analyses.
In order to mimic the behaviour of protein-bound FMN during enzyme turnover, it is necessary to observe the FRET process in the presence of an FMN-reducing agent. In order to investigate this, a solution containing PTIR 475 phosphors (0.01 wt%) and FMN (100 μM) in TRIS buffer was prepared and deoxygenated by bubbling with N2. To this solution was added sodium dithionite (600 μM), also prepared as a separate anaerobic solution in TRIS buffer. As anticipated, upon reduction of the FMN, the FRET process between the flavin and UCPs is effectively turned off and the original UCP emission spectrum is restored (Fig. S3a†). The spectral effects of this reduction can also be observed physically, with a loss of the deep yellow colour of the flavin in solution. The FRET process can be restored simply by shaking the sample in air to reoxidise the FMN. The reduction and subsequent oxidation can be cycled multiple times (>3) with no apparent negative effect to either flavin or phosphors. These processes can also be analysed experimentally by observing direct flavin emission upon 475 nm irradiation (Fig. S3b†).
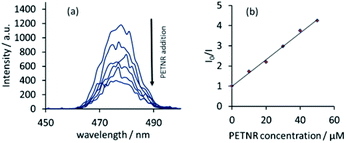
These promising studies with FMN led to investigations into the interactions between the full enzyme system and UCPs. PETNR was prepared following literature procedures.17 The FRET process between the two systems was achieved by irradiating a solution of UCPs (0.01 wt%) with CW 980 nm excitation, followed by incremental addition of PETNR. A similar response was observed to that with flavin, with a reduction in the 475 nm UCP emission band with increasing PETNR concentration (Fig. 4). Comparison of the Stern–Volmer analyses over the relevant concentration range (0–50 μM) suggests electrostatic binding in solution is much stronger in the enzyme-UCP system than in the FMN-UCP system (Fig. S4†).
FRET-induced flavin emission can also be observed upon addition of the enzyme (Fig. S5†). However, due to the broad nature of the flavin emission in comparison the narrow, line-like luminescence of the UCPs, this emission appears very weak in comparison, highlighting an advantage of using lanthanide-based emission over standard organic chromophores.
Due to the weak nature of this FRET-induced flavin emission band, FRET efficiency calculations (discussed in detail for related systems elsewhere)18 using changes in the acceptor excited state lifetimes proved unfeasible. No significant change in the UCP emission lifetime (τ475 nm = 88 ± 1 μs, τ800 nm = 172 ± 1 μs) was observed upon the addition of μM aliquots of either FMN or PETNR. Combined with the results from the Stern–Volmer analysis, these data suggest predominantly static quenching is occurring in this system, particularly when PETNR or FMN is at low concentration. UV-vis absorption spectra were unsuitable for monitoring this quenching due to the high level of scatter caused by the UCPs in solution.
Next, to model enzyme turnover, reduction of the protein was investigated with the addition of NADPH under anaerobic conditions (Fig. 5). As with the sodium dithionite reduction of FMN, an initial reduction in the 475 nm UCP emission band is observed upon addition of 100 μM PETNR. As the enzyme is reduced by NADPH (200 μM), the FRET process between PETNR and the UCPs is far less efficient, resulting in almost full recovery of the 475 nm emission. By exposing the sample to air, the enzyme is reoxidised and the FRET process is restored. Again, this behaviour can be confirmed by observing direct enzyme emission (Fig. S6†). These redox states can be repetitively cycled (>5 times) with no apparent degradation of either the UCPs or the enzyme, indicating the stable reversibility of the system. Incremental addition of NADPH under anaerobic conditions can also be measured as a ratiometric response (Fig. S9†), establishing the basis for the system to act as a responsive probe for various species in solution.
Conclusions
This study indicates a proof-of-principle basis for the use of UCPs to report on the function of biological systems; in this case the redox state of a flavoenzyme. As the emission profile of the UCPs can be tuned by altering the rare-earth dopant concentrations, this technique can be expanded to monitor other biological chromophores. Investigations are underway to improve concentration limits by covalently binding UCPs to biological compounds, in order to develop functional in vitro bioassay systems. This work establishes the basis for the development of a new class of responsive luminescent probe systems that combine the advantages of UCPs (NIR excitation, no autofluorescence, etc.) with the high sensitivity and selectivity of enzymes for ratiometric detection of enzyme-substrate activity e.g. during substrate and/or enzyme variant screens in biocatalysis.We thank Phosphor Technology Ltd for the UCPs, Prof. Nigel Scrutton for the PETNR expression plasmid and the EPSRC (LN, PH, CO) and BBSRC (SH, MD) for financial support. SH is a David Phillips Fellow and LN is a Career Acceleration Fellow.
Notes and references
- L. Xiong, Z. Chen, M. Yu, F. Li, C. Liu and C. Huang, Biomaterials, 2009, 30, 5592 CrossRef CAS PubMed; C. Carling, J. Boyer and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 10838 CrossRef PubMed; W. J. Kim, M. Nyk and P. N. Prasad, Nanotechnology, 2009, 20, 185301 CrossRef PubMed; P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526 CrossRef PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- L. S. Natrajan, A. Toulmin, A. Chew and S. W. Magennis, Dalton Trans., 2010, 39, 10837 RSC.
- F. Wang, D. Banerjee, Y. Liu, X. Chen and X. Liu, Analyst, 2010, 135, 1839 RSC.
- L. Cheng, K. Yang, M. Shao, X. Lu and Z. Liu, Nanomedicine, 2011, 6, 1327 CrossRef CAS PubMed.
- S. Sivakumar, P. R. Diamente and F. C. J. M. van Veggel, Chem.–Eur. J., 2006, 12, 5878 CrossRef CAS PubMed; L. Wang, R. Yan, Z. Huo, L. Wang, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 6054 CrossRef PubMed.
- L. Sun, H. Peng, M. I. J. Stich, D. Achatz and O. S. Wolfbeis, Chem. Commun., 2009, 5000 RSC; D. E. Achatz, R. J. Meier, L. H. Fischer and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 260 CrossRef CAS PubMed; Q. Liu, J. Peng, L. Sun and F. Li, ACS Nano, 2011, 5, 8040 CrossRef PubMed; J. Zhang, B. Li, L. Zhang and H. Jiang, Chem. Commun., 2012, 48, 4860 RSC; Y. Ding, H. Zhu, X. Zhang, J. Zhu and C. Burda, Chem. Commun., 2013, 49, 7797 RSC.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed; C. Li and J. Lin, J. Mater. Chem., 2010, 20, 6831 RSC; J. F. Suyver, A. Aebischer, D. Biner, P. Gerner, J. Grimm, S. Heer, K. W. Krämer, C. Reinhard and H. U. Güdel, Opt. Mater., 2005, 27, 1111 CrossRef.
- H. N. Barnhill, S. Claudel-Gillet, R. Ziessel, L. J. Charbonnière and Q. Wang, J. Am. Chem. Soc., 2007, 129, 7799 CrossRef CAS PubMed.
- R. E. Williams and N. C. Bruce, Microbiology, 2002, 148, 1607 CrossRef CAS PubMed.
- A. D. N. Vaz, S. Chakraborty and V. Massey, Biochemistry, 1995, 34, 4246 CrossRef CAS PubMed.
- C. E. French, S. Nicklin and N. C. Bruce, J. Bacteriol., 1996, 178, 6623 CAS.
- H. Khan, R. J. Harris, T. Barna, D. H. Craig, N. C. Bruce, A. W. Munro, P. C. E. Moody and N. S. Scrutton, J. Biol. Chem., 2002, 277, 21906 CrossRef CAS PubMed; P. R. Binks, C. E. French, S. Nicklin and N. C. Bruce, Appl. Environ. Microbiol., 1996, 62, 1214 Search PubMed; C. E. French, S. Nicklin and N. C. Bruce, Appl. Environ. Microbiol., 1998, 64, 2864 Search PubMed.
- O. A. Blackburn, M. Tropiano, T. J. Sorenson, J. Thom, A. Beeby, L. M. Bushby, D. Parker, L. S. Natrajan and S. Faulkner, Phys. Chem. Chem. Phys., 2012, 38, 13378 RSC.
- D. G. Smith, B. K. McMahon, R. Pal and D. Parker, Chem. Commun., 2012, 48, 8520 RSC; J. Liu, Q. Liu, C. Li, L. Sun and F. Li, J. Am. Chem. Soc., 2011, 133, 15276 CrossRef CAS PubMed.
- The change in emission over the relevant concentration range of added PETNR (0 to 60 μM) was found to be comparable for both 0.01 and 0.1 wt% UCPs. This is characteristic of ratiometric analysis.
- H. S. Toogood, A. Fryskowska, M. Hulley, M. Sakuma, D. Mansell, G. M. Stephens, J. M. Gardiner and N. S. Scrutton, ChemBioChem, 2011, 12, 738 CrossRef CAS PubMed.
- L. J. Charbonniere and N. Hildebrandt, Eur. J. Inorg. Chem., 2008, 21, 3241 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and experimental details. See DOI: 10.1039/c4dt00356j |
| This journal is © The Royal Society of Chemistry 2014 |