 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A phosphoramidite-based [FeFe]H2ase functional mimic displaying fast electrocatalytic proton reduction†
Sofia
Derossi‡
a,
René
Becker‡
a,
Ping
Li
a,
František
Hartl
*ab and
Joost N. H.
Reek
*a
aVan't Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH, Amsterdam, The Netherlands. E-mail: j.n.h.reek@uva.nl; Fax: (+31)205255604; Tel: (+31)205255265
bDepartment of Chemistry, University of Reading, Whiteknights, Reading RG6 6AD, UK. E-mail: f.hartl@reading.ac.uk; Fax: +44 (0)1183786331; Tel: +44 (0)1183786795
First published on 8th April 2014
Abstract
A phosphoramidite modified [FeFe]H2ase mimic is studied as a model for photodriven production of H2. On cathodic activation, the pyridyl–phosphoramidite complex exhibits a strongly enhanced rate of proton reduction over the previously reported pyridylphosphine model at the same overpotential. Analysis of the cyclic voltammograms shows an apparent H2 evolution rate strongly influenced by the presence of both side-bound pyridyl and phosphorous-bound dimethylamino moieties at the phosphoramidite ligands. This difference is ascribed to the basic amines acting as proton relays.
Efficient photochemical generation of molecular hydrogen is one of the key technologies our society needs in order to move to a sustainable hydrogen-based economy.1 As a consequence, a great deal of attention has been devoted to (photo-redox)catalysts performing proton reduction, and both homogeneous and heterogeneous systems have been developed.2 [FeFe] hydrogenases ([FeFe]H2ase) are natural-occurring enzymes that catalyse reduction of protons in an extremely efficient way (TOF ∼ 9000 s−1).3 Models of their bimetallic core based on cheap and abundant materials are easily synthesised, which has led to a plethora of diiron mimics.4 So far, the majority of studies of such bioinspired systems have focused on electrocatalysis,5 while the direct photodriven catalysis, using a light-harvesting chromophore coupled to the [FeFe] core, has only recently been addressed.6,7
The few initial attempts to assess light-driven H2 production with homogeneous systems are roughly based on four approaches: (i) simple mixing of the chromophore with the catalyst in solution, (ii) electron-transfer mediation by addition of an electron relay in donor–mediator–catalyst systems, (iii) covalent attachment of the chromophore to the catalyst or (iv) supramolecular coordination of the catalyst to the chromophore.7 The first two strategies offer ease of screening but limit the control over the spatial arrangement between the moieties. However, advantages of a well-defined system in the covalent case come at the price of tedious syntheses and more rapid charge recombination.
We envisaged a supramolecular approach combining the chromophore with the precatalyst to be most advantageous. With this philosophy in mind, we recently introduced 1·ZnTPP, a supramolecular dyad which (after disproportionation into a disubstituted complex, Fig. 1) is capable of a photocatalytic conversion of protons into H2.7 In that case, ZnTPP was chosen as the photosensitizer, while the catalytic mimic 1 was the [Fe2(μ-pdt)(CO)5L] precursor (Fig. 2). L represents the template ligand8pPyPPh2 (pPy = 4-pyridyl), which is coordinated to the diiron centre via the phosphorus atom and to ZnTPP via the pPy group (Fig. 1). Under photocatalytic conditions, i.e. upon irradiation in the presence of a sacrificial proton- and electron donor, the supramolecular assembly 1·ZnTPP exhibits H2 evolution, whereas the reference complex (3; L = PPh3) is inactive.
 | ||
| Fig. 1 The active [FeFe]H2ase biomimetic supramolecular assembly formed under photoreductive conditions from complex 1 in the presence of two different porphyrins. | ||
 | ||
| Fig. 2 The novel [FeFe]H2ase biomimetic catalyst 2 based on the phosphoramidite ligand appended with two 3-pyridyl groups (mPyPA) and the reference complexes 1, 3 and 4. | ||
Herein, we report development of our supramolecular dyad approach, using mPyPA, a recently reported phosphoramidite template ligand based on the binol motif and decorated with two pyridyl groups (Fig. 2, complex 2).9 We have been intrigued by the properties such ligand could impart onto the diiron core. Phosphoramidites have better π-acceptor properties compared to the related phosphines, likely removing electron density from the diiron core. This would lead to less negative reduction potentials of the complex and, therefore, smaller overpotential for H2 production. Furthermore, the two pyridyl side groups on the ligand may facilitate the association of macrocyclic chromophores to the metal centre and/or participate in proton transfer.
Complex 2 [Fe2(μ-pdt)(CO)5(mPyPA)] was synthesised together with the pyridyl-free complexes 3 [Fe2(μ-pdt)(CO)5(PPh3)] and 4 [Fe2(μ-pdt)(CO)5(PhPA)] serving for control experiments (Fig. 2). The syntheses of 2 and 4 involve substitution of one carbonyl ligand in [Fe2(μ-pdt)(CO)6] for the phosphoramidite ligand: 31P NMR spectra as well as MS of the new compounds revealed single CO displacement and phosphoramidite coordination to the Fe centre via the phosphorus atom, leaving, in each case, both pyridyl groups on the phosphoramidite free for coordination to the photosensitizer.
The IR spectra show a characteristic ν(CO) pattern of [Fe2(μ-pdt)(CO)5L] (Table 1) and carbonyl stretching shifted ca. 30 cm−1 to smaller wavenumbers with respect to [Fe2(μ-pdt)(CO)6]. Surprisingly, the ν(CO) values are very close to those for the pyridylphosphine derivative 1, demonstrating that the electronic difference between the pyridylphosphine and phosphoramidite ligands is not translated into Fe-to-CO π-back-donation and hence into differences in electron density at the iron core.
In line with this observation, cyclic voltammetry shows that 1–4 have their (irreversible) first cathodic waves placed at roughly the same electrode potential (Table 2). The electrocatalytic activity of these complexes towards proton reduction was studied at a static mercury drop electrode by examining the growth of their cathodic waves upon addition of acetic acid under identical experimental conditions (Fig. 3). Again, catalytic waves for all four compounds were found at roughly the same potential (−2.4 V to −2.5 V vs. Fc/Fc+).§ Adsorption of the pyridine-functionalized complexes to the mercury surface was not observed.¶
| Complex | E pc1 [V] | E pc2 [V] | n | k′ | k obs [s−1] |
|---|---|---|---|---|---|
| 1 | −1.78 | −2.24 | 1.79 | 17.8 | 580 |
| 2 | −1.88 | −2.28 | 3.58 | 6.00 | 6340 |
| 3 | −1.81 | −2.26 | 2.31 | 1.44 | 130 |
| 4 | −1.90 | −2.38 | 2.85 | 0.93 | 240 |
Although both cathodic and catalytic wave potentials for all four studied complexes are similar, the current maxima and shapes of the catalytic waves have indicated that there is a remarkable difference in activity. To understand this difference, in-depth analysis was performed on the cyclic voltammograms for each complex. The observed rate constant kobs has been determined by the method of DuBois and co-workers (eqn (1)),11 using the ratio between the second cathodic peak current (ipc2) and the catalytic peak current (icat) under the assumption that H2 formation is irreversible (ErCi mechanism). Remarkably, catalytic efficiency is much higher for catalyst precursor 2, as reflected in the much larger kobs (∼6000 s−1 at 7 mM acetic acid) compared to 1, 3 and 4 (Fig. 4). Since all catalysts operate at similar potentials, the effect of differences in overpotential on the catalytic rate can be neglected,12 and therefore the rate increase might well be caused by differences in particular mechanisms of the H2 evolution.
 | (1) |
 | (2) |
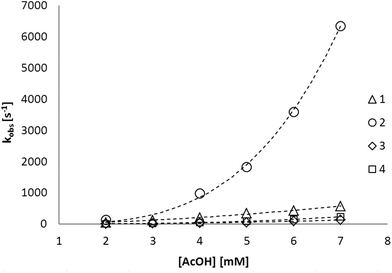 | ||
| Fig. 4 Plotted dependence of the rate constant kobs on acetic acid concentration for complexes 1 to 4. Dashed lines are curve fits of the form y = axn. For fit parameters, R2 values and zoomed-in graph (kobs < 600 s−1) see the ESI.‡ | ||
In line with behaviour of the natural system and observations from recent model complexes,13 it seems eminent that proton preorganization plays a role in accelerating the catalytic reduction. To get more insight into the proton-reactive behaviour, a relation between the rate of the H2 formation and acid concentration was sought by equating kobs to ∂[H2]/∂t. The obtained curve (kobsvs. [H+]) is then characterised by the rate constant k′ and reaction order n in a pseudo rate equation proportional to the kinetics of the reaction under study. Fitting our data points to a power function (eqn (2)) has yielded values for k′ and n for each complex under study (Table 2) with R2 values between 0.993 and 0.999.
The obtained pseudo rate equation for (electro)catalysis takes into account the step(s) just before the rate determining step, including additional protonation equilibria (if any).14 On this basis a correlation between rate order and constant, and Brønsted basic sites has been found: Phosphine complexes 1 and 3 show a reaction order close to 2, whereas for phosphoramidite complexes 2 and 4, n has been found close to 3, suggesting additional protonation equilibria. Since phosphoramidites can be protonated on the dimethylamine moiety, proton preorganization might explain the differences in the reaction order. It has been shown for multiple proton reduction catalysts that a proton relay close to the active site can indeed increase their activity, supporting the hypothesis of proton preorganization in the case of the phosphoramidite complexes.15
Still, this does not explain why complex 2 is much more active than its phenyl analogue 4. However, it has been found that the pseudo rate constants for phenyl-functionalized 3 and 4 are considerably smaller than those for pyridyl-functionalized 1 and 2. This pyridyl-induced rate increase might be explained by an electronic communication between the ligand and iron core or by stabilisation of the mono-reduced intermediate.8a,9
From this analysis, it is believed that for the high electrocatalytic rate observed for 2, both phosphoramidite and pyridyl functionalities are mandatory. The dimethylamino moiety might act as a proton relay, whereas the role of the pyridyl functionality remains unclear. This distinct ligand effect also implies that the active species contains at least one ligand moiety. However, since it is known that Fe2(μ-pdt) complexes undergo a variety of transformations on one-electron reduction, it is unclear whether the active species is in fact one-electron reduced 2 or one of its secondary reduction products.|| Although its exact structure remains elusive, the active species formed after reduction of precatalyst 2 shows a much higher electrocatalytic activity than its phosphine analogue.
Having established that compound 2 is a precursor of a good proton reduction catalyst, we analysed its photocatalytic behaviour when combined with zinc tetraphenylporphyrin (ZnTPP). Comparison of the catalytic potential of 2 (roughly −2.5 V vs. Fc/Fc+) with the second reduction potential of the porphyrin belonging to its singlet excited state (−1.75 V vs. Fc/Fc+) shows that the quenching of ZnTPP* by 2 is thermodynamically uphill, making photocatalysis for this system unfeasible.**
Despite this, we still trust that the overall supramolecular strategy is worth investigating, since using e.g. aromatic dithiolates instead of the propanedithiolate bridge in the studied complexes (Fig. 2), the catalytic potential may well be shifted towards a range well-accessible for ZnTPP.16 Therefore, we studied the supramolecular assemblies of the pyridyl-functionalised phosphoramidite ligand and its diiron pentacarbonyl complex with ZnTPP. First, the binding of ZnTPP to the pyridyl groups at the mPyPA ligand in complex 2 was determined by means of UV-Vis titration (ESI†). Free mPyPA binds two ZnTPP macrocycles with equal association constants (2Ka1 = 0.5Ka2 = 8.6 × 103 M−1), which also applies for the assembly with complex 2 (2Ka1 = 0.5Ka2 = 9.5 × 103 M−1). These data suggest that the interaction is a regular pyridyl-ZnTPP association (typical value in dichloromethane 6.9 × 103 M−1).17
Conclusions
In summary, we report the synthesis and properties of a novel 3-pyridylphosphoramidite-ligated [FeFe]H2ase model. Compared to the previously reported 4-pyridylphosphine analogue, complex 2 is much more active in the reduction of protons at a similar overpotential, which could be attributed to the dimethylamine moiety acting as a proton relay. The pyridyl functionality on the phosphoramidite ligand also plays a crucial role in accelerating proton reduction, although its exact function has not yet been elucidated.Furthermore, the supramolecular host–guest–host assembly ZnTPP·2·ZnTPP forms in non-coordinating solvents without any cooperative behaviour. However, it was shown that complex 2 neither quenches the excited state of the chromophore, nor reaches the thermodynamic potential needed for photocatalysis. Further matching of redox levels of the catalyst core and the associated chromophore will show if photo-driven hydrogen formation is viable using the same phosphoramidite ligand. One way to achieve this goal would be the replacement of the propanedithiolate bridge by an aromatic bridge, effectively shifting the cathodic potential to less negative values.5,16
Acknowledgements
This work has been supported (S.D. and P.L.) by the Netherlands’ Organization for Scientific Research (NWO-CW, ECHO grant 700.57.042) and the BioSolar Cells program (R.B.). We acknowledge Dr A. M. Kluwer (InCatT BV) for fruitful discussions, and Dr R. Bellini (UvA) and Drs Bart van den Bosch (UvA) for ligand synthesis.Notes and references
- (a) N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21 CrossRef CAS PubMed; (b) N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed; (c) A. J. Esswein and D. G. Nocera, Chem. Rev., 2007, 107, 4022 CrossRef CAS PubMed.
- (a) M. P. McLaughlin, T. M. McCormick, R. Eisenberg and P. L. Holland, Chem. Commun., 2011, 47, 7989 RSC; (b) T. S. Teets and D. G. Nocera, Chem. Commun., 2011, 47, 9268 RSC; (c) M. L. Helm, M. P. Stewart, R. M. Bullock, M. Rakowski Dubois and D. L. Dubois, Science, 2011, 333, 863 CrossRef CAS PubMed; (d) P.-A. Jacques, V. Artero, J. Pecaut and M. Fontecave, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20627 CrossRef CAS PubMed; (e) V. Artero and M. Fontecave, Coord. Chem. Rev., 2005, 249, 1518 CrossRef CAS PubMed; (f) X. Zong, Y. Na, F. Wen, G. Ma, J. Yang, D. Wang, Y. Ma, M. Wang, L. Sun and C. Li, Chem. Commun., 2009, 4536 RSC.
- R. K. Thauer, Eur. J. Inorg. Chem., 2011, 919 CrossRef CAS.
- (a) J.-F. Capon, F. Gloaguen, F. Y. Pétillon, P. Schollhammer and J. Talarmin, Coord. Chem. Rev., 2009, 253, 1476 CrossRef CAS PubMed; (b) J. C. Fontecilla-Camps, A. Volbeda, C. Cavazza and Y. Nicolet, Chem. Rev., 2007, 107, 4273 CrossRef CAS PubMed; (c) M. Y. Darensbourg, E. J. Lyon and J. J. Smee, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3683 CrossRef CAS PubMed; (d) M. Y. Darensbourg, E. J. Lyon and J. J. Smee, Coord. Chem. Rev., 2000, 206, 533 CrossRef; (e) F. Gloaguen and T. B. Rauchfuss, Chem. Soc. Rev., 2009, 38, 100 RSC; (f) M. Schmidt, S. M. Contakes and T. B. Rauchfuss, J. Am. Chem. Soc., 1999, 121, 9736 CrossRef CAS.
- G. A. N. Felton, C. A. Mebi, B. J. Petro, A. K. Vannucci, D. H. Evans, R. S. Glass and D. L. Lichtenberger, J. Organomet. Chem., 2009, 694, 2681 CrossRef CAS PubMed.
- (a) L.-C. Song, M.-Y. Tang, S.-Z. Mei, J.-H. Huang and Q.-M. Hu, Organometallics, 2007, 26, 1575 CrossRef CAS; (b) M. Wang, Y. Na, M. Gorlov and L. Sun, Dalton Trans., 2009, 6458 RSC; (c) F. Wang, W. G. Wang, X. J. Wang, H. Y. Wang, C.-H. Tung and L. Z. Wu, Angew. Chem., Int. Ed., 2011, 50, 3193 CrossRef CAS PubMed; (d) R. Lomoth and S. Ott, Dalton Trans., 2009, 9952 RSC; (e) M. Wang, L. Chen, X. Lia and L. Sun, Dalton Trans., 2011, 40, 12793 RSC.
- A. M. Kluwer, R. Kapre, F. Hartl, M. Lutz, A. L. Spek, A. M. Brouwer, P. W. N. M. van Leeuwen and J. N. H. Reek, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10460 CrossRef CAS PubMed.
- For template-ligand approach in other reactions see: (a) V. F. Slagt, M. Röder, P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, J. Am. Chem. Soc., 2004, 126, 4056 CrossRef CAS PubMed; (b) M. Kuil, P. E. Goudriaan, P. W. N. M. van Leeuwen and J. N. H. Reek, Chem. Commun., 2006, 4679 RSC; (c) M. Kuil, P. E. Goudriaan, A. W. Kleij, D. M. Tooke, A. L. Spek, P. W. N. M. van Leeuwen and J. N. H. Reek, Dalton Trans., 2007, 2311 RSC; (d) A. W. Kleij, M. Lutz, A. L. Spek, P. W. N. M. van Leeuwen and J. N. H. Reek, Chem. Commun., 2005, 3661 RSC; (e) A. W. Kleij, D. M. Tooke, A. L. Spek and J. N. H. Reek, Eur. J. Inorg. Chem., 2005, 4626 CrossRef CAS; (f) A. W. Kleij and J. N. H. Reek, Chem. – Eur. J., 2006, 12, 4218 CrossRef CAS PubMed; (g) T. Gadzikwa, R. Bellini, H. L. Dekker and J. N. H. Reek, J. Am. Chem. Soc., 2012, 134, 2860 CrossRef CAS PubMed.
- R. Bellini, S. H. Chikkali, G. Berthon-Gelloz and J. N. H. Reek, Angew. Chem., Int. Ed., 2011, 50, 7342 CrossRef CAS PubMed.
- P. Li, M. Wang, C. He, G. Li, X. Liu, C. Chen, B. Åkermark and L. Sun, Eur. J. Inorg. Chem., 2005, 2506 CrossRef CAS.
- A. D. Wilson, R. H. Newell, M. J. McNevin, J. T. Muckerman, M. Rakowski DuBois and D. L. DuBois, J. Am. Chem. Soc., 2006, 128, 358 CrossRef CAS PubMed.
- C. Costentin, S. Drouet, M. Robert and J.-M. Savéant, J. Am. Chem. Soc., 2012, 134, 11235 CrossRef CAS PubMed.
- D. Schilter and T. B. Rauchfuss, Angew. Chem., Int. Ed., 2013, 51, 13518 CrossRef PubMed.
- D. H. Pool and D. L. DuBois, J. Organomet. Chem., 2009, 694, 2858 CrossRef CAS PubMed.
- U.-P. Apfel, Models for the Active Site of the [FeFe]-Hydrogenase, Ph.D. Thesis, Friedrich-Schiller-Universität Jena, Jena (D), 2010, pp. 22–25 Search PubMed.
- L. Schwartz, P. S. Singh, L. Eriksson, R. Lomoth and S. Ott, C. R. Chim., 2008, 11, 875 CrossRef CAS PubMed.
- A. Satake and Y. Kobuke, Tetrahedron, 2005, 61, 13 CrossRef CAS PubMed.
- G. A. N. Felton, R. S. Glass, D. L. Lichtenberger and D. H. Evans, Inorg. Chem., 2006, 45, 9181 CrossRef CAS PubMed.
- L. D. Klyukina and B. B. Damaskin, Bull. Acad. Sci. USSR, 1963, 12, 931 CrossRef.
- H. Gerischer and D. A. Scherson, J. Electroanal. Chem. Interfacial Electrochem., 1985, 188, 33 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Syntheses and characterisation of complexes 2 and 4; detailed description of experiments, Fig. S1–S17 and Tables S1–S2. See DOI: 10.1039/c3dt53471e |
| ‡ Both authors contributed equally to this work. |
| § Cyclic voltammograms of 1 in ref. 7 showed electrocatalytic proton reduction at the potential coinciding with the first cathodic wave. However, these voltammograms were recorded using a glassy carbon working electrode, which gives competing direct reduction, thereby increasing the observed “catalytic” current.18 Repeating the same experiments using a static mercury drop electrode has revealed that the true catalytic response is in fact not at the first cathodic wave, but shifted more negatively by ca. 0.6 V. |
| ¶ It is known that pyridine (and pyridine-functionalized molecules) can adsorb to mercury surfaces.19 If this were the case for complexes 1 and 2, their cyclic voltammograms would diverge significantly from those recorded for complexes 3 and 4, respectively. Namely, the cathodic peaks would be broadened20 and diffusion-related behaviour would be suppressed. However, we have observed almost identical cyclic voltammograms for 1 and 3, and 2 and 4 (cf. ESI, Fig. S11†) which are in their turn similar to cyclic voltammograms published in literature (on Pt and glassy carbon).10 Furthermore, we have conducted cyclic voltammetry of complex 2 at different scan rates (0.05 to 5.0 V s−1) using both static mercury drop and platinum microdisc working electrodes and found a linear relationship between the cathodic peak current and the square root of the scan rate (cf. ESI, Fig. S13 and S14†). Since this behaviour corresponds with the diffusion control of mass transport in the double-layer region of the working electrode in line with the Randles–Sevcik equation, we assume no significant surface adsorption for the major species in the electrolyte solution. |
| || Complex 2 shows an irreversible one-electron reduction wave, consistent with previously reported mono-substituted pentacarbonyl Fe2(μ-pdt) complexes.10 Furthermore, on one-electron reduction, complex 1 has been shown to disproportionate into the parent hexacarbonyl and the disubstituted tetracarbonyl complex.7 Spectroelectrochemistry (SEC) on 2 shows an identical behaviour (cf. ESI, Fig. S15 to S17†). However, on the short time scale of cyclic voltammetry (defined by ν = 100 mV s−1 as opposed to 2 mV s−1 for SEC), it is reasonable to assume that during catalysis a rather complex chemical mixture is present, consisting of precatalyst 2, one-electron reduced 2−, its disproportionation products (the hexacarbonyl and the disubstituted complexes 5, see ESI†), their respective decomposition products (a mixture of Fe2 and Fe4 clusters with or without bridging CO and thiolate ligands) plus all possible protonated species. |
| ** A luminescence titration was carried out, monitoring the light emission of ZnTPP in the presence of an increasing amount of 2. Instead of static quenching by electron transfer, a red shift of the emission was observed which can be assigned to emission of the assembly 2·ZnTPP. Preliminary photocatalytic experiments using this system led, in all cases, to decomposition of the catalyst with evolution of 0.5 equivalents of H2 with respect to the catalyst. |
| This journal is © The Royal Society of Chemistry 2014 |

