DFT studies on the mechanism of palladium-catalyzed carbon–silicon cleavage for the synthesis of benzosilole derivatives†
Abstract
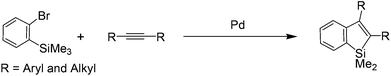
DFT calculations have been carried out to study the detailed mechanism of Pd-catalyzed intermolecular coupling reactions of 2-silylaryl bromides with alkynes via selective cleavage of C(sp3)–Si bonds. Through our calculations, we found that, starting from the alkenylpalladium intermediate derived from oxidative addition of the substrate C–Br bond followed by alkyne insertion, there are two possible pathways leading to the formation of the benzosilole product. Furthermore, these two pathways were found to be competitive. In this paper, we will present the detailed mechanistic study and analyze the results we have obtained.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...