Preparation and evaluation of carborane-derived inhibitors of prostate specific membrane antigen (PSMA)†
Abstract
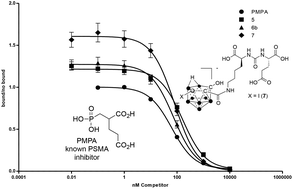
A series of C-hydroxy carborane derivatives of (S)-2-(3-((S)-5-amino-1-carboxypentyl)ureido)-pentanedioic acid were prepared as a new class of boron rich inhibitors of prostate specific membrane antigen (PSMA), which is overexpressed on prostate cancer tumours and metastases. Closo-, nido- and iodo-carborane conjugates were prepared and screened in vitro where the water soluble iodinated cluster had the highest affinity with an IC50 value (73.2 nM) that was comparable to a known PSMA inhibitor 2-(phosphonomethyl)-pentanedioic acid (PMPA, 63.9 nM). The radiolabeled analogue was prepared using 123I and the biodistribution determined in a prostate cancer model derived from a PSMA positive cell line (LNCaP) at 1, 2, 4, 6 and 24 h post injection (n = 4 per time point). The results showed good initial tumour uptake of 4.17% at 1 h, which remained at that level only decreasing somewhat at 6 h (3.59%). At the latter time point tumour-to-blood and tumour-to-muscle ratios peaked at 3.47 at 25.52 respectively. There was significant off-target binding particularly in the liver and gall bladder and a surprising amount of deiodination in vivo. Notwithstanding, this work demonstrates that carboranes can be used to prepare potent ligands for PSMA creating the opportunity to develop a new class of BNCT agents for prostate cancer.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...