Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
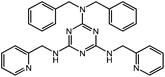
A semi-flexible aminotriazine-based bis-methylpyridine ligand for the design of nickel(II) spin clusters†
Yen-Wen
Tzeng
a,
Chang-Jui
Lin
a,
Motohiro
Nakano
b,
Chen-I
Yang
*a,
Wun-Long
Wan
c and
Long-Li
Lai
*c
aDepartment of Chemistry, Tunghai University, Taichung 407, Taiwan. E-mail: ciyang@thu.edu.tw; Fax: +886-4-23590426
bDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, 565-0871, Japan
cDepartment of Applied Chemistry, National Chi Nan University, Nantou 545, Taiwan. E-mail: lilai@ncnu.edu.tw
First published on 5th November 2013
Abstract
The self-assembly of a semi-flexible aminotriazine-based bis-methylpyridine ligand, N2,N2-dibenzyl-N4,N6-di(pyridylmethyl)-1,3,5-triazine-2,4,6-triamine (H2L), with NiCl2 and NiBr2 afforded two new nickel(II) clusters, (H2NMe2)2[Ni5(OH)2(H2L)2Cl10] (1) and [Ni6(OH)2(H2L)2Br10(THF)2] (2) showing a high spin ground state of S = 3.
The development of new molecule-based magnets is an important research topic in the fields of chemistry and physics, due to their impressive structural diversity and intriguing physical properties as well as complicated magneto-structural correlations.1 One of the major challenges in the area of molecular magnetism is the construction of a polynuclear metal cluster that exhibits interesting magnetic properties, such as the high-spin ground state and/or single-molecule magnet (SMM) behavior.2–5 For the preparation of such metal clusters, using a polychelating ligand with an unused arm or a donor site has been recognized.6,7 An alternative preparation method is to utilize the flexidentate behavior of a multidentate ligand and judicial choice of bridging ligands, such as carboxylate and azide.8,9 Among the bridging ligands, halide ions have been known for their versatile bridging coordination modes that generate polymeric compounds.10
Poly-pyridyl ligands had a major impact in the field of supramolecular chemistry for decades, which have led to a variety of metal/ligand supramolecular ensembles to be obtained such as double and triple helices, grids, ladders, and so forth.11 However, the flexible poly-pyridyl ligands are rarely exploited in the formation of polynuclear metal clusters; especially the resulting structures may potentially exhibit interesting magnetic properties.
We herein report the self-assembly of two Ni(II) clusters, (H2NMe2)2[Ni5(OH)2(H2L)2Cl10] (1) and [Ni6(OH)2(H2L)2Br10(THF)2] (2), using a semi-flexible aminotriazine-based bis-methylpyridine ligand, N2,N2-dibenzyl-N4,N6-di(pyridylmethyl)-1,3,5-triazine-2,4,6-triamine (H2L). The designed ligand, H2L (Scheme 1), contains an aminotriazine ring and two flexible methylpyridine arms, which could chelate metal ions into clusters 1 and 2, exhibiting an S = 3 spin ground state arising from the uncanceled spin arrangement of the antiferro- and ferromagnetic interactions in 1 and ferromagnetic interaction in 2, respectively.
X-ray crystal structure analysis showed that 1 and 2‡ crystallize in the monoclinic space groups P21/n and in the triclinic space groups P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. In complex 1, the geometry of the centrosymmetric NiII5 cluster can be described as two corner-sharing μ3-OH-centred NiII3 triangles with bowtie topology (Fig. 1). Two H2L groups connect the central NiII atom (Ni1) and two peripheral metal ions in the two sides of a bow tie (Ni2 and Ni3) in a μ3-H2L-κ5-N,N′:N′′:N′′′,N′′′′ coordination mode, in which two methylpyridine groups exhibit in a trans-conformation. The base (Ni2⋯Ni3) of each triangle is bridged by two μ2-Cl− anions. The μ3-OH− group links the central Ni1 to the two peripheral metal ions on either side of the molecule and the O atom of OH− lie out of the plane of the Ni3 triangle about 0.402 Å. Peripheral ligations around each Ni centers are completed by terminal Cl− anions.
, respectively. In complex 1, the geometry of the centrosymmetric NiII5 cluster can be described as two corner-sharing μ3-OH-centred NiII3 triangles with bowtie topology (Fig. 1). Two H2L groups connect the central NiII atom (Ni1) and two peripheral metal ions in the two sides of a bow tie (Ni2 and Ni3) in a μ3-H2L-κ5-N,N′:N′′:N′′′,N′′′′ coordination mode, in which two methylpyridine groups exhibit in a trans-conformation. The base (Ni2⋯Ni3) of each triangle is bridged by two μ2-Cl− anions. The μ3-OH− group links the central Ni1 to the two peripheral metal ions on either side of the molecule and the O atom of OH− lie out of the plane of the Ni3 triangle about 0.402 Å. Peripheral ligations around each Ni centers are completed by terminal Cl− anions.
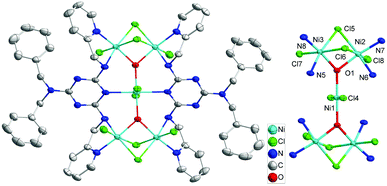 | ||
| Fig. 1 Crystal structure of the anion complex 1 (left) and its NiII5 core structure (right). The Me2NH2 cations and H atoms were omitted for clarity. | ||
The structure of complex 2 reveals a dimer of [NiII3(μ3-OH)(μ3-Br)(μ2-Br)3]+ core which is connected by two bis-chelating H2L ligands (Fig. 2). The structure [NiII3(μ3-OH)(μ3-Br)(μ2-Br)3]+ adopts a near-equilateral NiII3 triangle core, which is bonded by a μ3-oxide (O1) and a μ3-Br− (Br1) on both sides of the central planar where the central OH− and Br− bridges are located 0.902 and 2.056 Å above the Ni3 plane. Each base of the NiII3 triangle is connected by μ2-Br− anions (Br2–Br4). Two H2L ligands in complex 2 exhibit a μ2-H2L-κ4-N,N′:N′′,N′′′ coordination mode with trans-conformation of their two methylpyridine groups and connects the two Ni3 triangles into a hexanuclear dimer of Ni3 structure. Peripheral ligations around each Ni2 centers are ended by one terminal Br− anion and one THF molecule.
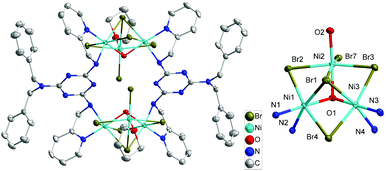 | ||
| Fig. 2 Crystal structure of the complex 2 (left) and its NiII3 core structure (right). The H atoms were omitted for clarity. | ||
The solid-state, variable-temperature magnetic susceptibility measurements were performed on microcrystalline samples of complexes 1 and 2 in the 2–300 K range in a 1 kOe magnetic field, which was suspended in eicosane to prevent torquing.
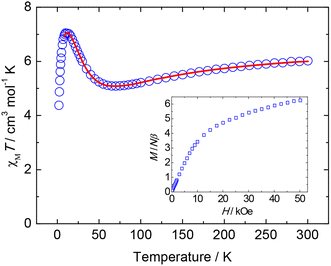
For complex 1, the χMT value of 6.01 cm3 K mol−1 at 300 K decreases gradually with decreasing temperature in the range of 300 to 70 K, then abruptly increases, reaching a maximum of 7.05 cm3 K mol−1 at 10 K, and decreases to 4.38 cm3 K mol−1 at 2 K (Fig. 3). The change in χMT value indicates that antiferromagnetic dominated in the Ni5 unit with a non-canceled spin ground state and the χMT value at 10 K is consistent with S = 3 (g = 2.2). Below 10 K, the χMT values slowly decrease, probably due to weak intermolecular antiferromagnetic interactions, zero field splitting and/or small anisotropy.
In order to understand the magnetic coupling of complex 1, the magnetic susceptibility data were fitted using a NiII5 Heisenberg–van Vleck model. Based on the structure analysis, the number of magnetic interactions can be reduced significantly: J1 for NiII⋯NiII through one μ3-OH and one H2L bridgings and J2 for NiII⋯NiII through one μ3-OH− and two μ2-Cl− bridges (see Fig. S5 in the ESI†), hence the Hamiltonian can be written as:
| H = −2J1(S1S2 + S1S3 + S1S4 + S1S5) − 2J2(S2S3 + S4S5) |
The χMT data could be well fitted by this Heisenberg–van Vleck model with the addition of an intermolecular interaction by the mean-field approximation (zJ′). The results from fitting the experimental data are shown as solid lines in Fig. 3, with final parameters being g = 2.30, J1 = −11.7 cm−1, J2 = 3.5 cm−1 and zJ′ = −0.10 cm−1. This set of parameters leads to the conclusion that the ground state is ST = 3 and the first excited state is S = 2 at 24 cm−1 above the ground state (Fig. S6†). The estimated values, for the intracluster magnetic exchange interactions, indicate that the antiferro- and ferromagnetic interactions are provided within the NiII5 cluster in 1, and are associated with an S = 3 spin ground state. Both interactions (J1 and J2) are close to the reported exchange interactions of NiII⋯NiII through the similar pathways.12 The magnetization curve recorded at 2 K of complex 1 shows a continuous increase up to the saturation value of 6.3Nβ (Fig. 3 inset), which corresponds well to a ground-state spin S = 3, in agreement with the χMT data. However, this magnetization curve cannot be nicely fitted by the Brillouin equation for S = 3, probably due to the presence of intermolecular interaction, zero field splitting and/or anisotropy.
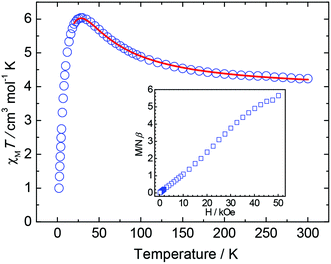
For complex 2, the value of χMT increases steadily from 4.24 cm3 mol−1 K at 300 K as the temperature decreases to reach a maximum of 6.03 cm3 mol−1 K at 18 K, and then decreases to 1.00 cm3 mol−1 K at 2.0 K (Fig. 4). The χMT value at 300 K is slightly larger than 4.00 cm3 mol−1 K, the expected value for a NiII3 complex with noninteracting metal centers with g = 2.3. This behavior clearly indicates the ferromagnetic coupling within complex 2 and the decrease in χMT at low temperature (<28 K) is likely due to the intermolecular (Ni3⋯Ni3) interaction, the Zeeman effect or zero-field splitting in the ground state. In order to describe the coupling within the cluster, the magnetic susceptibility data were fitted using a NiII3 Heisenberg–van Vleck model: H = −2J(S1S2 + S2S3 + S1S3) with an interunit interaction by the mean-field approximation (zJ′) (Fig. S7†). The data below 20 K were omitted in the fitting, because zero-field splitting and Zeeman effect likely dominate in this temperature range. The fitting result of dc data in 1 kOe gave the best fit parameters of g = 2.26, J = 8.10 cm−1 and zJ′ = −0.50 cm−1. This set of parameters gives the ground state of ST = 3 and the first excited state S = 2 at −48 cm−1 above the ground state (Fig. S8†). Although the magnetic interaction between NiII ions through such bridges (one μ3-OH, one μ3-Br and one μ2-Br) has not been reported in the literature, it is believed that the ferromagnetic interactions ensue from 6-coordinate geometry and the Ni–X–Ni bridging angles close to 90°.13 The magnetization curve recorded at 2 K of 2 is shown in Fig. 4 inset, in which the magnetization slowly increases with the increase of field and becomes saturated around 50 kOe with a value of 5.65Nβ. The less rapid saturation of magnetization at low field may result from the antiferromagnetic interaction of Ni3⋯Ni3 interunit and the saturation magnetization value corresponds well to a ground-state spin S = 3, in agreement with the χMT data. Again, this magnetization curve cannot be well fitted by the Brillouin equation for S = 3, due to the presence of intermolecular interaction and/or zero field splitting.
To investigate whether 1 and 2 might be a SMM, ac susceptibility measurements were performed with a zero applied dc field. Representative results for 1 and 2 are shown in Fig. S9 and S10.† At lower temperatures, the in-phase signal (χM′T) increases to ∼6.8 and 6.5 cm3 K mol−1 for 1 and 2, respectively, confirming the spin ground state of S = 3 for both complexes. For complex 1, a weak χM′′ signal appears below 5 K, which is indicative of a slow magnetic relaxation within 1. However, the peak maxima clearly lie in the temperatures below 1.8 K, the operating limit of our instrument. These data thus suggest that compound 1 indeed exhibits a slow magnetic relaxation or long-range magnetic ordering at temperatures below 1.8 K. In contrast, the complex 2 shows no SMM behavior from the absence of χM′′ signal.
In conclusion, the use of semi-flexible aminotriazine-based bis-methylpyridine ligands (H2L) has allowed the access of two novel Ni clusters with interesting magnetic properties. The H2L ligand represents a ‘proof of feasibility’ for the belief that such ligands may provide a rich source of new transition-metal clusters. Further studies are in progress.
Notes and references
- Magnetism: Molecules to. Materials, ed. J. S. Miller and M. Drillon, Wiley-VCH, Weinheim, Germany, 2001–2004, vol. I–V Search PubMed.
- (a) G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66–71 CrossRef CAS; (b) R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804–1816 CrossRef CAS; (c) A. Caneschi, D. Gatteschi, R. Sessoli, A. L. Barra, L. C. Brunel and M. Guillot, J. Am. Chem. Soc., 1991, 113, 5873–5874 CrossRef CAS; (d) M. Nakano and H. Oshio, Chem. Soc. Rev., 2011, 40, 3239–3248 RSC.
- (a) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141–143 CrossRef CAS; (b) D. Gatteschi, R. Sessoli and A. Cornia, Chem. Commun., 2000, 725–732 RSC; (c) Z. Sun, C. M. Grant, S. L. Castro, D. N. Hendrickson and G. Christou, Chem. Commun., 1998, 721–722 RSC; (d) E. C. Yang, D. N. Hendrickson, W. Wernsdorfer, M. Nakano, L. N. Zakharov, R. D. Sommer, A. L. Rheingold, M. Ledezma-Gairaud and G. Christou, J. Appl. Phys., 2002, 91, 7382–7384 CrossRef CAS; (e) C.-I. Yang, W. Wernsdorfer, Y.-J. Tsai, G. Chung, T.-S. Kuo, G.-H. Lee, M. Shieh and H.-L. Tsai, Inorg. Chem., 2008, 47, 1925–1939 CrossRef CAS PubMed.
- (a) G. Christou, Polyhedron, 2005, 24, 2065–2075 CrossRef CAS PubMed; (b) D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford University Press, New York, 2006 Search PubMed; (c) G. Aromí and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef and references therein.
- (a) T. N. Nguyen, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2011, 133, 20688–20691 CrossRef CAS PubMed; (b) A. Saha, K. A. Abboud and G. Christou, Inorg. Chem., 2011, 50, 12774–12784 CrossRef CAS PubMed; (c) Z. Wang, J. Van Tol, T. Taguchi, M. R. Daniels, G. Christou and N. S. Dalal, J. Am. Chem. Soc., 2011, 133, 17586–17589 CrossRef CAS PubMed.
- (a) A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 4926–4929 CrossRef CAS PubMed; (b) S. S. Tandon, S. D. Bunge, J. Sanchiz and L. K. Thompson, Inorg. Chem., 2012, 51, 3270–3282 CrossRef CAS PubMed; (c) Z. E. Serna, M. K. Urtiaga, M. G. Barandika, R. Cortés, S. Martin, L. Lezama, M. I. Arriortua and T. Rojo, Inorg. Chem., 2001, 40, 4550–4555 CrossRef CAS PubMed.
- (a) E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146–16155 CrossRef CAS PubMed; (b) C. C. Stoumpos, O. Roubeau, G. Aromi, A. J. Tasiopoulos, V. Nastopoulos, A. Escuer and S. P. Perlepes, Inorg. Chem., 2010, 49, 359–361 CrossRef CAS PubMed; (c) M. Murugesu, J. Raftery, W. Wernsdorfer, G. Christou and E. K. Brechin, Inorg. Chem., 2004, 43, 4203–4209 CrossRef CAS PubMed; (d) T. C. Stamatatos, C. G. Efthymiou, C. C. Stoumpos and S. P. Perlepes, Eur. J. Inorg. Chem., 2009, 2009, 3361–3391 CrossRef.
- (a) C. Papatriantafyllopoulou, T. C. Stamatatos, W. Wernsdorfer, S. J. Teat, A. J. Tasiopoulos, A. Escuer and S. P. Perlepes, Inorg. Chem., 2010, 49, 10486–10474 CrossRef CAS PubMed; (b) M. Murugesu, M. Habrych, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 4766–4767 CrossRef CAS PubMed; (c) T. C. Stamatatos and G. Christou, Inorg. Chem., 2009, 48, 3308–3322 CrossRef CAS PubMed.
- (a) G. Aromí, M. J. Knapp, J.-P. Claude, J. C. Huffman, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1999, 121, 5489–5499 CrossRef; (b) M. Charalambous, E. E. Moushi, C. Papatriantafyllopoulou, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Chem. Commun., 2012, 48, 5410–5412 RSC; (c) J. Esteban, L. Alcázar, M. Torres-Molina, M. Monfort, M. Font-Bardia and A. Escuer, Inorg. Chem., 2012, 51, 5503–5505 CrossRef CAS PubMed.
- (a) J.-M. Lehn, Supramolecular Chemistry, Wiley-VCH, New York, 1995 Search PubMed; (b) C. Piguet, G. Berbardinelli and G. Hopfgartner, Chem. Rev., 1997, 97, 2005–2062 CrossRef CAS PubMed; (c) M. Albrecht, Chem. Rev., 2001, 101, 3457–3498 CrossRef CAS PubMed.
- (a) J. Esteban, P. E. Ruiz, D. M. Font-Bardia, D. T. Calvet and A. Escuer, Chem.–Eur. J., 2012, 18, 3637–3648 CrossRef CAS PubMed; (b) P. L. Pawlak, A. Y. S. Malkhasian, B. Sjlivic, M. J. Tiza, B. E. Kucera, R. Loloee and F. A. Chavez, Inorg. Chem. Commun., 2008, 11, 1023–1026 CrossRef CAS PubMed.
- G. N. Newton, H. Sato, T. Shiga and H. Oshio, Dalton Trans., 2013, 42, 6701–6704 RSC.
- A. Bencini and D. Gatteschi, Inorg. Chim. Acta, 1978, 31, 11 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional crystallographic diagrams and magnetic diagram. CCDC 947250 and 947251. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3dt52903g |
‡ The complexes analyzed as (C, H, N) 1, calcd (found): C, 42.52 (42.17); H, 4.26 (4.79); N, 14.40 (14.35)% and 2, calcd (found): C, 34.37 (34.03); H, 3.23 (3.34); N, 9.72 (9.72)%. Crystal-structure data for 1, C62H70Cl10N18Ni5O2, M = 1747.31, monoclinic, P21/n, a = 15.7038(12) Å, b = 9.9564(7) Å, c = 23.8274(18) Å, β = 93.4680(10)°, V = 3718.7(5) Å3, T = 150(2) K, Z = 2. (Rint = 0.0426), 8215 parameters, R(Rw) = 0.0382(0.0865) with [I > 2σ (I)] and for 2, C66H74Br10N16Ni6O4, M = 2306.56, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 11.9623(7) Å, b = 13.3874(8) Å, c = 14.2964(9) Å, α = 65.3520(10)°, β = 72.9400(10)°, γ = 75.1400(10)°, V = 1965.5(2) Å3, T = 150(2) K, Z = 1. (Rint = 0.0291), 9051 parameters, R(Rw) = 0.0256(0.0481) with [I > 2σ(I)]. , a = 11.9623(7) Å, b = 13.3874(8) Å, c = 14.2964(9) Å, α = 65.3520(10)°, β = 72.9400(10)°, γ = 75.1400(10)°, V = 1965.5(2) Å3, T = 150(2) K, Z = 1. (Rint = 0.0291), 9051 parameters, R(Rw) = 0.0256(0.0481) with [I > 2σ(I)]. |
| This journal is © The Royal Society of Chemistry 2014 |