Cross-coupling reaction between arylboronic acids and carboranyl iodides catalyzed by graphene oxide (GO)-supported Pd(0) recyclable nanoparticles for the synthesis of carboranylaryl ketones†
Abstract
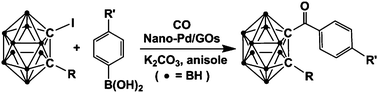
Well-dispersed palladium(0) nanoparticles with small and narrow size distributions were synthesized conveniently on a graphene oxide (GO) surface. The GO-supported nano-Pd0 was found to be a highly efficient and recyclable catalyst for the carbonylative cross-coupling reaction between arylboronic acids and aryl and carboranyl iodides, respectively. Benzophenone and a series of carboranylaryl ketones, 1-R-2-[C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Ar]-1,2-C2B10H10 (R = H, Me, Ph; Ar = C6H5, C6H4-4-OMe and C6H4-4-F), were synthesized and fully characterized. The catalyst was recyclable at least three times with sustained activity.
O)Ar]-1,2-C2B10H10 (R = H, Me, Ph; Ar = C6H5, C6H4-4-OMe and C6H4-4-F), were synthesized and fully characterized. The catalyst was recyclable at least three times with sustained activity.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...