Transformations of polyols to organic acids and hydrogen in aqueous alkaline media
Abstract
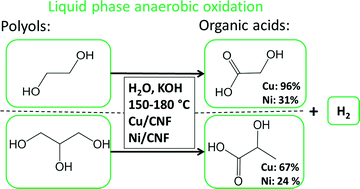
In this paper we show that carbon nanofiber supported copper and nickel nanoparticles can selectively transform ethylene glycol and glycerol into value added oxygenates (organic acids) under anaerobic aqueous conditions. During aqueous phase oxidation Cu based catalysts showed a nearly quantitative yield (96% selectivity at 82% conversion) of glycolic acid from ethylene glycol. The reaction was carried out under alkaline conditions at relatively mild temperatures (150–180 °C) and produced H2 as co-product. The high selectivity towards glycolic acid was independent of the temperature. For glycerol oxidation a high selectivity (67% at full conversion) towards lactic acid was observed using Cu with competitive formation of glyceric acid, 1,2-propanediol tartronic acid and formation of H2 as co-product. The activity of Ni was comparable to that of Cu but it was less selective for the formation of desired oxygenates, glycolic acid (31%) and lactic acid (24%), due to the formation of formic acid.
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...