Open Access Article
Open Access ArticleOrganocatalytic strategies for enantioselective metal-free reductions
Sergio
Rossi
,
Maurizio
Benaglia
*,
Elisabetta
Massolo
and
Laura
Raimondi
Dipartimento di Chimica, Università degli Studi di Milano, via Golgi 19, 20133 Milano, Italy. E-mail: maurizio.benaglia@unimi.it; Fax: +39 02 50314159
First published on 4th April 2014
Abstract
One of the most important chemical transformations is the reduction of multiple bonds, carbon–carbon as well as carbon–heteroatom double bonds, since it leads very often to the generation of new stereocenters in the molecule. The replacement of metal-based catalysts with equally efficient metal-free counterparts is very appealing in view of possible future applications of non toxic, low cost, and environmentally friendly promoters on an industrial scale. This perspective will focus specially, but not exclusively, on the enantioselective reduction of the carbon nitrogen double bond; despite the historical need for and continued interest in chiral amines, their synthesis remains challenging. Three metal-free catalytic methodologies available for the reduction of carbon–nitrogen double bond will be discussed: i) binaphthol-derived phosphoric acids catalyzed reductions, with dihydropyridine-based compound as the reducing agent; ii) trichlorosilane mediated reductions, in the presence of catalytic amounts of chiral Lewis bases; iii) metal-free hydrogenation of imines through FLP (Frustrated Lewis Pair) methodology, that involves the use of a combination of a strong Lewis acid with a variety of sterically encumbered Lewis bases, for examples phosphines or tertiary amines, to activate hydrogen at ambient conditions. Special attention will be devoted to the most recent applications of the last five years.
1. Introduction
Though a large number of chiral molecules that find their application in pharmaceutical, flavors, and agrochemicals industries are in pipeline, their development and commercialization is not without problems. A key reason responsible for this is the lack of general solutions to address issues related to chirality. In addition, development is mostly focused upon cost effectiveness, rather than on application and research of advanced, state-of-the-art technologies.Despite several difficulties and many open questions, enantioselective catalysis is rapidly becoming more and more popular also at the industrial level: examples come from technologies such as asymmetric hydrogenation.1 As stereoselective methodologies develop over time, declining costs enable companies to access public-domain technologies. Enantioselective catalysis has witnessed increased activity in terms of extensive academic and industrial investment. Industries are keen on further growth in the market for “chiral technologies”, as new processes and reactions are discovered and applied in pharmaceutical synthesis. Furthermore, the rising complexity of new chemical entities calls for the evolution of advanced catalytic chemo- and stereoselective technologies.2
Indeed, the importance of catalysis to the pharmaceutical industry has steadily increased over the past two decades.3 Catalysis in pharmaceutical R&D has been attracting continuously increasing attention due to increasing regulatory requirements that force companies to develop and study single-enantiomer drugs, environmental protection laws and the pressure to reduce drug development cost and time; the picture is completed by the continued discovery of new practical catalysts from both academia and industry, that make available possible new solutions for production.4 Catalysis is one of the solutions that companies are exploring to address the problem of the increasing complexity of chemical targets. The average number of manipulations required to synthesize an active pharmaceutical ingredient (API) continues to grow and currently amounts to an average of 12 synthetic steps. Finally catalysis plays a fundamental role in companies’ strategies aimed to set up a second generation process to develop environmentally favorable API manufacturing processes, without compromising the safety, efficacy and quality of the final product.5
In this context, the advent of organocatalysis6 brought new attractive possibilities, to realize stereoselective catalytic synthesis of complex chiral molecules, bearing several functional groups, with metal-free processes. One of the most important chemical transformations is the reduction of multiple bonds, carbon–carbon as well as carbon–nitrogen double bonds, since it leads very often to the generation of new stereocenters in the molecule. The outcome of a reduction process is therefore the production of different stereoisomers and the control of the reaction, in the attempt to obtain preferentially one stereoisomer over the others, which requires the use of a “chiral technology”. The replacement of metal-based catalysts with equally efficient metal-free counterparts is very appealing in view of possible applications in the future of non toxic, low cost, and environmentally friendly promoters on industrial scale.
The perspective will focus specially, but not exclusively, on the enantioselective reduction of the carbon–nitrogen double bond. Despite the historical need for and continued interest in chiral amines, their synthesis remains challenging.7 This is especially true in the context of introducing nitrogen into a pharmaceutical compound, with all its complexity and multifunctionality, or into an advanced intermediate via an operationally simple, preferably one-step, procedure allowing high chemo-, regio-, diastereo-, and enantiocontrol. It is obvious that catalytic asymmetric hydrogenation of prochiral unsaturated compounds, such as olefins, ketones, and imines, has been intensively studied and is considered a versatile method for access to chiral compounds. However, stereoselective hydrogenation of carbon–nitrogen double bonds, including those inserted in heteroaromatic compounds, is much less explored, presumably because of different problems, the main being the deactivation and/or poisoning of catalysts by compounds containing nitrogen and sulfur atoms. Despite the difficulties cited above, the search for catalysts enabling efficient asymmetric hydrogenation of aromatic/heteroaromatic compounds continues and it is driven by the prospect of straightforward and efficient routes to optically active saturated or partially saturated chiral heterocyclic compounds.8 On the other hand enantioselective, metal-catalyzed hydrogenations suffer from other drawbacks: catalysts are generally quite expensive species, typically constituted by an enantiomerically pure ligand (whose synthesis may be costly, long and difficult) and a metal species, in many cases a precious element. Since chiral amines are finding applications in an increasing number of fields,7 such as pharmaceuticals, agrochemicals and fragrances, the possibility to develop an organocatalytic approach has attracted much attention because it might represent a solution to the problems due to the presence of toxic metal, whose leaching could contaminate the product.
Today three metal-free catalytic methodologies are available for the reduction of the carbon–nitrogen double bond,9 especially of ketoimines: i) binaphthol-derived phosphoric acids catalyzed reductions with a dihydropyridine-based compound as the reducing agent; ii) trichlorosilane mediated reductions, in the presence of catalytic amounts of chiral Lewis bases; iii) metal-free hydrogenation of imines through FLP (Frustrated Lewis Pair) methodology, which involves the use of a combination of a strong Lewis acid, like tris(perfluorophenyl)borane B(C6F5)3 with a variety of sterically encumbered Lewis bases, for examples phosphines or tertiary amines, to activate hydrogen at ambient conditions. The most representative examples of all three methodologies will be presented in the discussion section, with special attention on the most recent applications of the last four–five years.
2. Frustrated Lewis Pairs (FLP)-catalyzed reductions
Hydrogenation catalysis is the most common transformation used in the chemical industry and it is employed in the preparation of scores of commercial targets, including natural products, commodities and fine chemicals.10 Recently, studies have been directed to the exploitation of non-transition metal systems for the activation of H2 and subsequent use in hydrogenation. A novel and promising approach to the utilization of hydrogen in catalysis has emerged from studies related to the use of a proper combination of a Lewis acid and a Lewis base, in which steric demands preclude classical adduct formation. Such systems have been termed “frustrated Lewis pairs” or “FLPs”.11 In these unique Lewis acid–base (LA–LB) adducts, the steric hindrance precludes the formation of stable donor–acceptor complexes, on account of which these pairs are able kinetically to promote various unprecedented reactions with organic and inorganic molecules. Their most remarkable reactivity is the heterolytic cleavage of hydrogen at room temperature, the capacity of which was long thought to be the exclusive characteristic of transition metals (Scheme 1).Computational studies12 suggest the generation of a phosphine–borane “encounter complex”, stabilized by H⋯F interactions. In this “species” the boron and phosphorus centers are close but fail to form a P to B dative bond as a result of steric congestion. Interaction of H2 in the reactive pocket between the donor and acceptor sites results in heterolytic cleavage of H2. However more recent studies12b of the quasi-linear P⋯H–H⋯B activation mechanism cast some doubts on the corresponding transition state. According to these new results, a transition state in a linear arrangement only appears for rather large P⋯B distances over 4.5 Å. Such values seem to be artificially induced by the quantum chemical method (B3LYP) which is well known to overestimate steric congestion. With a properly dispersion-corrected density functional no linear transition state exists and only one minimum with a rather large H–H distance of about 1.67 Å could be found. This points to an alternative bimolecular mechanism in which H2 access into the frustrated P⋯B bond is the rate-determining step. Further theoretical studies to address this important question are needed in order to elucidate fully the mechanism.
Following the initial studies on metal-free catalytic hydrogenation of imines,13a it was proposed that the substrate could serve as the base-partner of an FLP and thus only a catalytic amount of a Lewis acid, as tris-pentafluorophenyl borane, should be required. Indeed, a series of differently substituted imines were reduced by hydrogen using a catalytic amount of B(C6F5)3 (Scheme 2).13b For poorly basic imines, addition of catalytic amount of sterically encumbered phosphine accelerated the reduction. This presumably results from the greater ease with which phosphine/borane cleaves hydrogen heterolytically.
The application to asymmetric synthesis is a logical and highly desirable extension of these findings. Indeed, stereoselective methodologies can be designed based on the consideration that FLP reductions can be viewed as a catalytic version of borohydride reductions. In 2011 Stephan studied the catalytic hydrogenation of chiral ketimines using tris-pentafluorophenyl borane as a catalyst.14 Using imines derived from camphor and menthone, the reductions proceed with quantitative yields and high diastereoselectivities (up to 99% d.e.). Generally, the reduction of chiral imines with B(C6F5)3 resulted in excellent diastereoselectivities when the stereogenic center was close to the unsaturated carbon center, probably due to the proximity of the stereocenter to the approach of the sterically bulky reductive [HB(C6F5)3]− species.
Recently, an important breakthrough was obtained by Klankermayer, who developed the first stereoselective catalytic FLP hydrogenation, employing sterically crowded chiral boranes as Lewis acids (Scheme 3). After early experiments with pinene-derived chiral boranes,15a able to promote the reduction of acetophenone derived ketimines with only 13% e.e., in 2010 a chiral borane derived from camphor allowed a high enantiomeric excess (up to 83%) in the enantioselective reduction of imines.15b The hydroboration of a 2-phenyl bicycloheptene derivative 1 using bis(perfluorophenyl)borane in toluene or pentane gave the diastereomeric boranes 2 and 3 in a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 ratio as confirmed by multinuclear NMR spectroscopy. Treatment of an n-pentane solution of the boranes mixture with hydrogen at 25 °C in the presence of tri-tert-butylphosphine resulted in the precipitation of a colorless solid in 53% yield, which multinuclear NMR spectroscopy confirmed to be a mixture of the activated FLP salts 2′ and 3′.
80 ratio as confirmed by multinuclear NMR spectroscopy. Treatment of an n-pentane solution of the boranes mixture with hydrogen at 25 °C in the presence of tri-tert-butylphosphine resulted in the precipitation of a colorless solid in 53% yield, which multinuclear NMR spectroscopy confirmed to be a mixture of the activated FLP salts 2′ and 3′.
In the presence of a 5 mol% amount of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the two diastereoisomers at 65 °C and 25 bar hydrogen, imine N-(1-phenylethylidene)aniline was transformed into the corresponding secondary amine with a 20% e.e. Obviously, the use of the diastereomerically pure salts as catalysts for the hydrogenation process gave more encouraging results. By using 3′, full conversion into the product was achieved in 48% e.e. for the S enantiomer, while 2′ led to the R enantiomer with higher enantioselectivity (79% e.e.) and up to 83% e.e. for N-PMP substituted imines. More recently, Klankermayer and co-workers developed a modified version of the previously described camphor borane featuring a strongly enhanced stability.16 After the first hydrogenation experiment, which yielded the desired amine with full conversion and 76% enantiomeric excess, the recycled solid catalyst 4 was subsequently retransferred to the autoclave, mixed with toluene and substrate and pressurized with 25 bar hydrogen. Four consecutive runs demonstrated constant levels of conversion and enantioselectivity, confirming the effectiveness and stability of this novel chiral FLP catalyst.
1 mixture of the two diastereoisomers at 65 °C and 25 bar hydrogen, imine N-(1-phenylethylidene)aniline was transformed into the corresponding secondary amine with a 20% e.e. Obviously, the use of the diastereomerically pure salts as catalysts for the hydrogenation process gave more encouraging results. By using 3′, full conversion into the product was achieved in 48% e.e. for the S enantiomer, while 2′ led to the R enantiomer with higher enantioselectivity (79% e.e.) and up to 83% e.e. for N-PMP substituted imines. More recently, Klankermayer and co-workers developed a modified version of the previously described camphor borane featuring a strongly enhanced stability.16 After the first hydrogenation experiment, which yielded the desired amine with full conversion and 76% enantiomeric excess, the recycled solid catalyst 4 was subsequently retransferred to the autoclave, mixed with toluene and substrate and pressurized with 25 bar hydrogen. Four consecutive runs demonstrated constant levels of conversion and enantioselectivity, confirming the effectiveness and stability of this novel chiral FLP catalyst.
It should be also mentioned that in 2012 the same group reported the enantioselective hydrosilylation of various imines using a slightly modified version of the previous camphor derived catalyst.17 Hydrosilylation of sterically hindered imine afforded only negligible conversion and the introduction of an electron-withdrawing group in the acetophenone moiety led to relatively low conversion, albeit with high enantioselectivity. However, the presence of a methoxy donor group strongly enhanced the conversion (up to 90%), while retaining high levels of enantioselectivity (up to 85% e.e.).
The field of chiral FLP catalysts is clearly in its infancy, but it is undoubtedly attracting the interest of several groups. Du envisioned that direct hydroboration of chiral dienes bearing two terminal olefins with HB(C6F5)2 could provide simple access to a new class of chiral borane catalysts for the asymmetric hydrogenation of imines.18 In this strategy, binaphthyl based chiral diene 5 acts like a “ligand” to generate the borane catalyst in situ without further isolation, thus ensuring easy operation and rapid evaluation (Scheme 4). Moreover, terminal olefins offer the advantage of generating enantiomerically pure boranes by hydroboration, instead of the diastereoisomer mixture which would be obtained in the case of internal olefins. After proper tuning of the reaction conditions and of the substituents at the 3,3′-positions of binaphthyl framework, a variety of imines was smoothly hydrogenated in good yields and high enantioselectivities (up to 89% e.e.).
One of the main issues related to FLP catalytic systems is the reduced stability in the presence of air and moisture, thus complicating their effective recycling. Recently, Rieger and Repo described a chiral intramolecular FLP catalyst (cat. 6, Scheme 4) with high air and moisture stability, foreshadowing the extended applicability of these systems.19 This catalyst was used in the hydrogenation of imines with a moderate catalytic loading, leading to an enantiomeric excess up to 37% e.e.
These results represent only the beginning of a very promising area, that calls for the design and the development of new, more efficient, highly stereoselective FLP catalytic systems for the activation of hydrogen and other small organic molecules.20
3. Chiral phosphoric acids-catalyzed reductions
Another opportunity for realizing enantioselective metal-free reductions comes from the use of chiral phosphoric acids. The electrophilic activation of a substrate by means of a Brønsted acid is, undoubtedly, the most straightforward and common approach employed to promote a reaction and hence they have been widely utilized as efficient catalysts for numerous organic transformations.However, their synthetic utility as chiral catalysts for stereoselective reactions has been quite limited until recently. The key to successfully realizing enantioselective transformations using a chiral Brønsted acid is to activate chemically the substrate via protonation and to control stereochemically the reaction outcome through the chiral environment created by the chiral conjugated base, which exists in the proximity of the substrate.21 Among the various organic Brønsted acids, chiral phosphoric acids have gained great attention in the last few years and have proved to be very successful catalysts.22
The great popularity of these compounds originates from their several positive characteristics. One important feature is that the phosphoryl oxygen would function as a Brønsted basic site; therefore an acid/base dual function can be anticipated, even for monofunctional phosphoric acid catalysts (Fig. 1). Furthermore, two substituents can be directly introduced at the phosphorous atom, thus creating a chiral environment close to the catalytically active site. Due to their symmetry, the commercial availability of both the enantiomers and the numerous protocols for introducing substituents at the 3,3′-position of the binaphthyl backbone, BINOL derivatives were generally selected as chiral scaffold to construct enantiomerically pure phosphoric acids.
In 2005 Rueping reported the first enantioselective Brønsted acid-catalysed hydrogenation of ketoimines.23 A screening of various phosphoric acids selected compound 7 as the best performing catalyst, showing that not only steric but also electronic effects of the 3,3′ substituents on the binaphthol scaffold played a role in this transformation, while screening of solvents established that nonpolar solvents were essential (68–84% e.e. in benzene, Scheme 5). The phosphoric acid acts as a bifunctional catalyst, not only activating the ketoimines but also coordinating the Hantzsch ester through hydrogen bonding with the Lewis basic site P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
At the same time, independently List and MacMillan described novel, modified chiral phosphoric acids for performing the same reaction efficiently. List group observed that a differently substituted catalyst (8, (R) 3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate (TRIP)) under optimized conditions showed significantly improved performance in many aspects, e.g. shorter reaction times, lower temperature, higher yields and e.e. values (80–98% yields, 80–93% e.e.) and, most notably, much lower catalyst loading.24 Moreover, this catalyst was also able to reduce highly enantioselectively aliphatic ketoimines and could be employed also in the first enantioselective organocatalytic reductive amination reaction.
Soon after, MacMillan's group explored properly this organocatalytic reductive amination,25 observing that the ortho-triphenylsilyl phosphoric acid 9 in the presence of MS 5 Å facilitates the desired coupling of acetophenone and 4-OMe-aniline in high conversion and with excellent levels of enantiocontrol at 40 °C (87% yield, 94% e.e.). The scope of this reaction is quite wide, as a variety of substituted acetophenone derivatives can be successfully coupled, including electron-rich, electron-deficient, as well as ortho, meta, and para substituted aryl ketone systems. Notably, methyl alkyl substituted ketones are also suitable substrates, thus highlighting a key benefit of reductive amination versus imine reduction: imines derived from alkyl–alkyl ketones are unstable to isolation, a fundamental limitation that is bypassed using this route.
In 2010 Wang and co-workers reported the first examples of enantioselective transfer hydrogenation of ortho-hydroxyaryl alkyl unprotected N–H ketimines using a chiral phosphoric acid as a catalyst and Hantzsch ester as the hydrogen source.26 The hindered (S)-3,3′-bis(triphenylsilyl)-substituted phosphoric acid 9 turned out to be the most effective in terms of transfer of the stereochemical information. Under optimal conditions, the unsubstituted amine was isolated in 94% yield with 92% e.e., while the presence of either an electron-withdrawing or an electron-donating group at the C-3, C-4, and C-5 positions of the aromatic ring did not affect significantly the enantioselectivity (Scheme 6).
It is remarkable to observe that previously only N-Ar imines derived from acetophenone were used as substrates in this catalytic reduction methodology. Based on NMR studies, the authors proposed transition state A (Scheme 6) wherein the phosphoric acid formed H-bonds with both the hydroxyl and the imine functions of the substrate. The hydride transfer would then occur from the Re face of the imines to deliver the amines with the observed (S) configuration.
One important breakthrough in the field was achieved by Antilla and Li, when they reported the enantioselective hydrogenation of enamides by exploiting chiral phosphoric acid catalysis.27 Although highly efficient examples of reductive amination of ketones and hydrogenation of ketoimines catalysed by chiral Brønsted acids were already reported, these reactions were limited primarily to reactants derived from aniline and its analogues. As a result, the deprotection of the aromatic group to release the amino group could be relatively difficult, rendering these methods less synthetically appealing. On the contrary, when considering N-acyl enamide substrates, the acyl group of the reduction product can be removed easily under standard procedures in good yield. Indeed phosphoric acid 10 was able to promote efficiently the reaction. In the hypothesized catalytic cycle, in the presence of the catalyst and the cocatalyst acetic acid, the enamide is tautomerized to the corresponding imine, which is activated by the acid via an iminium intermediate. In the following step, only the chiral phosphoric acid is active enough to catalyse the reduction of the imine, while acetic acid probably contributes merely to keep a sufficient concentration of iminium intermediate.
The phosphoric acid-catalysed reduction protocol was extended to α-imino esters and their derivatives, and employed even in a gram scale synthesis of a chiral intermediate.28 The enantioselectivity was highly dependent on the steric size of the ester R′′ group; high e.e. were obtained for substrates bearing bulky ester groups such as i-Pr and t-Bu, whereas only 33% e.e. was observed for the methyl ester substrate. As for the scope of R′, several substituted phenyl isopropyl esters containing either electron-donating or electron-withdrawing groups all led to good yields and excellent e.e. (Scheme 7). However, a low reactivity was observed in the case of the alkyl-substituted imino ester.
Independently, Antilla and co-workers developed an organocatalytic reduction process for the enantioselective synthesis of protected α-amino acids, by employing a VAPOL-derived phosphoric acid.29 Derivative 11 was found to be superior in this reaction to BINOL-derived phosphoric acid, as well as to a small library of alternative chiral phosphoric acid catalysts. However, the analogous reductive amination process involving in situ imino ester formation was not efficient and was selective only when starting materials bearing aliphatic substituents were used. Enders has recently described the reduction of ketoimines and α-imino esters with catecholborane via Brønsted acid catalysis.30 Under optimized conditions, various electron-rich as well as electron-deficient aromatic α-imino esters with different substitution patterns were reduced in very good enantioselectivity and high chemical yield.
List's group discovered an interesting variation on this theme, realizing the direct reductive amination of α-branched aldehydes via an efficient dynamic kinetic resolution (DKR).31 Under the reductive amination conditions an α-branched aldehyde undergoes a fast racemization in the presence of the amine and acid catalyst via an imine/enamine tautomerization. The reductive amination of one of the two imine enantiomers would then have to be faster than that of the other, resulting in an enantiomerically enriched product via a dynamic kinetic resolution (Scheme 8). TRIP catalyst 8 once again turned out to be the most effective and enantioselective catalyst for this transformation and provided the chiral amine product in 50% yield and an enantiomeric excess of 68%, which could be raised to 87% yield and 96% e.e. under optimized conditions. The efficient removal of water formed during the reaction seems to be important as the enantiomeric ratio improved considerably upon using 5 Å molecular sieves; furthermore, oxygen-free conditions are required as substantial acetophenone and p-formylanisidine formation was observed in the presence of oxygen, presumably via an oxidative cleavage of the enamine intermediate.
A few years later, List reported the use of DKR in the catalytic asymmetric reductive amination of racemic α-branched ketones.32 An important feature of this process is its tolerance of a variety of different substituents whilst maintaining excellent enantioselectivity. Simple alkyl-substituted substrates are particularly reactive, requiring only a very low amount of catalyst, while sterically more-demanding substrates, as well as aromatic substrates, require slightly higher catalyst loadings; α,β-unsaturated, α-branched ketones could be converted into the desired product in reasonable yields and excellent selectivity.
In 2013, Akiyama's group developed the Brønsted acid-catalyzed asymmetric hydrogen transfer reaction of indolines, by employing imines as hydrogen acceptors; the work represents the first example of an efficient oxidative kinetic resolution of secondary amines.33 This approach allowed the isolation of 2-substituted and 2,3-disubstituted indolines in high yields and excellent enantioselectivities, allowing at the same time the synthesis of chiral amines in a nearly enantiopure form (Scheme 9).
From a mechanistic point of view, one enantiomer of the indoline would preferentially participate in this hydrogen transfer reaction and be converted into a cyclic imine, which would immediately isomerize to a stable indole. On the basis of the bifunctional nature of the phosphoric acid, the authors hypothesized a dicoordinated cyclic transition state, where the Brønsted acidic proton activates the ketoimine and the Lewis basic phosphoryl oxygen coordinates to the indoline N–H. The most favorable TS resulting from DFT calculations shows the N-aryl group of the ketoimine and the 2-phenyl group of the indoline to have no unfavorable steric interactions. In contrast, the steric hindrance between the 3,3′-substituents of the chiral phosphoric acid and the two aryl groups of the substrates destabilizes the other possible transition states.
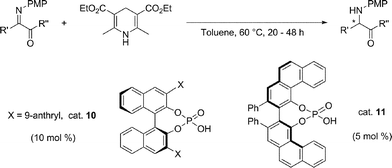
The phosphoric-acid-catalysed methodology was successfully used also in the reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds of heterocyclic systems. Examples of efficient catalysts for the asymmetric hydrogenation of aromatic and heteroaromatic compounds are quite rare, even amongst the chiral Rh, Ru, and Ir complexes. It was therefore an important breakthrough the development by Rueping's group in 2006 of an enantioselective organocatalytic partial reduction of quinoline derivatives34 which are of great synthetic importance in the preparation of pharmaceuticals and agrochemicals, as well a structural key element of many alkaloids. This represented the first example of a metal-free reduction of heteroaromatic compounds. After screening of a variety of sterically congested phosphoric acids, (R)-(−)-9-phenanthryl-1,1′-binaphthyl-2,2′-diyl hydrogen-phosphate was selected as catalyst of choice to generate 2-phenyltetrahydroquinoline in 97% e.e. (Scheme 10).
N bonds of heterocyclic systems. Examples of efficient catalysts for the asymmetric hydrogenation of aromatic and heteroaromatic compounds are quite rare, even amongst the chiral Rh, Ru, and Ir complexes. It was therefore an important breakthrough the development by Rueping's group in 2006 of an enantioselective organocatalytic partial reduction of quinoline derivatives34 which are of great synthetic importance in the preparation of pharmaceuticals and agrochemicals, as well a structural key element of many alkaloids. This represented the first example of a metal-free reduction of heteroaromatic compounds. After screening of a variety of sterically congested phosphoric acids, (R)-(−)-9-phenanthryl-1,1′-binaphthyl-2,2′-diyl hydrogen-phosphate was selected as catalyst of choice to generate 2-phenyltetrahydroquinoline in 97% e.e. (Scheme 10).
The first step in the enantioselective cascade hydrogenation is the protonation of quinoline through a Brønsted acid catalyst to generate the iminium ion, followed by the transfer of the first hydride from dihydropyridine to generate the corresponding enamine, which reacts in a second cycle with the Brønsted acid and is reduced to the desired tetrahydroquinoline. Subsequent proton transfer will then restore the phosphoric acid. The absolute configuration of the products can be explained by a stereochemical model, where the approach of the hydride nucleophile is favoured from the less hindered Si face since the Re face is shielded by the large phenanthryl group of the catalyst.
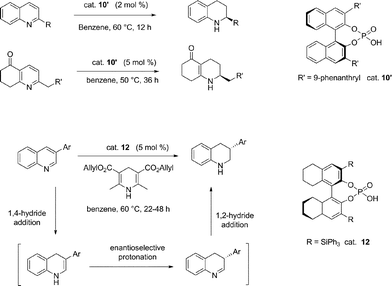
The methodology was then applied to the enantioselective reduction of pyridine derivatives.35 Catalyst 10 worked efficiently in the hydrogenation of this class of compounds to furnish the corresponding hexahydroquinolinones with high enantioselectivities and with analogous mechanism. However, the mechanistic picture is quite different in the case of 3-substituted quinolones. The key step of the transfer hydrogenation of 3-substituted quinolines is the enantioselective protonation of the intermediary enamine, which, after 1,2-hydride addition, leads to 3-substituted tetrahydroquinolines with good enantioselectivities (Scheme 10).36a Moreover, the strategy was successful also in the reduction of 2,3-substituted quinolines, which allowed isolation of the octahydroacridine in good yield and with excellent diastereo- and enantioselectivities.36b
At the end of this section it is worth mentioning that chiral phosphoric acids have been employed also for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
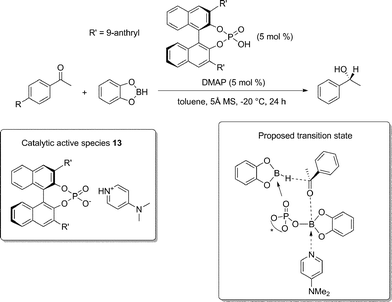
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds stereoselective reductions. About the carbonyl reduction, in 2011, Antilla reported the first example of enantioselective reduction of ketones catalyzed by a chiral phosphoric acid derivative.37 The reduction of variously substituted acetophenones with catecholborane promoted by a series of BINOL-derived phosphoric acids gave the desired product with only modest enantioselectivity, catalyst 8 being the best performing one.
C bonds stereoselective reductions. About the carbonyl reduction, in 2011, Antilla reported the first example of enantioselective reduction of ketones catalyzed by a chiral phosphoric acid derivative.37 The reduction of variously substituted acetophenones with catecholborane promoted by a series of BINOL-derived phosphoric acids gave the desired product with only modest enantioselectivity, catalyst 8 being the best performing one.
However, it was found that an increase in the enantiomeric excess could be obtained by lowering the reaction temperature to −20 °C and by using 4-(dimethylamino)pyridine (DMAP) as an additive; this pyridine derivative is likely to form the corresponding pyridium phosphate salt, a very weak acid (species 13, Scheme 11). The substrates bearing either electron-donating or electron-withdrawing groups on the phenyl ring furnished the resulting chiral alcohols with good selectivity; labile functional groups, such as nitrile, nitro, ester, iodide and bromide, were generally well tolerated.
The boron center is believed to act as a Lewis acid to activate the carbonyl, while the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety can act as a Lewis base to increase the nucleophilicity of catecholborane. Simultaneously, the hydride from unreacted catecholborane is added to the activated carbonyl under the influence of a chiral environment to form the hydroboration product and regenerate the catalyst.
O moiety can act as a Lewis base to increase the nucleophilicity of catecholborane. Simultaneously, the hydride from unreacted catecholborane is added to the activated carbonyl under the influence of a chiral environment to form the hydroboration product and regenerate the catalyst.
Regarding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C reductions, the reduction of unsaturated carbonyl derivatives was already described in 2006. The organic salt of (R)-3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate 8 (TRIP) and morpholine was able to promote transfer hydrogenation via Hantzsch dihydropyridine of α,β-unsaturated aldehydes with high levels of enantioselectivity, ranging between 96% and >98% e.e. (Scheme 12).38 The reaction proceeds via an iminium ion intermediate since salts of tertiary amines are ineffective, and stereoselection presumably occurs in the cationic transition state of the reaction by means of a stereochemical communication with the chiral phosphate counteranion, possibly through CH⋯O hydrogen-bonding interaction. Notably the procedure was used successfully to convert citral into (R)-citronellal with an e.e. value of 90%, the highest enantioselectivity reported for this reaction until then.
C reductions, the reduction of unsaturated carbonyl derivatives was already described in 2006. The organic salt of (R)-3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate 8 (TRIP) and morpholine was able to promote transfer hydrogenation via Hantzsch dihydropyridine of α,β-unsaturated aldehydes with high levels of enantioselectivity, ranging between 96% and >98% e.e. (Scheme 12).38 The reaction proceeds via an iminium ion intermediate since salts of tertiary amines are ineffective, and stereoselection presumably occurs in the cationic transition state of the reaction by means of a stereochemical communication with the chiral phosphate counteranion, possibly through CH⋯O hydrogen-bonding interaction. Notably the procedure was used successfully to convert citral into (R)-citronellal with an e.e. value of 90%, the highest enantioselectivity reported for this reaction until then.
The result represented an advancement compared to the previous studies by List and MacMillan, where chiral imidazolidinone-based catalysts did not give satisfactory results in the reduction of sterically non-hindered aliphatic substrates. Indeed it should be mentioned that in 2004 the first example of an enantioselective metal-free transfer hydrogenation of an olefin was described, the reduction of β-methyl, α,β-unsaturated aldehyde, in the presence of a substoichiometric amount of the HCl salt of a chiral imidazolinone (81% yield and 81% e.e.).39
The phosphoric-acid catalysed protocol was successfully employed also in the reduction of unsaturated ketones (Scheme 12).40
The combination of (R)-(TRIP) (acid 8) and valine was selected as best performing catalyst; the effect of the amino acid seems to be important as corresponding glycine derived catalyst gave significantly reduced enantioselectivity, as well as the reaction promoted by the phosphoric acid alone. When the opposite enantiomeric counteranion ((S)-TRIP) was used, the same major enantiomer was formed but with much lower enantioselectivity, thus demonstrating the existence of a matched/mismatched catalyst–ion pair combination effect.
4. Trichlorosilane-promoted reductions
The third organocatalytic approach to perform a stereoselective reduction involves the use of trichlorosilane. This reagent is a very cheap, colorless, volatile liquid, easily supplied from the silicon industry. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry; it is the basic ingredient used in the production of purified polysilicons. Because of its reactivity and wide availability, it is frequently used in the synthesis of silicon-containing organic compounds, for example in the preparation of perfluoroalkyltrichlorosilane, employed in surface science and nanotechnology to form self-assembled monolayers. In order to act as reagent able to reduce C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, trichlorosilane needs to be activated by coordination with bases, such as N,N-dimethylformamide, acetonitrile and trialkylamines, to generate hexacoordinated hydridosilicate, the real active reducing agent that operates under mild conditions.41 The use of chiral Lewis bases offers the possibility to control the absolute stereochemistry of the process and it has been widely explored in the last few years, leading to the development of some very efficient catalysts. Since two reviews have recently covered the topic,42 only the most representative classes of chiral catalysts will be presented here, with the intent to show the wide applicability of trichlorosilane to a great variety of substrates and also to introduce properly the argument to non-experts in the field.
N bonds, trichlorosilane needs to be activated by coordination with bases, such as N,N-dimethylformamide, acetonitrile and trialkylamines, to generate hexacoordinated hydridosilicate, the real active reducing agent that operates under mild conditions.41 The use of chiral Lewis bases offers the possibility to control the absolute stereochemistry of the process and it has been widely explored in the last few years, leading to the development of some very efficient catalysts. Since two reviews have recently covered the topic,42 only the most representative classes of chiral catalysts will be presented here, with the intent to show the wide applicability of trichlorosilane to a great variety of substrates and also to introduce properly the argument to non-experts in the field.
Most of the chiral Lewis bases developed for trichlorosilane activation derive from natural α-aminoacids. After seminal works by Matsumura with proline,43 the first successful catalyst for trichlorosilane-mediated enantioselective reductions of ketoimines was reported by Malkov and Kočovský (e.e. up to 93%).44 They identified as organocatalyst of choice the N-methyl-(S)-valine-derived type 14 compounds, commercially available since 2009. The screening of a variety of N-methyl-(S)-amino acids highlighted valine as chiral element of choice to perform stereocontrol and allowed to individuate key features of the catalytic system: the N-methyl formamide moiety of the catalyst is fundamental to achieve high levels of enantioselectivity, while the anilide moiety must have a NH group; arene–arene interactions may play an important role in determining the stereoselectivity of the catalyst, and bulkier groups in the 3,5-positions of the aromatic ring (diisopropyl and di-tert-butyl) have a positive effect on the enantioselectivity of the process. In the proposed transition state coordination of the silicon atom by the two carboxyamide groups guarantees chemical activation of the reducing agent, whereas the formation of a hydrogen-bond between the secondary amide group of the catalyst and the substrate represents an element of stereocontrol. The N-aryl group is believed to play an important role in the stereocontrol because it should be involved in π–π stacking interaction between the catalyst and the substrate (Scheme 13). The general applicability of catalyst 14, known as Sigamide, was then successfully investigated in the reduction of multifunctionalized ketimines bearing heterocyclic and aliphatic moieties.45
The popularity of this catalytic species is demonstrated also by the development of immobilized versions of 14 on different supports, in order to realize a recoverable system. Heterogeneous materials were employed; Merrifield, Wang, TentaGel and Marshall resins, were all employed to immobilize the organocatalysts through an ethereal bond (catalyst type 15).46 By operating under the best experimental conditions, with the Merrifield-anchored catalyst, the product was isolated in good yield and in an e.e. about 10% lower than the enantioselectivity obtained with the non-supported catalyst. Other recovery strategies have been employed, including the use of gold nanoparticles,47 block polymethacrylate polymers48 and a fluorous tag.49
Later Sun and co-workers developed a N-formyl derivative of (S)-pipecolinic acid (catalyst 16), able to promote the reduction of N-aryl ketimines with trichlorosilane with high yields and good enantioselectivities.50 Switching from the five-membered ring of proline to a six-membered ring had a beneficial effect on enantioselectivity. The reduction of aliphatic ketoimines51 was also accomplished in this work, where the independence of the imine geometry on the selectivity of the reaction was demonstrated.
It is interesting to note that in 2006 Matsumura reported the activity of N-formyl proline derivatives in the reduction of imines and ketones in the presence of trichlorosilane.52 Secondary alcohols could be synthesized with high enantioselectivity (up to 97%) employing a catalytic amount of N-formyl-α′-(2,4,6-triethylphenyl)-(S)-proline (catalyst 17). The selection of the best performing compound was the result of the screening of a series of α′-arylproline derivatives. Both the carbonyl group at the α-position and a 2,4,6-triethylphenyl group at the 5 position in the proline ring are crucial in determining the high enantioselectivity. In a closely related work,53 our group reported a similar use of chiral amino acids, whose ability to form hydrogen bonds with the substrate provides an easy route to induce enantioselectivity. This approach was applied for the first time to the HSiCl3 mediated reduction of carbon–nitrogen double bonds employing proline-derived Lewis bases as catalysts (catalyst 18, Scheme 13), leading to high chemical yields and enantiomeric excess up to 75%. It is important to point out that in 2009 Schreiner published a detailed investigation of the influence that non aromatic groups in N-formylprolinamide may have on the enantiomeric excesses of ketoimine reductions, also employing computational methods in the attempt to get some mechanistic insights in the process.54 By working with a series of novel chiral organocatalysts derived from proline, valine, and pipecolinic acid, the dominant role of the amino acid scaffold in the enantiodifferentiating step was demonstrated. DFT mechanistic studies seem to confirm that the catalyst not only coordinates to trichlorosilane, but also reacts as a proton donor in the crucial transition structure; indeed, the importance of the presence of acidic NH proton of a secondary amide group, able to bind to the basic nitrogen of the reacting imine, has been demonstrated. Although the authors suggest that the stereoselective steps for proline, pipecolinic acid and valine-derived catalysts may be different, from computational studies they propose a general picture for the catalytic reduction of ketoimines with trichlorosilane, that could be described as a formal H+/H− transfer to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. However other, more refined computational studies are needed to elucidate further the mechanism of these reactions.
N double bond. However other, more refined computational studies are needed to elucidate further the mechanism of these reactions.
A contribution by Matsumura in 2006 opened the way towards the development of a novel class of catalysts for trichlorosilane-mediated reductions, derived from chiral amino alcohols, showing that N-picolinoylpyrrolidine derivatives were able to activate trichlorosilane in the reduction of aromatic imines.55 Catalyst 19 gave the best results, leading to enantioselectivities up to 80%. The authors proposed that both the nitrogen atom of the picolinoyl group and the carbonyl oxygen were involved in the coordination and activation of silicon atoms. Based on these seminal works, our group has recently focused on the design and synthesis of a wide class of catalysts prepared by simple condensation of a chiral aminoalcohol with picolinic acid derivatives. We56 and Zhang57 reported independently in a preliminary communication the use of ephedrine and pseudoephedrine-derived picolinamides in the reduction of N-aryl and N-benzyl ketimines promoted by trichlorosilane. The pyridine ring, the free hydroxyl group and N-alkyl substitution in the aminoalcohol portion were identified as key structural elements, necessary to secure good stereocontrol; the presence of two stereogenic centers on the aminoalcohol moiety with the correct relative configuration, as in (1R,2S)-(−)-ephedrine, as well as of the methyl groups on the amide nitrogen and on the stereocenter in position 2 of the amino alcohol chain are also necessary to direct efficiently the stereochemistry of the imine attack by trichlorosilane. In the proposed stereoselection model (Scheme 14), leading to the experimentally observed preferred formation of the R isomer of the product, the steric interaction between the pyridine ring and N-aryl group is much less significant than that observed in the model leading to the opposite enantiomer. 4-Bromo and 4-chloro picolinic derivatives showed remarkable catalytic properties. Working at 0 °C with catalyst 20 the chiral amine was obtained in quantitative yield and 88% enantiomeric excess; a further improvement was observed by performing the reaction at −20 °C: enantioselectivity reached 95% e.e. and at the same time no erosion of the chemical yield was observed, with the reduction product isolated in quantitative yield. Even by working with a 1% mol amount, catalyst 20 promoted the reduction in 90% yield after only 2 hours. Good results were also obtained in the enantioselective reduction of N-alkyl imines, a transformation only recently accomplished organocatalytically.
A very convenient enantioselective organocatalytic three-component methodology was also developed; the reductive amination process, starting simply from a mixture of a ketone and an aryl amine, opens an easy access to chiral amines with a straightforward experimental methodology. The hydrosilylation of a range of substrates derived from (R)-1-phenylethylamine was also examined (eq. (b), Scheme 14). When chiral picolinamide 20 was employed as a catalyst, the control of the stereoselectivity was total, as demonstration of the presence of a cooperative effect between the Lewis basic catalyst and the (R)-methyl benzyl residue at the imine nitrogen.58 The approach was extended to the synthesis of several enantiomerically pure secondary amines with C1 or C2 symmetry. Also the imine derived from methyl isopropyl ketone was readily reduced in >98% yield, to afford an enantiomerically pure direct precursor of (R)-isopropyl methyl amine. At the same time Jones reported the use of the N-methyl imidazole catalyst 21 derived from prolinol.59 This was employed in the reduction of a wide range of aromatics and aliphatics ketimines with just 1 mol% of catalyst and a short reaction time, obtaining up to 96% yield and 87% e.e. The same catalyst was then reported for the high selective reductive amination of a large variety of ketones and aryl or aliphatic amines.60
However the search for novel enantiomerically pure Lewis bases able to act as catalysts in enantioselective reduction processes is endless. For example in Fig. 2 new organocatalysts, based on a different chiral scaffold developed by Malkov and Kočovský were reported. Chiral oxazolines containing isoquinoline 22 were employed in the reduction of both aromatic ketones and imines with trichlorosilane.61 The best enantiomeric excess reached in the reduction of ketones was 87%, while even better results were achieved in the ketimines’ reduction (92% e.e.). The authors hypothesized coordination of trichlorosilane by the catalyst would generate a hexacoordinated silicon species that would act as the actual reducing species. When a ketone is the reactive substrate, further activation would be provided by coordination of a molecule of trichlorosilane by the carbonyl oxygen.
Almost at the same time, Sun published a novel catalyst featuring a sulfinamide group as the stereocontrolling element.62 This family of organocatalysts was found to be able to activate trichlorosilane for the stereoselective reduction of N-aryl ketimines with good yields and enantioselectivities, catalyst 23 being the most successful compound in terms of stereoselection. Based on the assumption that the mechanism would involve two molecules of Lewis base for the activation of HSiCl3, a novel chiral bis-sulfinamide was then developed.63 After a screening of different derivatives, the compound of choice was found to be a bis-sulfinamide bearing a five-methylene linkage. Catalyst 24 promoted the reduction of the model substrate, N-phenylimine of acetophenone, with 96% e.e. (Fig. 2).
The success of the trichlorosilane-mediated reduction methodology is demonstrated by its application to the reduction of different classes of compounds, leading to the formation of highly functionalized chiral molecules. Indeed trichlorosilane was used to reduce both α-and β-imino esters (Scheme 15).
Zhang recently reported the first highly efficient protocol for the organocatalytic synthesis of α-amino esters.64 Notably the prolinol-derived catalyst of choice, compound 25, exhibited only moderate enantioselectivities in the hydrosilylation of N-aryl β-enamino esters, but promoted the reduction of α-imino esters with high enantioselectivities (up to 93% e.e.). The introduction of a bulky group at C4 of the pyrrolidine ring was decisive in order to obtain high stereoselectivities. The addition of small quantities of pentanoic acid was crucial for the efficiency of the process.
More studies have been performed on the reduction of β-imino esters. N-Sulfinyl L-proline amide 26 has been used for the enantioselective reduction of a range of N-alkyl β-enamino esters.65 The use of water as an additive proved to be crucial to obtain high levels of reactivity and enantioselectivity, accelerating the enamine–imine tautomerization and increasing the electrophilicity of the imine through protonation of the nitrogen atom. At the same time our group employed successfully ephedrine-derived catalyst 20 to reduce a series of N-benzyl and N-α-methylbenzyl-β-enaminoesters. Then, hydrogenolysis of the enantiomerically enriched N-benzyl-β-aminoesters, followed by LDA-promoted ring closure, afforded enantiomerically pure 4-aryl or 4-alkyl substituted β-lactams.66 Furthermore, we reported a novel class of chiral prolinol derivatives to promote the hydrosilylation of α-imino and β-imino esters.67 In nearly all cases, catalyst 27 was the most effective in the reduction of a range of electron rich and electron deficient substrates. Good results were obtained also with catalyst 28 at −30 °C for 48 h. In the presence of 10 mol% of Lewis base, β-enamino esters were reduced in high yields and enantioselectivities typically ranging from 90% to 95%.68 It is worth mentioning that N-acyl β-enamino esters were totally inactive in the present organocatalytic system. The reaction is supposed to proceed through the imine tautomer rather than its enamine counterpart. In the proposed mechanism the nitrogen atom of the pyridine ring and the carbonyl oxygen atom of the catalyst are coordinated to HSiCl3, while the imine is activated by the hydroxyl group of the Lewis base through hydrogen bonding.
More recent studies from this group have focused on α-substituted-β-enamino esters. It should be mentioned that a few years ago catalyst 14 had been used to develop a new protocol for the enantioselective synthesis of β-aminoacids derivatives from enamine precursors.69 Treatment of the β-ketoester or β-ketonitrile with p-anisidine afforded enamines, which as such cannot be reduced by HSiCl3. Since the enamine–imine equilibration is facilitated by Brønsted acids, a number of acid additives were examined, among which AcOH (one mol equivalent) emerged as a good compromise between reactivity and selectivity. Enamine was reduced to give the amino ester in high yield and 89% e.e. More recently, a novel class of chiral Lewis base catalysts, prepared from a readily available chiral source, was developed by Zhang (catalyst 29).70 A wide variety of N-aryl β-aryl and -heteroaryl substrates were reduced in very good yields (up to 98%) and selectivity (up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 syn/anti and 99% e.e.). This methodology was used in the synthesis under very mild reaction conditions of both the taxol C13 side chain and of a potent hypocholesterolemic agent; the removal of water and oxygen from the reaction system was not necessary, suggesting the generation of a Brønsted acid that promoted enamine tautomerisation. Lewis base-catalyzed asymmetric hydrosilylation of α-acetamido-β-enamino esters was also reported in the presence of catalyst 30, to afford smoothly the corresponding products with high yields (up to 99%), excellent enantioselectivities (up to 98% e.e.) and moderate diastereoselectivities (up to 80
1 syn/anti and 99% e.e.). This methodology was used in the synthesis under very mild reaction conditions of both the taxol C13 side chain and of a potent hypocholesterolemic agent; the removal of water and oxygen from the reaction system was not necessary, suggesting the generation of a Brønsted acid that promoted enamine tautomerisation. Lewis base-catalyzed asymmetric hydrosilylation of α-acetamido-β-enamino esters was also reported in the presence of catalyst 30, to afford smoothly the corresponding products with high yields (up to 99%), excellent enantioselectivities (up to 98% e.e.) and moderate diastereoselectivities (up to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 d.r.).71 The lower diastereocontrol observed in this study is ascribed by the authors to the role of the hydrogen of the α-acetamide group. Indeed, the enamine isomerization forms preferentially the E-imine; however in this case the Z-imine can be stabilized by hydrogen bonding between the hydrogen of the α-acetamide group and the nitrogen of the imine. In this way, considerable amounts of Z-imine could be generated, resulting in lowers levels of diastereoselectivity.
20 d.r.).71 The lower diastereocontrol observed in this study is ascribed by the authors to the role of the hydrogen of the α-acetamide group. Indeed, the enamine isomerization forms preferentially the E-imine; however in this case the Z-imine can be stabilized by hydrogen bonding between the hydrogen of the α-acetamide group and the nitrogen of the imine. In this way, considerable amounts of Z-imine could be generated, resulting in lowers levels of diastereoselectivity.
Finally it is worth mentioning that the methodology has been already applied to the reductions of heterocyclic systems. For example, Sun reported the first direct enantioselective hydrosilylation of prochiral 1H-indoles by combined Brønsted acid/Lewis base activation.72 The key factor for this methodology is the addition of one equivalent of water to react with HSiCl3 to generate a controlled amount of strong Brønsted acid, HCl. In this way the reaction proceeds through the generation of electrophilic indolenium ions by C3 protonation with the in situ formed HCl, and subsequent chiral Lewis base 31 catalyzed enantioselective hydrosilylation with HSiCl3 (Scheme 16). The same group has recently studied the reduction of 3-aryl-1,4-benzooxazines,73 that compares favorably with the phosphoric acid-based approach. The catalyst of choice was found to be the N-sulfinyl L-phenylalanine derived amide 32, which allowed the production of a broad range of 3-substituted 3,4-dihydro-2H-1,4-benzooxazines in very good chemical yield and high enantioselectivity, even with a low catalyst loading (2 mol%). A stereochemical match between the carbon and the sulfur stereogenic centers is crucial for the enantiocontrol. The stereoselective synthesis of chiral heterocyclic building blocks such as dihydrobenzodiazepinones was also accomplished, by using picolinamides 25.74 The corresponding products were obtained in excellent yields (up to 99%) and enantioselectivities (up to 98%).
5. Outlook and perspectives
The advent of organocatalysis brought new attractive possibilities, to realize stereoselective catalytic synthesis of complex chiral molecules, even bearing several functional groups, with metal-free processes. The enantioselective organocatalytic methodologies described in the present chapter are a clear demonstration of the enormous potentiality of metal-free catalytic reductions. But it is also evident that this is only the beginning of the story; even if both pharmaceutical companies and fine chemicals suppliers continue to invest heavily in chiral technologies, the chiral market is still steadily growing and always calls for new stereoselective catalytic strategies for the synthesis of chiral molecules. FLP-based catalytic chiral methods are almost a totally unexplored field. On the other hand in trichlorosilane-mediated reductions the search for new and more efficient chiral Lewis bases is still very active. In this context recently the use of enantiomerically pure phosphine oxides75 as catalysts in HSiCl3-mediated reactions has been introduced. The reduction of 1,3-diphenylbutenone promoted by catalytic amounts of 2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl dioxide 33 ((S)-BINAPO) at 0 °C led to the corresponding saturated compound in 97% yield and 97% e.e.76 As a further development, it was shown that, after performing the 1,4-reduction, the generated trichlorosilyl enolate should react with the electrophilic aldehyde to afford the aldol product (Fig. 3).Indeed, the possibility to design organocatalytic cascade reactions and to realize a one-pot multi-step synthesis of complex chiral molecules is a frontier research field. Another major issue that needs to be seriously tackled is the catalyst loading; too often large amounts of the catalyst (10–20 mol%) are necessary to guarantee high levels of stereoselectivity. Recently our group has reported a highly efficient reduction protocol for trifluoromethyl aryl and alkyl ketoimines, leading to the corresponding fluorinated amines with high chemical and stereochemical efficiency (typically in >90% yield and up to 98% e.e.) in the presence of 1 mol% only of catalyst 20.77
It can be anticipated that many more exciting results and developments will occur in this field, both in the discovery of new chiral organocatalysts and in the design of innovative synthetic catalytic stereoselective methodologies.
Acknowledgements
M. B. thanks FIRB project RBFR10BF5V “Multifunctional hybrid materials for the development of sustainable catalytic processes” (Rome) and the European COST Action CM0905-Organocatalysis.Notes and references
- For general reviews on asymmetric hydrogenation, see: (a) A. M. Palmer and A. Zanotti-Gerosa, Curr. Opin. Drug Discovery Dev., 2010, 13, 698–719 CAS; (b) G. Shang, W. Li and X. Zhang, Transition Metal-Catalyzed Homogeneous Asymmetric Hydrogenation, in Catalytic Asymmetric Synthesis, ed. I. Ojima, Wiley, Hoboken, 3rd edn, 2010, pp. 343–436 Search PubMed.
- J. Aleman and S. Cabrera, Chem. Soc. Rev., 2013, 42, 774–793 RSC.
- C. A. Busacca, D. R. Fandrick, J. J. Song and C. H. Senanayake, Adv. Synth. Catal., 2011, 353, 1825–1864 CrossRef CAS.
- V. Farina, J. T. Reeves, C. H. Senanayake and J. J. Song, Chem. Rev., 2006, 106, 2734–2763 CrossRef CAS PubMed.
- P. J. Dunn, Green Chem., 2013, 15, 3099–3104 RSC.
- Enantioselective Organocatalysis. Reactions and Experimental procedures, ed. P. I. Dalko, Wiley VCH, Weinheim, 2007 Search PubMed.
- For a recent review on chiral amine synthesis see: T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753–819 CrossRef CAS.
- For a review on hydrogenation of heteroaromatic compounds see: Y.-G. Zhou, Acc. Chem. Res., 2007, 40, 1357–1366 CrossRef CAS PubMed . See also F. Glorius, Org. Biomol. Chem., 2005, 3, 4171–4175 Search PubMed.
- Recent reviews: (a) J. G. de Vries and N. Mršić, Catal. Sci. Technol., 2011, 1, 727–735 RSC; (b) M. Rueping, J. Dufour and F. R. Schoepke, Green Chem., 2011, 13, 1084–1105 RSC.
- For a special issue on hydrogenation and transfer hydrogenation see: M. J. Krische and Y. Sun, Acc. Chem. Res., 2007, 40, 1237–1419 CrossRef CAS.
- Reviews: (a) D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 46–76 CrossRef CAS PubMed; (b) D. W. Stephan, Chem. Commun., 2010, 46, 8526–8533 RSC; (c) J. Paradies, Synlett, 2013, 24, 777–780 CrossRef CAS PubMed; (d) D. Chen and J. Klankermayer, Top. Curr. Chem., 2013, 334, 1–26 CrossRef; (e) Pioneering work: G. C. Welch, R. R. San Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124–1126 CrossRef CAS PubMed.
- (a) T. A. Rokob, A. Hamza, A. Stirling and I. Papai, J. Am. Chem. Soc., 2009, 131, 2029–2036 CrossRef CAS PubMed; (b) S. Grimme, H. Kruse, L. Goerigk and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 1402–1405 CrossRef CAS PubMed.
- (a) P. A. Chase, G. C. Welch, T. Jurca and D. W. Stephan, Angew. Chem., Int. Ed., 2007, 46, 8050–8053 CrossRef CAS PubMed; (b) P. A. Chase, T. Jurca and D. W. Stephan, Chem. Commun., 2008, 1701–1703 RSC.
- S. J. Geier, P. A. Chase and D. W. Stephan, Chem. Commun., 2010, 46, 4884–4886 RSC.
- (a) D. J. Chen and J. Klankermayer, Chem. Commun., 2008, 2130–2131 RSC; (b) D. J. Chen, Y. T. Wang and J. Klankermayer, Angew. Chem., Int. Ed., 2010, 49, 9475–9478 CrossRef CAS PubMed.
- G. Ghattas, D. J. Chen, F. Panb and J. Klankermayer, Dalton Trans., 2012, 41, 9026–9028 RSC.
- D. J. Chen, V. Leich, F. Pan and J. Klankermayer, Chem. – Eur. J., 2012, 18, 5184–5187 CrossRef CAS PubMed.
- Y. Liu and H. Du, J. Am. Chem. Soc., 2013, 135, 6810–6813 CrossRef CAS PubMed.
- V. Sumerin, K. Chernichenko, M. Nieger, M. Leskelä, B. Rieger and T. Repo, Adv. Synth. Catal., 2011, 353, 2093–2110 CrossRef CAS.
- For some very recent advancements in the field see: T. Wiegand, H. Eckert, O. Ekkert, R. Fröhlich, G. Kehr, G. Erker and S. Grimme, J. Am. Chem. Soc., 2012, 134, 4236–4249 CrossRef CAS PubMed; T. Mahdi, Z. Heiden, S. Grimme and D. W. Stephan, J. Am. Chem. Soc., 2012, 134, 4088–4091 CrossRef PubMed.
- Reviews: (a) K. Brak and E. N. Jacobsen, Angew. Chem., Int. Ed., 2013, 52, 534–561 CrossRef CAS PubMed; (b) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518–533 CrossRef CAS PubMed.
- For recent reviews see: (a) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed; (b) M. Terada, Synthesis, 2010, 12, 1929–1982 CrossRef PubMed; (c) J. Yu, F. Shi and L. Gong, Acc. Chem. Res., 2011, 44, 1156–1171 CrossRef CAS PubMed; (d) For mechanistic studies, see: C. Zheng and S.-L. You, Chem. Soc. Rev., 2012, 41, 2498–2518 RSC; (e) L. Simon and J. M. Goodman, J. Am. Chem. Soc., 2008, 130, 8741–8747 CrossRef CAS PubMed; T. Marcelli, P. Hammar and F. Himo, Chem. – Eur. J., 2008, 14, 8562–8571 Search PubMed.
- M. Rueping, E. Sugiono, C. Azap, T. Theissmann and M. Bolte, Org. Lett., 2005, 7, 3781–3784 CrossRef CAS PubMed.
- S. Hoffmann, A. M. Seayad and B. List, Angew. Chem., Int. Ed., 2005, 44, 7424–7427 CrossRef CAS PubMed.
- R. I. Storer, D. E. Carrera, Y. Ni and D. W. C. MacMillan, J. Am. Chem. Soc., 2006, 128, 84–86 CrossRef CAS PubMed.
- T. B. Nguyen, H. Bousserouel, Q. Wang and F. Guéritte, Org. Lett., 2010, 12, 4705–4707 CrossRef CAS PubMed.
- G. Li and J. C. Antilla, Org. Lett., 2009, 11, 1075–1078 CrossRef CAS PubMed.
- Q. Kang, Z. Zhao and S. You, Adv. Synth. Catal., 2007, 349, 1657–1660 CrossRef CAS.
- G. Li, Y. Liang and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 5830–5831 CrossRef CAS PubMed.
- D. Enders, A. Rembiak and B. A. Stockel, Adv. Synth. Catal., 2013, 355, 1937–1942 CrossRef CAS.
- S. Hoffmann, M. Nicoletti and B. List, J. Am. Chem. Soc., 2006, 128, 13074–13075 CrossRef CAS PubMed.
- V. N. Wakchaure, J. Zhou, S. Hoffmann and B. List, Angew. Chem., Int. Ed., 2010, 49, 4612–4614 CrossRef CAS PubMed.
- K. Saito, Y. Shibata, M. Yamanaka and T. Akiyama, J. Am. Chem. Soc., 2013, 135, 11740–11743 CrossRef CAS PubMed.
- M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 3683–3686 CrossRef CAS PubMed.
- M. Rueping and A. P. Antonchick, Angew. Chem., Int. Ed., 2007, 46, 4562–4565 CrossRef CAS PubMed.
- (a) M. Rueping, T. Theissmann, S. Raja and J. W. Bats, Adv. Synth. Catal., 2008, 350, 1001–1006 CrossRef CAS . For a recent contribution on the reduction of benzodiazepinones see: M. Rueping, E. Merino and R. Koenigs, Adv. Synth. Catal., 2010, 352, 2629–2634 Search PubMed; (b) other selected recent contributions for the same group: M. Rueping, C. Brinkmann, A. P. Antonchick and I. Atodiresei, Org. Lett., 2010, 12, 4604 CrossRef CAS PubMed; M. Rueping, E. Sugiono, A. Steck and T. Theissmann, Adv. Synth. Catal., 2010, 352, 281–287 Search PubMed; M. Rueping, B. J. Nachtsheim, R. M. Koenigs and W. Ieawsuwan, Chem. – Eur. J., 2010, 16, 13116–13126 Search PubMed; (c) For other recent works in the field of reduction of heteroaromatic systems see, among others: J. Zhou and B. List, J. Am. Chem. Soc., 2007, 129, 7498–7499 CrossRef CAS PubMed; Z.-Y. Han, H. Xiao and L.-Z. Gong, Bioorg. Med. Chem. Lett., 2009, 19, 3729–3733 Search PubMed; Q. Yin, S. G. Wang and S. L. You, Org. Lett., 2013, 15, 2688–2691 Search PubMed.
- Z. Zhang, P. Jain and J. C. Antilla, Angew. Chem., Int. Ed., 2011, 50, 10961–10964 CrossRef CAS PubMed . See also: D. Enders, A. Rembiak and M. Seppelt, Tetrahedron Lett., 2013, 54, 470–473 CrossRef PubMed.
- S. Mayer and B. List, Angew. Chem., Int. Ed., 2006, 45, 4193–4195 CrossRef CAS PubMed.
- J. W. Wang, M. T. Hechavarria Fonseca and B. List, Angew. Chem., Int. Ed., 2004, 43, 6660–6662 CrossRef CAS PubMed . Immediately after, reduction of β,β-disubstituted aldehydes was described, see: J. W. Wang, M. T. Hechavarria Fonseca and B. List, Angew. Chem., Int. Ed., 2005, 44, 108–110 CrossRef PubMed; S. G. Ouellet, J. B. Tuttle and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 32–33 CrossRef PubMed.
- N. J. A. Martin and B. List, J. Am. Chem. Soc., 2006, 128, 13368–13369 CrossRef CAS PubMed.
- Review: M. Benaglia, S. Guizzetti and L. Pignataro, Coord. Chem. Rev., 2008, 252, 492–512 CrossRef CAS PubMed . For a comprehensive review on Lewis bases see: S. E. Denmark and G. L. Beutner, Angew. Chem., Int. Ed., 2008, 47, 1560–1638 CrossRef PubMed.
- (a) S. Guizzetti and M. Benaglia, Eur. J. Org. Chem., 2010, 5529 CrossRef CAS; (b) S. Jones and C. J. A. Warner, Org. Biomol. Chem., 2012, 10, 2189–2200 RSC.
- F. Iwasaki, O. Onomura, K. Mishima, T. Maki and Y. Matsumura, Tetrahedron Lett., 1999, 40, 7507–7511 CrossRef CAS.
- A. V. Malkov, A. Mariani, K. N. MacDougal and P. Kočovský, Org. Lett., 2004, 6, 2253–2256 CrossRef CAS PubMed.
- A. V. Malkov, M. Figlus, S. Stončius and P. Kočovský, J. Org. Chem., 2009, 74, 5839–5849 CrossRef CAS PubMed.
- A. V. Malkov, M. Figlus and P. Kočovský, J. Org. Chem., 2008, 73, 3985–3995 CrossRef CAS PubMed.
- A. V. Malkov, M. Figlus, G. Cooke, S. T. Caldwell, G. Rabani, M. R. Prestly and P. Kočovský, Org. Biomol. Chem., 2009, 7, 1878–1883 CAS.
- M. Figlus, S. T. Caldwell, D. Walas, G. Yesilbag, G. Cooke, P. Kočovský, A. V. Malkov and A. Sanyal, Org. Biomol. Chem., 2010, 8, 137–141 CAS.
- A. V. Malkov, M. Figlus, S. Stoncius and P. Kočovský, J. Org. Chem., 2007, 72, 1315–1325 CrossRef CAS PubMed.
- Z. Wang, X. Ye, S. Wei, P. Wu, A. Zhang and J. Sun, Org. Lett., 2006, 8, 999–1002 CrossRef CAS PubMed.
- S. Guizzetti, M. Benaglia and G. Celentano, Eur. J. Org. Chem., 2009, 3683–3687 CrossRef CAS.
- Y. Matsumura, K. Ogura, Y. Kouchi, F. Iwasaki and O. Onomura, Org. Lett., 2006, 8, 3789–3792 CrossRef CAS PubMed.
- M. Bonsignore, M. Benaglia, L. Raimondi, M. Orlandi and G. Celentano, Beilstein J. Org. Chem., 2013, 9, 633–640 CrossRef CAS PubMed.
- Z. Zhang, P. Rooshenas, H. Hausmann and P. R. Schreiner, Synthesis, 2009, 9, 1531–1544 Search PubMed.
- O. Onomura, Y. Kouchi, F. Iwasaki and Y. Matsumura, Tetrahedron Lett., 2006, 47, 3751–3754 CrossRef CAS PubMed.
- S. Guizzetti and M. Benaglia, European Patent, Application November 30 2007; PCT/EP/2008/010079, WO2009068284, Nov. 27, 2008 CrossRef CAS PubMed; S. Guizzetti, M. Benaglia, R. Annunziata and F. Cozzi, Tetrahedron, 2009, 65, 6354–6363 CrossRef CAS PubMed.
- H. Zheng, J. Deng, W. Lin and X. Zhang, Tetrahedron Lett., 2007, 48, 7934–7937 CrossRef CAS PubMed.
- S. Guizzetti, M. Benaglia and S. Rossi, Org. Lett., 2009, 11, 2928–2931 CrossRef CAS PubMed.
- F. M. Gautier, S. Jones and S. J. Martin, Org. Biomol. Chem., 2009, 7, 229–231 CAS.
- S. Jones and X. Li, Org. Biomol. Chem., 2011, 9, 7860–7866 Search PubMed.
- A. V. Malkov, A. J. P. S. Liddon, P. Ramírez-López, L. Bendová, D. Haigh and P. Kočovský, Angew. Chem., Int. Ed., 2006, 45, 1432–1435 CrossRef CAS PubMed.
- D. Pei, Z. Wang, S. Wei, Y. Zhang and J. Sun, Org. Lett., 2006, 8, 5913–5915 CrossRef CAS PubMed.
- D. Pei, Y. Zhang, S. Wei, M. Wang and J. Sun, Adv. Synth. Catal., 2008, 350, 619–623 CrossRef CAS.
- Z.-Y. Xue, Y. Jiang, W.-C. Yuan and X.-M. Zhang, Eur. J. Org. Chem., 2010, 616–619 CrossRef CAS.
- X. Wu, Y. Li, C. Wang, L. Zhou, X. Lu and J. Sun, Chem. – Eur. J., 2011, 17, 2846–2848 CrossRef CAS PubMed.
- S. Guizzetti, M. Benaglia, M. Bonsignore and L. Raimondi, Org. Biomol. Chem., 2011, 9, 739–743 CAS.
- M. Bonsignore, M. Benaglia, R. Annunziata and G. Celentano, Synlett, 2011, 8, 1085–1088 Search PubMed.
- H.-J. Zheng, W.-B. Chen, Z.-J. Wu, J.-D. Deng, W.-Q. Lin, W.-C. Yuan and X.-M. Zhang, Chem. – Eur. J., 2008, 14, 9864–9867 CrossRef CAS PubMed.
- A. V. Malkov, S. Stončius, K. Vranková, M. Arndt and P. Kočovský, Chem. – Eur. J., 2008, 14, 8082–8085 CrossRef CAS PubMed.
- Y. Jiang, X. Chen, Y. Zheng, Z. Xue, C. Shu, W. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2011, 50, 7304–7307 CrossRef CAS PubMed.
- Y. Jiang, X. Chen, X. Hu, C. Shu, Y. Zhang, Y. Zheng, C. Lian, W. Yuan and X. Zhang, Adv. Synth. Catal., 2013, 355, 1931–1936 CrossRef CAS.
- Y.-C. Xiao, C. Wang, Y. Yao, J. Sun and Y.-C. Chen, Angew. Chem., Int. Ed., 2011, 50, 10661–10664 CrossRef CAS PubMed.
- X. Liu, C. Wang, Y. Yan, Y. Wang and J. Sun, J. Org. Chem., 2013, 78, 6276–6280 CrossRef CAS PubMed.
- X. Chen, Y. Zheng, C. Shu, W. Yuan, B. Liu and X. Zhang, J. Org. Chem., 2011, 76, 9109–9115 CrossRef CAS PubMed.
- For a recent review on the use of chiral phosphine oxides as organocatalysts, see M. Benaglia and S. Rossi, Org. Biomol. Chem., 2010, 8, 3824–3830 CAS.
- M. Sugiura, N. Sato, S. Kotani and M. Nakajima, Chem. Commun., 2008, 4309–4311 RSC.
- A. Genoni, M. Benaglia, E. Massolo and S. Rossi, Chem. Commun., 2013, 49, 8365–8367 RSC.
| This journal is © The Royal Society of Chemistry 2014 |