Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of mineral carbonation technologies to sequester CO2
A.
Sanna
*a,
M.
Uibu
b,
G.
Caramanna
a,
R.
Kuusik
b and
M. M.
Maroto-Valer
ac
aCentre for Innovation in Carbon Capture and Storage (CICCS), School of Engineering and Physical Sciences, Heriot-Watt University, 3.04 Nasmyth Building, Edinburgh EH14 4AS, UK. E-mail: A.Sanna@hw.ac.uk; Tel: +44 (0)131 451 3299
bLaboratory of Inorganic Materials, Tallinn University of Technology, 5 Ehitajate S., Tallinn 19086, Estonia
cInstitute of Petroleum Engineering, Heriot-Watt University, Edinburgh EH14 4AS, UK
First published on 1st July 2014
Abstract
Carbon dioxide (CO2) capture and sequestration includes a portfolio of technologies that can potentially sequester billions of tonnes of CO2 per year. Mineral carbonation (MC) is emerging as a potential CCS technology solution to sequester CO2 from smaller/medium emitters, where geological sequestration is not a viable option. In MC processes, CO2 is chemically reacted with calcium- and/or magnesium-containing materials to form stable carbonates. This work investigates the current advancement in the proposed MC technologies and the role they can play in decreasing the overall cost of this CO2 sequestration route. In situ mineral carbonation is a very promising option in terms of resources available and enhanced security, but the technology is still in its infancy and transport and storage costs are still higher than geological storage in sedimentary basins ($17 instead of $8 per tCO2). Ex situ mineral carbonation has been demonstrated on pilot and demonstration scales. However, its application is currently limited by its high costs, which range from $50 to $300 per tCO2 sequestered. Energy use, the reaction rate and material handling are the key factors hindering the success of this technology. The value of the products seems central to render MC economically viable in the same way as conventional CCS seems profitable only when combined with EOR. Large scale projects such as the Skyonic process can help in reducing the knowledge gaps on MC fundamentals and provide accurate costing and data on processes integration and comparison. The literature to date indicates that in the coming decades MC can play an important role in decarbonising the power and industrial sector.
1. Introduction
1.1 Carbon capture and storage
Our knowledge of the global carbon cycle is sufficiently extensive to conclude that natural processes cannot absorb all the anthropogenically produced carbon dioxide (CO2) in the coming centuries, so adaptation technologies are urgently required.1 Extensive evidence on the anthropogenic cause of climate change can be obtained from the comprehensive IPCC report published in 2007.2 There is a general agreement that to meet the ambitious target to stabilize atmospheric CO2 concentration at 500 ppm by 2050, a large portfolio of technologies need to be considered, where Carbon Dioxide Capture and Storage (CCS) represents a leading technology, particularly in the transition from a fossil fuel based economy to a renewable based economy.3CCS refers to a number of technologies which capture CO2 at some stage from processes such as combustion for power generation, cement manufacture, iron and steel making and natural gas treatment. Then, the captured CO2 is pressurised (≥100 bar) prior to being transported (by pipeline, ship, rail or road) to a storage site, where it is injected into stable geological sites (saline aquifers, depleted oil and gas fields, deep coal seams), trapping it for thousands of years.3,4
CO2 capture at power plants and other large point sources represents the most likely tool for the reduction of current CO2 emissions from fossil fuel use. CCS is not a new concept and a large number of different CO2 capture technologies are being developed, ranging from currently commercial technologies such as amine-scrubbing with geological storage through 2nd or 3rd generation technologies, such as chemical or carbonate looping. Recent improvements in amine scrubbing and processes have reduced the energy requirement from 450 kW h per tCO2 (2001) to about 200–300 kW h per t in 2012, which will result in a reduction of the power output by 20 to 30% in a typical coal-fuelled power plant (1000 kW h per tCO2 output).4,5
Even if most of the individual components of the CCS chain (e.g. capture) have been demonstrated, their integration into a single process is challenging and still to be demonstrated.4,6
Moreover, the delay of some large demonstration projects (e.g. Mongstad in Norway) due to higher complexity than expected and general public acceptance issues related to potential leakages and surface transport of supercritical CO2 are delaying the deployment of geological storage.4 Overall CO2 geological storage poses a great deal of uncertainty in terms of quantification of storage potential, monitoring injected CO2 and engineering challenges to ensure that the injected CO2 remains in the subsurface for hundreds or thousands of years.4
Under this scenario, Mineral Carbonation (MC) represents an alternative CCS option, which may be particularly suitable for small sources.
1.2 CO2 sequestration by mineral carbonation
Mineral carbonation (MC) is an accelerated form of weathering of naturally occurring silicate rocks and has been proposed as an alternative approach for CO2 sequestration since the 1990s.7 Some MC technologies have recently approached the commercial stage. MC is defined as the reaction of metal oxide bearing materials with CO2 to form insoluble carbonates:| Metal oxide + CO2 → Metal carbonate + Heat | (1) |
This reaction can take place either below (in situ) or above (ex situ) ground. In situ mineral carbonation involves the injection of CO2 into underground reservoirs to promote the reaction between CO2 and alkaline-minerals present in the geological formation to form carbonates.8Ex situ mineral carbonation relates to above-ground processes, which requires rock mining and material comminution as MC pre-requisites.9
MC can take advantage of different starting materials, which include Mg-silicate minerals and Ca− or Fe−-rich silicates. The reactions occurring in MC processes are listed below.9
| Mg2SiO4 + 2CO2 + 2H2O → 2MgCO3 + H4SiO4 | (2) |
| Mg3Si2O5(OH)4 + 3CO2 + 2H2O → 3MgCO3 + 2H4SiO4 | (3) |
| Fe2SiO4 + 2CO2 + 2H2O → 2FeCO3 + H4SiO4 | (4) |
| CaSiO3 + CO2 + 2H2O → CaCO3 + H4SiO4 | (5) |
CCS by geological storage represents the best CCS strategy for large emitters, while MC can play an important role by targeting small and medium emitters (<2.5 Mt CO2), which account for about 10–15% of the total CO2 emissions.10 Mineral carbonation is a permanent and safe way for storing CO2, which does not present potential concerns over long term monitoring and liability issues, such as geological storage. The inherent stability of mineral carbonation is confirmed by the distribution of carbon in the lithosphere of the Earth (Fig. 1), where approximately half of the total carbon is in the form of limestone (CaCO3) and other types of carbonates.
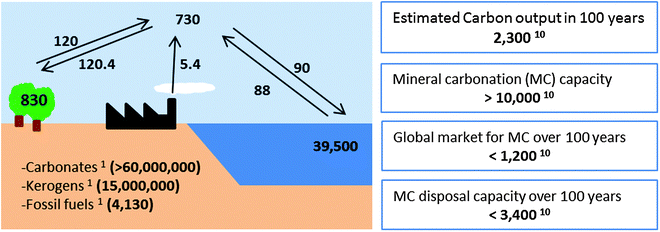 | ||
| Fig. 1 Global carbon reservoirs (GtC) and net fluxes (GtC per year) (modified from ref. 1, 10 and 17). | ||
Mineral carbonation resources have a large CO2 sequestration potential (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Gt C) due to the large abundance of silicates around the world, as shown in Fig. 2. MC could also be sourced by CO2 extracted directly from the air or the ocean. A method to extract CO2 from ambient air has been proposed adopting components and fabrication methods derived from cooling towers and using strong NaOH solution. The cost of this process (without taking into consideration the regeneration of NaOH) was $60 per tCO2.11 However, other works have quantified the costs of this option to be as high as $600–1000 per tCO2.12 Moreover, Goldberg et al. estimated that ∼75 Mt CO2 per year could be collected using air capture powered by wind energy and sequestered below seafloor in basalts formation at Kerguelen (see also Section 2.2), where regional reservoirs could hold over 1500 Gt CO2, sequestering a large fraction of 21st century emissions.13 In addition, CO2 has been extracted from seawater. A total of 59% of dissolved inorganic CO2 in seawater has been extracted using bipolar membrane electrodialysis with an energy input of ∼1527 kW h per tCO2.14 While the extraction of CO2 from air and seawater has been demonstrated, many challenges remain, including slow extraction rates, poor CO2 selectivity and high costs.
000 Gt C) due to the large abundance of silicates around the world, as shown in Fig. 2. MC could also be sourced by CO2 extracted directly from the air or the ocean. A method to extract CO2 from ambient air has been proposed adopting components and fabrication methods derived from cooling towers and using strong NaOH solution. The cost of this process (without taking into consideration the regeneration of NaOH) was $60 per tCO2.11 However, other works have quantified the costs of this option to be as high as $600–1000 per tCO2.12 Moreover, Goldberg et al. estimated that ∼75 Mt CO2 per year could be collected using air capture powered by wind energy and sequestered below seafloor in basalts formation at Kerguelen (see also Section 2.2), where regional reservoirs could hold over 1500 Gt CO2, sequestering a large fraction of 21st century emissions.13 In addition, CO2 has been extracted from seawater. A total of 59% of dissolved inorganic CO2 in seawater has been extracted using bipolar membrane electrodialysis with an energy input of ∼1527 kW h per tCO2.14 While the extraction of CO2 from air and seawater has been demonstrated, many challenges remain, including slow extraction rates, poor CO2 selectivity and high costs.
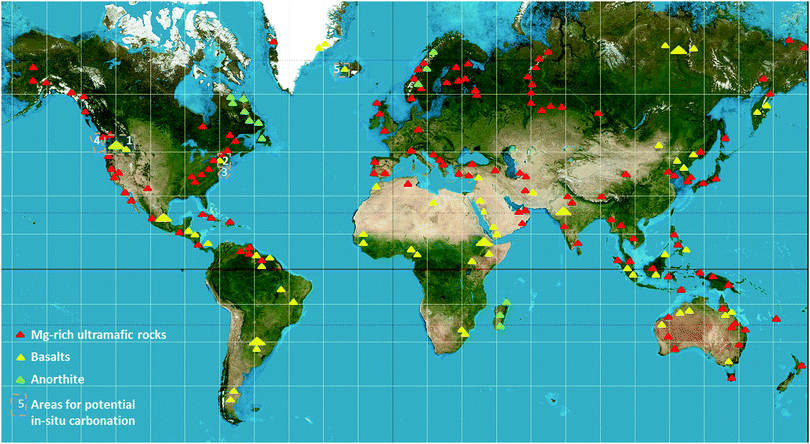 | ||
| Fig. 2 Mineral feedstock available for MC (modified from ref. 27). | ||
One of the issues associated with MC is the disposal/use of the products. Despite the fact that only a fraction of the MC products would be absorbed by current market for carbonates and silica as shown in Fig. 1, the disposal capacity considering mine and land reclamation projects around the world is considered feasible since large reclamation projects can involve 100–200 Gt of materials.10 Moreover, MC products (carbonates) may be used for ocean liming.15,16 It has been calculated that MC-ocean liming would require approximately 4.9 and 2.2 GJ of thermal and electrical energy ton−1 of CO2 sequestered.16 A lab-scale seawater/mineral carbonate gas scrubber was found to remove up to 97% of CO2 in a simulated flue gas stream at ambient temperature and pressure, with a large fraction of this carbon ultimately converted to dissolved calcium bicarbonate.15 However, manipulation of ocean chemistry may create an additional environmental impact on marine life, such as localized elevated pH or co-dissolution of trace metals.16
Mineral resource availability, scalability, applicability to regions without geologic storage capacity, inherent stability of the reaction products and the potential revenue from MC products support the on-going development of this technology. Also, mineral carbonation can operate on flue gases directly, without CO2 pre-separation, which typically stands for 70–75% of the cost of the CCS chain.18,19 A CO2 carbonation efficiency of ∼20% has been reported when SOx and NOx were present in the flue gas (15% CO2) using wollastonite at 40 bar and 150 °C.20 However, very few works have been published on MC in the presence of impurities to fully assess this option.
MC effectiveness for CO2 mitigation purposes has been limited due to the slow kinetics of the CO2–silicate reactions, energy intensive pre-treatments, logistic issues (e.g. locations of mineral resources and CO2 emitters, development of transport and storage facilities for waste carbonates on a large scale) and scalability issues (e.g. a coal-fired power station fitted with 100% mineral sequestration would require more tonnes of mineral feedstock than of coal).10,17
Several reviews on mineral carbonation technologies focused on a number of processes under development have recently been produced.8,17,21,22 Olajire21 focused his review on in situ technologies and the environmental impact of the reaction products with regard to their possible beneficial utilization. Zevenhoven et al.22 reviewed the state-of-the-art of ex situ mineralisation, illustrating the future prospects of CO2 mineralization and including a portfolio of CCS technologies under development worldwide. Salek and co-workers17 reviewed the potential sequestration of CO2 using environmental biotechnological processes, such as nitrification, anaerobic digestion (AD) and bio-electrochemical systems. The global sequestration potential of biodegradable solid waste and wastewater by AD employing silicates was estimated in 0.2–0.4 wt% of the total anthropogenic CO2 emissions. Bioelectrochemical systems such as microbial fuel cells can potentially sequester more CO2 than what is produced during the organic carbon oxidation (200 wt%). However, these systems suffer from extremely low current densities and therefore further development is required.17 Although these bio-based processes are promising, their development is still in its infancy and is not considered in this review. Also, a number of reviews on mineral carbonation of alkaline wastes have been recently published,23,24 providing an overview of the types of industrial wastes that can be used for mineral carbon sequestration and the process routes available. They concluded that industrial waste residues represent an alternative source of mineral alkalinity and are readily and cheaply available close to CO2 sources, and moreover, the carbonation of waste residues often improves their environmental stability.
The objective of this paper is to provide an up-to-date review and discussion on mineral carbonation technologies, including potential deployment for in situ and ex situ carbonation in the presence of silicate rocks and alkaline wastes and product applications. Technologies using mineral rocks as feedstock are treated in more depth, since previous studies clearly indicate that alkaline wastes represent a niche application and would only be able to marginally reduce the global CO2 emissions (<1%).25,26
In situ mineral carbonation is analysed in the first section of the work, followed by the evaluation of the silicate rocks for ex situ mineral carbonation technologies based on minerals and inorganic waste feedstocks. Finally, a section on the potential products from mineralisation and final remarks is presented.
2. In situ mineral carbonation
Carbonation is a natural process where CO2 reacts with different minerals forming solid precipitates leading to the weathering of the rocks. The reactions are spontaneous and exothermic and can be exemplified as (6) and (7) where calcium and magnesium oxides are considered to react with CO2.| CaO + CO2 = CaCO3 + 179 kJ mol−1 | (6) |
| MgO + CO2 = MgCO3 + 118 kJ mol−1 | (7) |
The most reactive compounds for CO2 mineralization are oxides of divalent metals, Ca and Mg, and their availability in nature is mainly in the form of silicates, such as olivine ((Mg,Fe)2SiO4) orthopyroxene (Mg2Si2O6–Fe2Si2O6), clinopyroxene (CaMgSi2O6–CaFeSi2O6) and serpentine ((Mg, Fe)3Si2O5(OH)4), the latter originated by the hydratation of olivine. When CO2 dissolves in water, it reacts with these silicates forming corresponding carbonates, where CO2 is fixed in a mineral form.28,29
Mantle peridotite and basalts deposits, enriched in Mg, Fe and Ca silicates, are the main targets for in situ CO2 mineralization projects, as discussed below.21
2.1 Peridotites
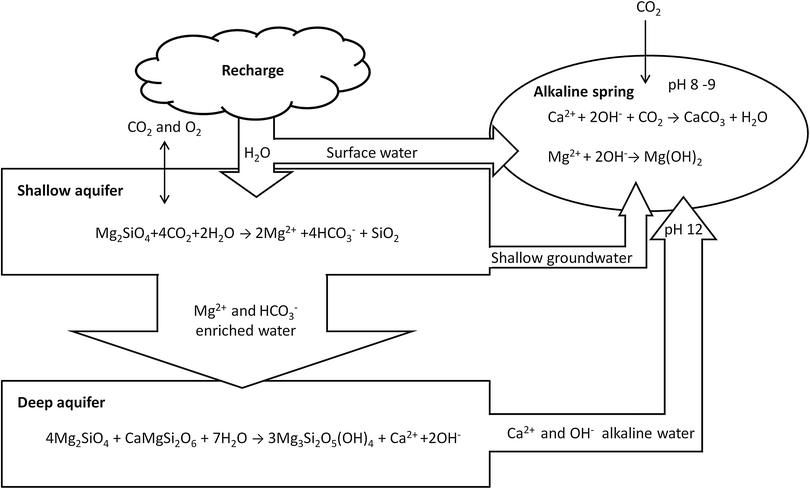
Peridotite is a component of ophiolites which are complex geological sequences representing the emplacement on land of sections of oceanic crust.30 The world's largest ophiolitic outcrop is the Samail Ophiolite in the Sultanate of Oman extending for about 350 km with a width around 40 km and average thickness of 5 km; about 30 vol% is composed of mantle peridotite. The mineralogical composition of this peridotite is 74% olivine (partially serpentinized), 24% orthopyroxene, 2% spinel (MgAl2O4) and traces of clinopyroxene.31,32 The Samail Ophiolite is characterized by the presence of an internal network of fractures hosting aquifers of variable volumes and chemical compositions, where several mineralized springs emit alkaline waters enriched in carbonates; the origin of those waters is linked to the natural carbonation process acting within the peridotite (Fig. 3).Surface water flows through fractures originating a shallow aquifer open to the atmospheric CO2 and O2 fluxes; the water reacts with the peridotite and the pre-existing carbonate rocks in an open system becoming enriched in Mg2+ and HCO3−. This water may infiltrate in the deeper regional aquifer which is isolated from the atmospheric fluxes. The chemical reactions with the peridotite will trigger the formation of serpentine, brucite, magnesite and dolomite; Ca2+ and OH− will accumulate in the water leading to a strong pH increase up to a value of 12. When these waters emerge at the surface in the alkaline springs the sudden intake of CO2 from the atmosphere will precipitate Ca-carbonates; the mixing with the shallow aquifer will further precipitate Ca-carbonates and brucite. The formation of carbonates, mostly in the form of terraced travertine around the springs, consumes some OH− decreasing the pH to values of 8–9. The total volume of carbonate in the Samail Ophiolite is 5.5 × 107 m3 with an average age of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years indicating that about 4 × 107 kg CO2 per year are consumed by the precipitation of carbonates. This natural process requires long times for the reactions to develop, in the order of magnitude of 50 years for the shallow-water aquifers and up to 5600 years for the deep reservoirs. Artificial enhancement of the carbonation can be achieved by injecting fluids with a higher concentration of CO2 and increasing the temperature. For example, when injecting CO2 at 90 °C with 100 bar pCO2, about 0.63 kg of CO2 can be permanently stored as carbonates for each kg of peridotite. A typical in situ mineralization project in the Samail Ophiolite could include the drilling of the peridotite, hydrofracturing of the hosting volume, injection of heated fluids to increase the temperature at 185 °C, which is the optimum temperature for olivine carbonation rates, followed by injection of pure CO2 at 25 °C. The exothermal reaction (producing 760 kJ kg−1) and the geothermal gradient (up to 20 °C km−1) will both contribute to the reduction in the energy needed for heating the fluids. The resulting enhancement of the carbonation rate following this process is considered to be one million times faster than the natural process pace.32,33
000 years indicating that about 4 × 107 kg CO2 per year are consumed by the precipitation of carbonates. This natural process requires long times for the reactions to develop, in the order of magnitude of 50 years for the shallow-water aquifers and up to 5600 years for the deep reservoirs. Artificial enhancement of the carbonation can be achieved by injecting fluids with a higher concentration of CO2 and increasing the temperature. For example, when injecting CO2 at 90 °C with 100 bar pCO2, about 0.63 kg of CO2 can be permanently stored as carbonates for each kg of peridotite. A typical in situ mineralization project in the Samail Ophiolite could include the drilling of the peridotite, hydrofracturing of the hosting volume, injection of heated fluids to increase the temperature at 185 °C, which is the optimum temperature for olivine carbonation rates, followed by injection of pure CO2 at 25 °C. The exothermal reaction (producing 760 kJ kg−1) and the geothermal gradient (up to 20 °C km−1) will both contribute to the reduction in the energy needed for heating the fluids. The resulting enhancement of the carbonation rate following this process is considered to be one million times faster than the natural process pace.32,33
2.2 Basalts
The largest presence of basalts is on the oceanic crust.34 Large outcrops of basalts are also present on the continental crust.35 Basalts can have a good degree of secondary permeability due to the formation of altered and brecciate horizons or networks of fractures during or after their deposition. The resulting pore space may be filled by circulating water originating from aquifers within the hosting rocks at different depths and mineral concentrations. These aquifers are often enriched in ions including Ca2+ and Mg2+,36 which can react with the injected CO2 precipitating carbonates and releasing H+ as in reaction (8):| (Ca2+, Mg2+) + CO2 + H2O = (Ca, Mg)CO3 + 2H+ | (8) |
The reaction rate is controlled by the concentration of H+ and will not proceed further until these ions are neutralized by the reaction with the hosting rock. Considering olivine and Ca-plagioclase basalts the neutralization process follows reactions (9) and (10).
| Mg2SiO4 + 4H+ = 2Mg2+ + SiO2 | (9) |
| CaAl2Si2O8 + 8H+ = Ca2+ + 2Al3+ + 2SiO2(aq) + 4H2O | (10) |
The availability of reactive Mg, Al and Ca silicates is therefore the controlling factor for the development of in situ CO2 mineralization.37
Following the injection of CO2 (both as supercritical fluids or as aqueous solution) the dissolution of some minerals and the precipitation of others, mostly carbonates, may change the porosity of the reservoir; carbonate deposition during the first stages of the injection may have adverse effects on the storage potential due to the reduction in available pore space which is progressively filled by minerals, and thus, clogging the surrounding of the injection well. Mineral deposition in a more advanced phase of the injection and during the post-injection phase instead is considered an advantage enhancing the trapping potential of the hosting structure.
Injecting CO2 within the basalts of the ocean seafloor would benefit from a further series of trapping mechanisms in addition to the geochemical transformation of CO2 in carbonates. The deep water environment, below 2700 m, and the cold temperature, below 2 °C, will make the injected CO2 denser than the surrounding seawater, allowing it to sink with a gravitation-trapping mechanism; the same environmental parameters are also favourable to the formation of CO2 hydrates, where the CO2 molecule is “encaged” within a lattice of ice strongly reducing its solubility in water in the case of seepage. Lastly, the thick sedimentary cover of the seafloor will form a low-permeability layer further reducing the possibility of leakage.38
Currently a few injection-test projects and feasibility studies are addressing the potential of basalts, both onshore and offshore, for CO2 storage (Table 1; Fig. 2).
| Location | Reservoir | Caprock | Storage potential | Ref. |
|---|---|---|---|---|
| New York state (USA) | Palisades sill. Dolerite with Ca-plagioclase and pyroxenes. Target zone porosity 5% | Lacustrine deposits of the Newark Basin; mudstone, arkoses, carbonate nodules shale and clastic sequences | An injection test was aimed to identify the buffering potential of dolerite | 37–39 |
| Washington, Oregon and Idaho states (USA) | Columbia River Basalt Group. Clinopyroxene, plagioclase and glass. Over 300 overlapping flows. Total volume in excess of 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km3. Target zone porosity 15 to 25% 000 km3. Target zone porosity 15 to 25% |
Basalts layers with very low permeability | 10 to 50 Gt CO2 | 40 and 41 |
| Offshore USA East Coast | Sandy Hook Basin basalt porosity 15% | Sedimentary cover, mudstone, silt, clay | 900 Mt of CO2 | 52 |
| Offshore USA West Coast | Juan de Fuca Plate basalts. Pillow lava and massive flows. Average porosity 10% | Fine-grained turbiditic sequences and clay deposits | 920 Gt of CO2 | 48 |
| Iceland | Ultrabasic to basic (45–49% SiO2) basalt flows and hyaloclastite of olivine–tholeiite composition | Low-porosity basalts | 12 Mt CO2 | 43–45 |
The Columbia River Basalt Group is a large continental flood basalt deposit covering an area of more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 in Washington, Oregon and Idaho states in the North-West US. Originated by a series of fissural eruptions between 17.5 and 6.0 million years ago, it is composed of over 300 different flows with a volume in excess of 224
000 km2 in Washington, Oregon and Idaho states in the North-West US. Originated by a series of fissural eruptions between 17.5 and 6.0 million years ago, it is composed of over 300 different flows with a volume in excess of 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km3. The flows can be divided into top and bottom areas, showing a vesicular and scoriaceous crust often brecciated, and an inner massive core. The mineral composition is clinopyroxene, plagioclase and glass, the latter ranging from a few percent to over 50%. The basalts host a regional deep aquifer of brackish water with high levels of sulphide and fluoride exceeding the limits for drinking water; this aquifer is therefore the target for CO2 injection at a depth between 663 m and 887 m through a well drilled in the Walla Walla County in south-eastern Washington State. The hosting rocks are part of a top layer area with permeability in the range of 75 to 150 md with 15 to 25% of porosity calculated from uncalibrated sonic logs. The water chemistry of the injection area is of sodium-bicarbonate type with pH 9.68, fluoride 4.98 mg L−1 and Fe 962 mg L−1. In July 2013 a test injection of 1000 tCO2 was started for one month. A model simulation forecasts that after one year 18% of CO2 will be dissolved in groundwater within a radius of 55 m from the bore-hole. The storage capacity of the total basalt deposits for CO2in situ mineralization is estimated from 10 to 50 Gt CO2.40,41
000 km3. The flows can be divided into top and bottom areas, showing a vesicular and scoriaceous crust often brecciated, and an inner massive core. The mineral composition is clinopyroxene, plagioclase and glass, the latter ranging from a few percent to over 50%. The basalts host a regional deep aquifer of brackish water with high levels of sulphide and fluoride exceeding the limits for drinking water; this aquifer is therefore the target for CO2 injection at a depth between 663 m and 887 m through a well drilled in the Walla Walla County in south-eastern Washington State. The hosting rocks are part of a top layer area with permeability in the range of 75 to 150 md with 15 to 25% of porosity calculated from uncalibrated sonic logs. The water chemistry of the injection area is of sodium-bicarbonate type with pH 9.68, fluoride 4.98 mg L−1 and Fe 962 mg L−1. In July 2013 a test injection of 1000 tCO2 was started for one month. A model simulation forecasts that after one year 18% of CO2 will be dissolved in groundwater within a radius of 55 m from the bore-hole. The storage capacity of the total basalt deposits for CO2in situ mineralization is estimated from 10 to 50 Gt CO2.40,41
Iceland has large volumes of basalt flows and hyaloclastites (volcanic breccias originated by the contact between the emitted lava and water or ice) associated with strong volcanic and geothermal activity with release of large volumes of CO2. There is evidence of natural carbonation processes within the aquifers hosted in the basaltic deposits. The Hekla volcano in Iceland originated from linear eruptions during the last 900 years and represents the deposition of basaltic andesitic tephra largely composed of volcanic glass. The water feeding a series of springs has high alkalinity and pH ranging from 7.7 to 9.3; the variation in DIC (Dissolved Inorganic Carbon) within the spring water and its correlation with changes in pH is considered a proof that CO2 is fixed as carbonates. Another relevant aspect is that the heavy metals ions which can be mobilized from the rocks by the acidification induced by the CO2 fluxes are incorporated in carbonates and oxy-hydroxides once the pH increases thus reducing the risk of environmental pollution.42
In Iceland the CarbFix project aims to assess the feasibility of in situ carbonation in basalt using as the CO2 source the gas emitted from the geothermal power plant of Hellisheidi. The gas is associated with the geothermal steam and is composed of 83% CO2, 16% H2S and the remaining 1% as H2, N2, and CH4; the gas stream is condensed separating CO2 and H2S, which is mostly re-injected in the deep geothermal reservoir. The resulting final gas is composed of 98% of CO2 plus 2% of H2S and it is to be injected in the deep basaltic aquifer. The hosting rocks are ultrabasic to basic (45–49% SiO2) basalt flows and hyaloclastites (a breccia rich in volcanic glass) of olivine–tholeiite composition. Crystalline lava flows were in place after the last glacial age and the hyaloclastites originated during the last glaciations under the ice cover in a time span between 116![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1500 years ago. The resulting structure is a sequence of more permeable lava flows hosting a shallow aquifer underlined by low-permeability layers of hyaloclastites separating and isolating a deeper aquifer. The shallow aquifer (above 400 m) has a temperature of 8–12 °C, pH 7.7–8.4, CO2 in balance with the atmospheric values, it is enriched in O2 and it is undersaturated in calcite. The groundwater of the deep aquifer has temperature between 18 and 33 °C, a pH of 8.4–9.4, CO2 concentration below the atmospheric balance and it is depleted in O2 and saturated in calcite. This deep aquifer between 400 and 800 m is the target area for the storage in a volume of about 1 km3; CO2 will be dissolved in water at PCO2 of 25 bar, pH 3.7, DIC 1 mol kg−1, requiring 22 t of water for each ton of CO2. A total of 2200 tCO2 per year will be injected. A monitoring program including geochemical analysis, tracers and isotopic concentration measures will assess the diffusion of the injected CO2 within the aquifer and the changes in chemistry associated with the mineralisation reaction. Models show that calcite precipitation will reduce the porosity of the reservoir of about 1%. The total storage potential is of about 12 Mt CO2, or 200 years considering the annual emission from the geothermal plant in 60
000 and 1500 years ago. The resulting structure is a sequence of more permeable lava flows hosting a shallow aquifer underlined by low-permeability layers of hyaloclastites separating and isolating a deeper aquifer. The shallow aquifer (above 400 m) has a temperature of 8–12 °C, pH 7.7–8.4, CO2 in balance with the atmospheric values, it is enriched in O2 and it is undersaturated in calcite. The groundwater of the deep aquifer has temperature between 18 and 33 °C, a pH of 8.4–9.4, CO2 concentration below the atmospheric balance and it is depleted in O2 and saturated in calcite. This deep aquifer between 400 and 800 m is the target area for the storage in a volume of about 1 km3; CO2 will be dissolved in water at PCO2 of 25 bar, pH 3.7, DIC 1 mol kg−1, requiring 22 t of water for each ton of CO2. A total of 2200 tCO2 per year will be injected. A monitoring program including geochemical analysis, tracers and isotopic concentration measures will assess the diffusion of the injected CO2 within the aquifer and the changes in chemistry associated with the mineralisation reaction. Models show that calcite precipitation will reduce the porosity of the reservoir of about 1%. The total storage potential is of about 12 Mt CO2, or 200 years considering the annual emission from the geothermal plant in 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2.43–45
000 tCO2.43–45
In 2011–2012 a test injection of 175 tons of pure CO2 and water was performed and in 2012–2013, 130 tons of CO2 mixed with H2S from the power plant were injected in a low-temperature (20–50 °C) aquifer at 400–800 m of depth and in deeper reservoir (below 800 m) at higher temperature (>250 °C).46
Another large accumulation of basalts is present along and offshore the eastern coast of North America where it was originated by a series of floods during subsequent eruptive events resulting in strata separated by alteration horizons with vesicular and brecciated layers.49 A series of deep sediments-filled basins have been identified offshore and, both from geophysical data and cores, basalt layers have been identified below the sedimentary cover.50,51 These offshore basalts could represent a large storage volume for the CO2 produced by point sources in the highly industrialized eastern shore belt of the USA.
As an example, the basalt present as bedrock of the Sandy Hook basin offshore New Jersey has an average porosity of 15% with an available pore volume for CO2 storage of about 1 km3 able to host 900 Mt of CO2, trapping of which will be enhanced by long-term mineral carbonation reactions.52
A general estimation of the overall potential storage capacity for ocean basalts is 8238 Gt of CO2; this value is calculated considering CO2 storage in brines hosted in 20 m thick horizons of relatively porous (10% average porosity) pillow lavas and flows at depth below 2700 m and overlaid by at least 200 m of ocean sediments. The water depth and sedimentary cover enhance the gravitational, hydrate formation and low-permeability sealing trapping mechanisms.53
3. Ex situ carbonation
Most processes under consideration for mineral carbonation focus on metal oxide (such as calcium and magnesium) bearing materials, whose corresponding carbonates are not soluble. Moreover, since waste materials rich in calcium oxide are conveniently located close to the CO2 emission source, they have also been targeted as MC feedstock. The following sections will review the processes developed for both rocks and waste resources.3.1 Processes developed for minerals
Since oxides and hydroxides of Ca and Mg are not abundant, silicate rocks containing the desired Mg and Ca have been targeted for mineral carbonation.9Table 2 summarises the main minerals available and their performance in terms of mass ratio of ore necessary to carbonate the unit mass of CO2 (Rreal) and reaction efficiency (ECO2). Serpentine, olivine, and to less extent wollastonite because of its lower abundance, are preferred based on performance and availability.54| Rock | Mineral | Mg | Ca | Fe2+ | R real | E CO2 (%) |
|---|---|---|---|---|---|---|
| Serpentine | Antigorite | 24.6 | <0.1 | 2.4 | 2.1 | 92 |
| Serpentine | Lizardite | 20.7 | 0.3 | 1.5 | 2.5 | 40 |
| Olivine | Fayalite | 0.3 | 0.6 | 44.3 | 2.8 | 66 |
| Olivine | Forsterite | 27.9 | 0.1 | 6.1 | 1.8 | 81 |
| Feldspar | Anorthite | 4.8 | 10.3 | 3.1 | 4.4 | 9 |
| Pyroxene | Augite | 6.9 | 15.6 | 9.6 | 2.7 | 33 |
| Basalt | 4.3 | 6.7 | 6.7 | 4.9 | 15 | |
| Oxide | Magnetite | 0.3 | 0.6 | 21.9 | 5.5 | 8 |
| Ultramafic | Talc | 15.7 | 2.2 | 9.2 | 2.8 | 15 |
| Ultramafic | Wollastonite | 0.3 | 31.6 | 0.5 | 2.8 | 82 |
The sequestration of CO2 in carbonates can be achieved through various process routes, which are described in this section: (1) direct carbonation (DC) is the simplest approach, where a Ca/Mg rich solid is carbonated in a single process step. DC can be further divided into gas–solid carbonation and direct aqueous mineral carbonation. The direct aqueous mineral carbonation-route with the aid of pre-treatments (DCP) is considered as the state of the art and is typically selected to compare other technologies. (2) Indirect carbonation (IC) consists of first extracting from the feedstock the reactive Mg/Ca oxide or hydroxide in one step and then, in a subsequent step, reacting the leached cations with CO2 to form the desired carbonate.8
For each process carbonation route, key parameters of a process are presented in Table 3 to give quick insight into the main features. The parameters considered are the feedstock material used; the mineral to CO2 ratio (Rreal); the mineral pre-treatment type (M-mechanical, C-chemical, T-thermal); operational temperature and pressure; chemical additives used and finally, the mineral cation reacted with CO2 (degree of conversion) (ECO2%). The different direct and indirect processes are discussed in detail in Section 3.1.2.
| Resource | R real | Process conditions | E CO2 (%) | Remarks |
|---|---|---|---|---|
| M = mechanical pre-treatment; T = thermal pre-treatment; n.a. = not available; DC = direct carbonation; DCP = direct carbonation with pre-treatments; IC = indirect carbonation. | ||||
| Serpentine | 3.39 | DCP: M, T (75 μm, 630 °C), 115 bar, 155 °C, 0.64 M NaHCO3, 1 M NaCl, 6 hours54,82 | 62 | Cost of 210 $ per tCO2 stored (lizardite) |
| Serpentine | 5.25 | DCP: M, T (75 μm:630 °C), 115 bar, 155 °C, 0.64 M NaHCO3, 1 M NaCl, 1 hour95,96 | 80 | Cost of about A$22 per tCO2 |
| Serpentine | n.a. | DCP: M, T serp. (650 °C), 30–45 bar, 140 °C, 150–200 μm, NaHCO318,19,91 | n.a. | Flue gas is used |
| Serpentine | 3.33 | IC: M (37 μm), 120 bar, 300 °C, NH3, 1 hour123 | 70 | |
| Serpentine | 2.33 | DCP: M (100 μm), 50 bar, 550 °C, weak acid/NH3, 1 hour125 | 90 | |
| Serpentine | 3 | IC: M (75 μm), 1 bar, 140 °C (300 °C regeneration), 1.4 M NH4HSO4, NH3, 2–3 hours68,72 | 70 | |
| Serpentine | 5.38 | IC: M (100 μm), 40 bar, 65 °C, H2SO4, NaOH, 3 hours109 | 55 | |
| Serpentine | 5.83 | IC: M, T (250–425 μm), 20 bar, 500–550 °C, (NH4)2SO4, NH3, 0.5 hour120–122 | 60 | Only 50–60% Mg is recovered |
| Serpentine | 8.97 | IC: M, T (50 μm:150), 1 bar, 325 °C, HCl/NaOH, 26 hours108 | 26 | |
| Serpentine | 3.5 | IC: M (<75 μm), 20 bar, 120 °C, 0.1 M citrate, EDTA, 2 hours110,111 | 60 | |
| Serpentine | 3.23 | IC: M (75 μm), 1 bar, 70 °C, 1 vol% orthophosphoric acid, 0.9 wt% oxalic acid, 0.1% EDTA, 1 hour113,114 | 65 | Internal agitation with grinding media is used in the dissolution stage costs between 50 and 100 $ per tCO2 stored |
| Olivine | 2.22 | DCP: M (38 μm), 150 bar, 185 °C, 0.64 M NaHCO3, 1 M NaCl, 3 hours54,82 | 68 | |
| Olivine | 1.82 | DCP: M (<200 μm), 30 bar, 140 °C, NaHCO3, 1–2 hours18,19 | 80–90 | |
| Olivine | 2.8 | DC: M, C (10 μm), 1.2 bar, 500 °C, bases, 0.5 hour76,77 | 25 | A Spray Dry Absorber (SDA) is used |
| Olivine | n.a. | DC: M (2.5–60 μm), 500 °C, 0.5 hour, moisture75 | 12 | Flue gas is used dissolution of olivine in carbonated aqueous solutions using a flow-through column reactor |
| Olivine | n.a. | IC: M (75 μm), 250 °C, 150 bar, 2 hours107 | 11 | |
| Olivine | n.a. | DC: M (37 μm), 97 bar, 80 °C, 20 hour78,79 | 8 | |
| Wollastonite | n.d. | DCP: M (38 μm), 40 bar, 100 °C, distilled water, 1 hour54,82 | 80 | |
| 4.06 | DC: M (38 μm), 20 bar, 200 °C, 0.25 hour70,80 | 75 | Cost of 102 euro per ton CO2 stored 90% dissolution was achieved in 1 hour, but carbonation was not tested | |
| Wollastonite | n.a. | IC: 80 °C, 30 bar, succinic acid112 | n.a. | |
| Brines | n.a. | DCP: 1 bar, 100 °C, seawater/brine/alkalinity (NaOH or Mg(OH)2 or MgO)99,100 | 70 | Amounts of NaOH and/or electricity |
| Brines | n.a. | DCP: 30 °C, NaOH, NaCl, electrolysis101 | 98 | Potential co-removal of SOx, NO2 |
The solid particle dissolution process is generally controlled by: (1) diffusion through a fluid film surrounding the particle, (2) diffusion through a solid product layer on the particle surface, or (3) chemical reaction at the particle surface.55 The rate of the overall process is controlled by the slowest of these sequential steps. Dissolution kinetics for olivine and serpentine, the two main source silicate minerals for mineral carbonation, have been studied for several decades; especially, olivine has attracted noticeable interest.56–60
Dissolution of mineral is the rate-limiting step in the direct aqueous mineral carbonation system, mainly due to the absence of protons at pH close to 7.61–63 In an aqueous–solid reaction system, the rate-limiting step is the dissolution of the mineral followed by product layer diffusion control (i.e. silica layer reduces diffusion of CO2 or the carbonate precipitate). In CO2–water–solid systems, the reaction rate of CO2 dissolution (gas diffusion through fluid film control) is the limiting-control step.63 Despite the fact that dissolution rates of minerals are commonly believed to be proportional to their crystals surface, the precipitation of secondary phases decreases the dissolution rates of those surfaces on which they precipitate; recent aqueous dissolution tests on diopside (25–70 °C, in the presence of NaHCO3) did not show any difference between the dissolution rate of the experiments with and without carbonate precipitation.64 It has been suggested that the precipitation of carbonate forms a porous coating on the mineral, which allows ions from the dissolving mineral to be transported readily to the bulk fluid.
The particle size is a key parameter affecting carbonation because the reduction of size increases surface area and consequently the availability of reactive Mg and Ca.65 The deposition of an inert layer such as SiO2 on the surface limits the diffusion of the extraction solution into the particles.66 Stirring or sonication during carbonation limits the formation of carbonate shells, allowing further dissolution of Mg and Ca and diffusion of CO2.65 Temperature influences both the dissolution of CO2 in water (dissolution decreases with temperature) and dissolution of calcium and magnesium from the minerals (dissolution increases when temperature increases). Low temperatures enhance the diffusion of CO2 in the carbonated shell, while high temperatures increase the magnesium and calcium available.65,67 For example, temperatures of 90–100 °C are able to extract 100% of magnesium from serpentine mineral.68
High pressure (40–150 bar) can be used to enhance both the dissolution of CO2 in the water media and the diffusion of the gas into the solid matrix.69 CO2 pressure and the stirring rate (1500 rpm) can significantly influence the reaction rate in direct aqueous carbonation at the optimum temperature range (150–200 °C). Operating above those values, carbonation was considered independent of the stirring rate and CO2 pressure. The carbonation of wollastonite at constant temperature (150 °C) remains constant between 10 and 40 bar and decreases at CO2 pressure below 10 bar due to deficiency of (bi)carbonate activity. On the other hand, the wollastonite carbonation increases when the CO2 pressure is increased from 20 to 40 bar at 200–225 °C, due to deficiency of (bi)carbonate activity.70
The control mechanisms of carbonation of pure CO2 and flue gas carbonation are expected to be similar, but the reaction rate of diluted CO2 is slower because its dissolution rate is slower compared to that of pure CO2.71 The liquid/solid (L/S) ratio is an important parameter because carbonation requires specific L/S ratios to be efficient.65L/S-ratios lower than 2 cannot be stirred sufficiently in an autoclave reactor and may result in poor CO2 gas–liquid and solid–liquid mass transfer rates. Therefore, the lowest liquid-to-solid ratio in an autoclave reactor is 2 kg kg−1, although the majority of aqueous carbonation experiments are carried out at a higher L/S-ratio to enhance the conversion efficiency.72 Also, the reduction of the L/S-ratio leads to a substantial improvement of the heat balance of the process and, thus, the overall CO2 sequestration efficiency. However, if the L/S-ratio becomes too low, pumping and stirring problems might arise because of an increased viscosity, which would lead to a significant decrease of the conversion. Theoretically, an industrial process might be operated to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 L/S ratios improving the overall CO2 sequestration efficiency of the process.70
1 L/S ratios improving the overall CO2 sequestration efficiency of the process.70
3.1.2.1 Direct carbonation without pre-treatments. Gas–solid carbonation: The most straightforward process route is the direct gas–solid carbonation,73 and it was first studied by Lackner and co-workers.74 Various reactions depending on the feedstock are possible. As an example, the direct gas–solid reaction of olivine is given:
| Mg2SiO4(s) + 2CO2(g) = 2MgCO3(s) + SiO2(s) | (11) |
High CO2 pressures (100–150 bar) are necessary in order to obtain reasonable reaction rates.
DaCosta and co-workers75 developed a direct dry process for the sequestration of CO2 where the flue gases pass through a bed of finely ground (2.5–60 μm) silicate rocks (mainly olivine, serpentine or wollastonite). As carbonation takes place, the mineral is replenished by either feeding fresh mineral with a pump or a conveyer. They reported that when using 5 g of olivine (surface of 2.5 m2 g−1) at temperature ranging from 100 to 500 °C and flue composition of 10% CO2, 8.3% H2O (balanced with N2), the storage capacity was 0.12 g CO2 per g olivine (12%) after 30 minutes.75 A higher capacity of 18% was achieved capturing flue gas with 15% CO2 in the presence of 8.3% water at 150 °C. The CO2 stored decreased when 5% or 20% CO2 gas stream were used, in the absence of moisture and at the higher temperatures tested, 175 and 200 °C.76,77 The enhanced CO2 stored capacity in the presence of moisture was related to the fact that water vapour can be useful to convert oxides that may be present to hydroxides which may then be carbonated as in eqn (12) and (13):
| MgO + H2O → Mg(OH)2 | (12) |
| Mg(OH)2 + CO2 → MgCO3 | (13) |
The above process is able to work in a dry environment, where moisture present in flue gas is assumed to be enough to convert the silicates oxides in the high reactive hydroxides and also requires only ∼10–30 minutes, which represent a time scalable to the industrial level. However, it would require a large amount of mineral per tonne of CO2 sequestered due to the low efficiency (<20 g CO2 per g olivine).76 Based on the data available, more than 8 tonnes of olivine would sequester 1 tonne of CO2. This would drastically reduce the applicability of this process to very small CO2 emitters in terms of process size and material handling. Also, particle size reduction to <60 μm is very energy intensive.
Aqueous carbonation: the carbonic acid route process involves CO2 reacting at high pressure (100–159 bar) in an aqueous suspension with olivine or serpentine.61,69 Firstly, CO2 dissolves in water and dissociates to bicarbonate and H+ resulting in a pH of about 5.0 to 5.5 at high CO2 pressure:
| CO2(g) + H2O(l) = H2CO3(aq) = H+(aq) + HCO3−(aq) | (14) |
Mg2+ is then liberated from the mineral matrix by H+:
| Mg2SiO4(s) + 4H+(aq) = 2Mg2+(aq) + SiO2(s) + 2H2O(l) | (15) |
Finally, Mg2+ reacts with bicarbonate and precipitates as magnesite:
| Mg2+(aq) + HCO3−(aq) = MgCO3(s) + H+(aq) | (16) |
Kwak and co-workers78,79 investigated the reaction pathways and reaction extent of direct aqueous carbonation of finely ground olivine (forsterite) (1 g) mixed with water (1 g) and fed into a batch reactor with a volume of 11.7 mL. The reaction was kept at 80 °C and 97 bar for 20 h with a final CO2 storage capacity of 8%. The capacity was increased to 67% but it required 7 days.
Huijgen and co-workers80 studied the direct aqueous carbonation of finely ground wollastonite mineral to particle size 38 μm that was suspended in distilled water. A CO2 stream was introduced into the reactor under continuous stirring to ensure dispersion of the gas. The carbonation reactions occur in the aqueous phase in two steps: calcium leaching from the CaSiO3 matrix and nucleation and growth of CaCO3. A promising conversion of 75% was attained after 15 minutes at 200 °C, 20 bar CO2 partial pressure, with estimated costs of 102 € per tCO2 sequestered, based on process simulation (Aspen). The major costs were associated with the feedstock and the electricity consumption for grinding and compression, with 54 and 26 € per tCO2 sequestered, respectively.70
Overall, direct routes present straightforward design and the absence of non-aqueous solvents. However, reaction conversions are low and high CO2 pressure and temperature are required, compared to processes where pre-treatments are used to enhance the CO2 storage capacity.73 To enhance reaction conversion, various pre-treatments have been employed.
3.1.2.2 Direct carbonation with pre-treatment. The purpose of the pre-treatment step is to promote and accelerate carbonation reaction rates and efficiencies through surface area increase. Two major processes have been developed: high energy mechanical grinding and chemical leaching, although other methods such as thermal- and mechano-chemical-pretreatments have also been reported.
3.1.2.2.1 Direct carbonation with mechanical pre-treatment. The mechanical grinding approach aims at destroying or disordering the mineral lattice, and thus, resulting in an increase of the surface area. Particle size reduction takes place in a sequence of crushing and grinding stages required to reduce the particle size to <300 μm which can be necessary to liberate valuable mineral grains. Crushing is normally performed on dry materials using compression equipment such as jaw or cone crushers. Instead, grinding is accomplished by abrasion and impact of the ore by the free motion of unconnected grinding media such as rods, balls, or pebbles.81
The US National Energy Technology Laboratory (NETL) developed a direct carbonation process (scheme shown in Fig. 4), involving grinding of magnesium (or calcium) silicates at 150–200 °C, 100–150 bar, where 0.64 M NaHCO3 and 1 M NaCl were added to the solutions.54,82 NaHCO3 was used to turn to slightly alkaline pH the solution in order to facilitate carbonate precipitation. Olivine carbonation proceeded to over 80% in 6 h. Wollastonite was found to be the most reactive, reaching over 70% in 1 h, and unlike the magnesium minerals, the wollastonite reaction proceeded rapidly in distilled water.82 The carbonation of olivine and wollastonite was controlled by the surface area consistent with the shrinking-core model, in which the particle surface reacts to release magnesium into solution, leaving a shrinking core. The higher wollastonite efficiency was related to the much higher precipitation rate for CaCO3 compared to MgCO3, which is four orders of magnitude lower than those of CaCO3.83
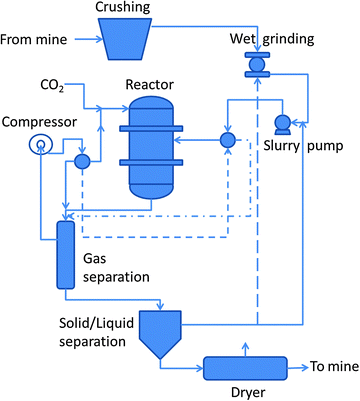 | ||
| Fig. 4 Scheme of the NETL process (modified from ref. 82). | ||
Various pre-treatment options such as ultrasonic treatment and wet grinding in caustic solution have also been tested, but they did not result in a higher reactivity.61 The major problem with many other pre-treatment options is the high energy input required.84 Extensive studies on the mechanical activation of silicates were performed at NETL85,86 and were reviewed later by Huijgen and Comans84 and Zevenhoven and co-workers.8 The major conclusions made were that high-energy attrition grinding of silicates resulted in a higher conversion rates but consumed too much energy.
Similar conclusions were obtained by Fabian et al.87 who studied the CO2 storage capacity of olivine mechanically activated using different conditions and by Haug et al.57 who reported dissolution (in 0.1 M HCl, and pressured CO2/H2O) and carbonation (115–128 °C and under 150–185 bar) rates of grinded olivine. Activation in a planetary mill, even if effective, was found to consume too much energy (see Table 4) for CO2 sequestration purposes. Therefore, it can be concluded that activation methods, such as thermal and chemical activation (discussed in the following sections), are preferred options to mechanical activation.
| Mechanical activation (olivine) | RPM | Time (min) | SA (m2 g−1) | Crystalline phase (%) | Energy consumption (kW h per t) |
|---|---|---|---|---|---|
| As-received | — | — | 0.25 | 100 | — |
| 1500 | 10 | 7.3 | 45 | 190 | |
| Attritor | 30 | 18.1 | 26 | 580 | |
| 120 | 35.2 | 12 | 2310 | ||
| Planetary | 450 | 10 | 4.8 | 37 | 2010 |
| 30 | 5.2 | 17 | 6030 | ||
| Nutating | 900 | 10 | 3 | 57 | 640 |
| Attritor | 1500 | 10 | 7.3 | n.a. | 170 |
| Attritor | 1500 | 30 | 18.1 | n.a. | 520 |
| Attritor | 1500 | 120 | 35.2 | n.a. | 2080 |
| Thermal activation (serpentine) | Carbonation efficiency (%) | Energy consumption (kW h per t) |
|---|---|---|
| Antigorite (75 μm) | 62 | 293 (306) |
| Antigorite (38 μm) | 92 | 293 (376) |
| Lizardite (75 μm) | 9 | 326 (339) |
| Lizardite (38 μm) | 40 | 326 (409) |
3.1.2.2.2 Direct carbonation with thermal pre-treatment. As previously mentioned, serpentine requires additional thermal-treatment to remove hydroxyl groups, resulting in the chemical transformation to pseudo-forsterite. Serpentine requires heating treatment above 630 °C to remove chemically bound water from the lattice.89
| Mg3Si2O5(OH)4 → (MgO)3(SiO2)2 + 2H2O | (17) |
The NETL findings indicate that the reaction rate for serpentine was slow if water (OH groups) was not removed. Thermally treated serpentine at 630 °C for 2 hours reached 65% CO2 storage capacity. Similar results were obtained with high-energy attrition grinding, but with a substantial associated energy penalty.54,82 The theoretical energy required for the heat-activation process is the sum of the energy to heat the mineral to 630 °C and the enthalpy of dehydroxylation. As shown in Table 4, this energy (as electrical power) was quantified in 293 and 326 kW h per t for antigorite and lizardite, respectively.54 Other authors have performed thermal-treatment optimisation studies.90–94 Sanna et al.94 reported that the energy requirement for 0.5 h activation at 610 °C could be lowered to 245 kW h per t instead of 326 kW h per t previously reported (630 °C for 2 h). This enhanced the subsequent dissolution of serpentine from 60% to 90% in just 5 minutes, where the Mg extracted was higher compared to another recent work where thermal activation was performed at 640–700 °C for 1 h.92
The direct use of thermal heat instead of electrical energy, coupled to partial dehydroxylation with heat integration (63% decrease in energy requirement for thermal-activation), has led to an overall mineral carbonation process estimated cost of A$ 70 per tCO2 avoided,91,93 compared to $ 210 per tCO2 avoided in the NETL process.82 Balucan et al.93 studied the thermal activation of serpentine from the Great Serpentinite Belt in New South Wales (Australia), in a Thermo-Gravimetric Analysis-Differential Scanning Calorimetry (TGA-DSC) apparatus. This serpentine was found to be particularly suitable for heat activation to 20% residual hydroxyl groups, as opposed to the partly serpentinised ultramafic minerals of the Coolac Serpentinite Belt. The activation strategy comprised heating serpentine particles with diameter < 34 μm to 680 °C (1.5 h) to produce an active material with 20% residual hydroxyl groups and the recovery of ∼80% of the sensible heat from the dehydroxylated mineral. This results in a thermal activation estimated cost of A$ 1.25 per t of serpentine.93 Serpentine from Coolac Serpentinite Belt used in the experiments had a much larger particle size (D90 127 μm) so the comparison is rather difficult. Also, particles size reduction to <34 μm is energy intensive (>220 kW h per t).82 However, if these preliminary studies are confirmed,91 technologies that use thermal-pretreatment may become more attractive, although they may be constrained by the specific reactivity of the serpentine used. Overall, on the data available, thermo-treatment is more effective in accelerating the Mg extraction than mechanical activation, although its associated energy penalty still remains significantly high.87,94
3.1.2.2.3 NETL derived processes. Brent and co-workers explored a better use of the system heat in order to avoid the drawbacks of serpentine thermal-chemical activation.95 The process is being exploited by Orica, a large Australian company interested in mineral carbonation. The energy savings were obtained after preheating the mineral feedstock in a combined series of heat exchangers utilising the exothermic heat from the carbonation reactor and low grade heat from the same power plant that provides the flue gas. The proposed mineral carbonation plant does not include CO2 capture and serpentine mining. Serpentine was reduced to a particle size of less than 75 μm. Since the recovered low grade heat from carbonation (120–150 °C) and power plant was not enough to reach the desired activation temperature (>580 °C), a hydrocarbonaceous fuelled furnace was used for the last heating step. The same chemical additives as in the NETL process were used. Carbonation was carried out at pressure in excess of 150 bar. This process has been claimed to be economically viable (Aspen modelling) for the permanent storage of 14.1 Mt per year of CO2 emissions from a conventional pulverised fuel electricity generation plant (15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 GW h per annum), which would consume about 41 Mt per year of serpentine and an additional 0.9 Mt per year coal to activate the serpentine at a claimed cost of about A$22 per tCO2.95,96 However, for the process neither experimental nor simulation work has been reported so far and it presents a very high grade of process integration, which may be difficult to achieve. Moreover, it does not consider some logistic issues, such as the long distance location of mineral and CO2 point sources.
500 GW h per annum), which would consume about 41 Mt per year of serpentine and an additional 0.9 Mt per year coal to activate the serpentine at a claimed cost of about A$22 per tCO2.95,96 However, for the process neither experimental nor simulation work has been reported so far and it presents a very high grade of process integration, which may be difficult to achieve. Moreover, it does not consider some logistic issues, such as the long distance location of mineral and CO2 point sources.
Shell has developed an aqueous slurry-based mineralisation technology suitable for both serpentine and olivine mineral rocks. The process comprises pre-treatment, leaching and precipitation steps, where activation of serpentine is achieved by both mechanical and thermal means.18,19 The overall process resembles that developed by NETL, but operates under lower process conditions. The slurry from the leaching step is pressurised (up to 45 bar) and heated up to 110–140 °C in the precipitation step. Here, precipitation of dissolved Mg(HCO3)2 takes place as well as transformation of hydromagnesite into magnesite. Two different concepts have been proposed as shown in Fig. 5, namely Shell's direct pure and flue gas mineralization technology (Fig. 5a and b). Since flue gas with 10% vol CO2 has a much lower solubility than pure CO2 under pressure, leaching of cations in the presence of CO2 will take place at a much slower rate. To avoid this, the flue gas is brought into contact with the mineral slurry prior to the precipitation stage in a separate slurry mill at ambient temperature.19 The slurry mill achieves both a huge reduction of particle size and the formation of carbonate intermediates other than bicarbonate, for instance hydromagnesite. Shell's thermal activation, which can reduce energy requirement up to 63%, has been employed in this process.91 It consists of heating the serpentine (preferably 150–200 μm) for 1 hour at a temperature of 650 °C in a fluidised bed. No data on the CO2 sequestration capacity and energy consumption are publically available. Technical feasibility of Shell's proposed direct flue gas mineralization concept using activated serpentine has been proved at the Energy Centre of the Netherlands (ECN) in a continuous pilot plant. The continuous experiments show that seawater accelerates the rate of leaching and subsequent precipitation, but requires particular material choice as expected. Also, it was shown that yielding dissolved magnesium bicarbonate by not using a magnesium carbonate precipitation unit has a large cost advantage, resulting in about 80% cost reduction. On the basis of the information available, the proposed process seems to be able to decrease the energy requirements for the serpentine pre-treatment, compared to the NETL process by employing proprietary thermo-treatment technology, but no information on potential costs is available yet. This technology would not get any benefit from the resultant products, since they are mixed in slurry, which would require energy intensive processing to isolate them.
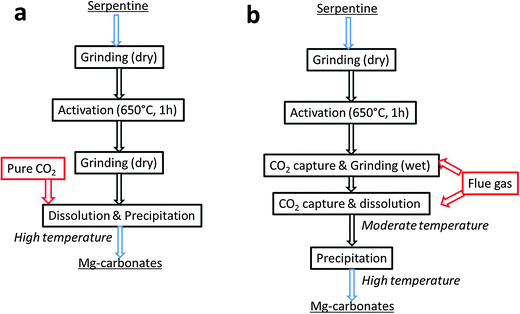 | ||
| Fig. 5 Mineralization process concept for pure CO2 and flue gas (modified from ref. 19). | ||
3.1.2.2.4 Brine-based processes. A different approach has been developed by Calera, which owns a demonstration plant at the gas fired Moss Landing power plant (USA). The Moss Landing plant has demonstrated to capture flue gas CO2 from a 10 MW power generator at 90% efficiency for about 2 years.96,97 A diagram of the Calera process is depicted in Fig. 6. The technology foresees the introduction of brines containing alkaline earth metal ions into a reactor, where the brine is contacted with CO2 containing gas. CO2 dissolves in water to produce carbonate and bicarbonate ions, resulting in a decrease in the pH of the solution. In order to produce a carbonate-containing precipitate, protons are removed from the solution so as to shift the equilibrium towards carbonate (which requires pH 9–11 to precipitate). The solution pH is then increased through the introduction of alkalinity to the point where the alkali metals are precipitated as carbonates, which may be suitable for cement manufacture. A 20% replacement in blended cement appeared to be technically feasible, but not yet demonstrated.98 An energy penalty ranging from about 10% to 40%, depending on power plant characteristics and availability of brines, has been associated with this process.98
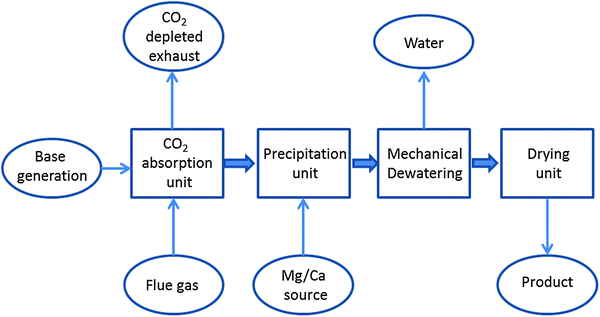 | ||
| Fig. 6 Calera process diagram (modified from ref. 98). | ||
Besides the fact that a large number of brines containing alkaline earth metal ions (Ca, Mg) are mentioned as potential feedstocks,99,100 the technical suitability of brines (e.g. initial proposal of using brines at the Latrobe Valley demonstration project was abandoned because of technically unsuitable brines), sea water and alkaline waste for the Calera process (design at the Moss Landing California pilot plant concluded that sea water required too much energy and alkaline industrial waste would be too limited for sustainable operations at a significant scale) reduces the wide application of this technology.98
Fig. 6 provides a scheme of the Calera process, where the final product is a cementitious material with the consistency of mud, which when de-watered, becomes an aggregate-like solid.98
Another method of sequestering carbon dioxide using brines, referred to as SkyMine, has been recently proposed.101 CO2 is absorbed into an aqueous caustic soda mixture to form carbonate and/or bicarbonate products.101 Flue gas from the power plant is cooled from 300 °C to 30 °C in a series of heat exchangers and then it is introduced at the bottom of an absorber, where NaOH is used. The latter is produced by brine electrolysis. The reaction taking place in the carbonation chamber is:
| 2NaOH + CO2 = Na2CO3 + H2O and Na2CO3 + H2O + CO2 = 2NaHCO3 | (18) |
The carbonate and bicarbonate formed are separated from the liquid solution by heating, which can be done by exchange of heat with the flue gas (93% purity of Na2CO3 is achieved) or heat derived from hydrogen produced in electrolysis. In the membrane cell processing units, the following inputs and products are obtained:
| At the anode: 26% NaCl + 2275 kW h per tCl2 = Cl2(g) + 24% NaOH | (19) |
| At the cathode: H2O + e = 30–33% NaOH + H2(g) | (20) |
This process claims to reach a conversion of 98% by using large amounts of NaOH and/or electricity.101 In addition to capturing and mineralizing CO2, the SkyMine process also claims the possibility to clean SOx and NO2 from the flue gas, and remove heavy metals, such as mercury. A joint venture namely Skyonic Corporation, which includes BP and ConocoPhillips, has started the construction of a commercial CO2 capture plant to remove 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tCO2 per year from a cement plant (130
000 tCO2 per year from a cement plant (130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 considering the reduced emissions in producing backing soda). The strength of the process is represented by the possibility to produce valuable carbon-negative products (e.g. hydrochloric acid and sodium bicarbonate) using low-cost chemical inputs in a low energy requirement capture-mineralisation plant.102 Despite the fact that the reliability of the process has been proved and large investment has been received ($128 millions), a comprehensive cost assessment is not publically available. Also, it has to be noticed that market for HCl and sodium bicarbonate is not large for a wide application of this technology.
000 considering the reduced emissions in producing backing soda). The strength of the process is represented by the possibility to produce valuable carbon-negative products (e.g. hydrochloric acid and sodium bicarbonate) using low-cost chemical inputs in a low energy requirement capture-mineralisation plant.102 Despite the fact that the reliability of the process has been proved and large investment has been received ($128 millions), a comprehensive cost assessment is not publically available. Also, it has to be noticed that market for HCl and sodium bicarbonate is not large for a wide application of this technology.
3.1.2.2.5 Organic acid direct processes. Organic acids and their anions may affect mineral weathering rates by three possible mechanisms: (1) changing the dissolution rate far from equilibrium either decreasing solution pH or forming complexes with cations at the mineral surface, which provides a new parallel reaction mechanism for the detachment of material from the mineral surface; (2) ability to make aqueous complexes with aqueous metals that would otherwise inhibit rates; and (3) changing the ions speciation in solution, which affects the dissolution rate of minerals.103,104 Far from equilibrium, the dissolution rates of most silicate minerals increase exponentially with increasing hydrogen ion concentration (low pH) in solution. The pH effect can be explained by the fact that sorption of protons on an oxide surface polarizes the metal–oxygen bonds, weakening the bonding with the underlying lattice.103
Recently, Bonfils et al.62 have proposed a direct mineral carbonation process where organic acids are used to enhance the dissolution of silicate rocks. The interactions between organic ligands and magnesium silicates have been reported in the geochemical literature since organic acids are more efficient than water in accelerating silicate leaching dissociation of Mg–O–Si bonds in the presence of protons.59,105 Bonfils work showed that the presence of disodium oxalate under 20 bar of CO2 pressure leads to the formation of strong oxalate–magnesium complexes in solution and precipitation of MgC2O4·2H2O (glushinskite), which impede the precipitation of magnesium carbonate. Contrary to oxalate, citrate and EDTA salts ligands do not form any solid by-products with magnesium, but also do not promote carbonation, arising strong doubts on the possibility of developing a direct aqueous mineral carbonation process using organic salts.62 Moreover, Declercq et al.104 investigated the effect of organic ligands on olivine (forsterite) dissolution at 25 °C and pH 3. The study included the evaluation of acetate, oxalate, citrate, EDTA, glutamate, gluconate, malonate, aspartate, tartrate, malate, alginate, salicylate and humate. Their study, in agreement with previous reports, concluded that aqueous organic ligands have at most a small effect on forsterite dissolution rates under strongly acidic conditions but may have an effect at higher pH (4–7).
The contrasting effects of organic acids on steady-state forsterite dissolution rates with increasing pH were related to their aqueous speciation, since these organic species are in the form of neutral species at acidic pH, but as negatively charged aqueous species under mild acidic and neutral conditions.104
Munz et al.107 demonstrated the principles of separating magnesite and silica after dissolution of olivine in carbonated aqueous solutions using a flow-through column reactor. The process consisted of three steps: (1) dissolution of 75 μm fine olivine at 130 °C and 150 bar; (2) precipitation of magnesite at 250 °C; and (3) precipitation of silica. Both precipitation steps were dependent on pH and temperature.107 A carbonation efficiency of 11% and 93% was obtained after mechanical pre-treatment (211 kW h per t) after 2 and 18 hours, respectively.60 However, as high carbonation efficiency was only obtained after long times, the process is not viable on an industrial scale.
A wide number of strong acids and bases such as HCl, H2SO4 and HNO3 have been employed for the dissolution of silicate rocks.2,23,56,108 Lin et al.108 proposed a 2-stage process, where serpentine is decomposed to magnesium hydroxide using HCl at 150 °C. The resulting Mg(OH)2 was then carbonated at 325 °C for 2 hours in a fixed bed at atmospheric pressure.108 However, because the authors did not address the recovery of the chemicals used in the process and the time required for dissolution was too long compared to the precipitation stage, the process was not attractive. Maroto-Valer et al.109 developed a process, where serpentine was chemically activated with H2SO4 at a temperature 20–65 °C for 3–12 hours. The resulting magnesium sulphate was reacted with sodium hydroxide to precipitate Mg(OH)2 following an exothermic reaction. Mg(OH)2 subsequently reacted with CO2 in aqueous suspension at 20 °C and 40 bar. A conversion of 55% was achieved in 10 minutes under these mild conditions. Sulphuric acid was regenerated by reacting CO2 with MgSO4.109 However, chemicals make up and intensive chemical regeneration hindered the deployment of this process. The effect of HCl, H2SO4 and HNO3 on serpentine dissolution at 20 °C and different solution concentrations (1, 2, and 4 M) revealed that their capacity in dissolving the mineral decreases in this order: H2SO4 > HCl > HNO3. Despite their effectiveness in extracting Mg from silicates, processes that employed strong acids did not result in viable MC processes due to the overall difficult and large energy penalties associated with their recovery.2,56 Organic acids have also been investigated in mineral carbonation to reduce the energy penalty associated with strong acids. Teir et al.56 found that acetic acid (CH3COOH) and formic acid (HCOOH) were able to leach a significant amount of magnesium from serpentine. Krevor and Lackner110 established that the sodium salts of citrate, oxalate, and ethylenediaminetetraacetic acid (EDTA) significantly enhance the dissolution of serpentine under weakly acidic conditions. In their process, finely ground serpentine of particle size less than 75 μm was reacted in a solution with dissolved salts under a CO2 atmosphere and at 120 °C.110,111 This energy penalty can be avoided dissolving the serpentine at an essentially neutral pH, i.e. in a solution more weakly acidic than carbonic acid. The reactions rates were several orders of magnitude higher in the presence of citrate than in the weakly acidic solution alone. Carbonation was performed at 20 bar and 120 °C. After 2 hours a conversion of 60% was achieved, while 80% was reached after 7 hours. EDTA, which forms magnesium complexes several orders of magnitude more stable than oxalate and citrate, cannot be used as a catalyst.110,111 The recovery and regeneration of the additive salts were not addressed in this work, which represents a major limit for its potential evaluation, as in the case of strong acids. Also, the long reaction times at the highest CO2 conversion would require a very large plant footprint to be economic. Succinic acid was also employed to extract reactive component (Ca2+) from wollastonite at 80 °C and 30 bar. A promising calcium dissolution of 90% was achieved after 1 hour, but the carbonation step was not tested.112 Park et al.113,114 demonstrated that a mixture of 1 vol% orthophosphoric acid, 0.9 wt% oxalic acid and 0.1% EDTA greatly enhanced the leaching of magnesium from serpentine at 70 °C and 1 bar. After 1 hour dissolution, the slurry was filtered to remove the SiO2 residue. The use of internal agitation with grinding media in the dissolution stage greatly improved the extraction of magnesium from serpentine. The filtrate rich in Mg2+ and Fe2+ was then carbonated by bubbling through CO2 at ambient temperature. Overall, the conversion achieved was 65%, but recovery of additives was not addressed even in this case.113,114
With the aim of improving the efficiency of mineral dissolution and recovering and re-using additives, Maroto-Valer and co-workers proposed a pH-swing CO2 mineralisation process using ammonium salts.68 At 100 °C, 1.4 M aqueous solution NH4HSO4 was found to extract 100% Mg from serpentine in 3 hours. The proposed process consists of five steps and the main reactions are presented in Fig. 7. In the first step, NH3 was used to capture CO2 from flue gas to produce NH4HCO3. In the mineral dissolution step, 1.4 M NH4HSO4 was used to extract Mg from serpentine ground to a particle size range 75–125 μm. The Mg-rich solution was then neutralised by adding NH4OH, after which impurities in the leaching solution were removed by adding NH4OH. The Mg-rich solution is then reacted with the product from the capture step NH4HCO3 to precipitate carbonates. Since the formation and stability of hydro-carbonates is temperature dependent, MgCO3·3H2O (nesquehonite) can be converted to 4MgCO3·Mg(OH)2·4H2O (hydromagnesite) at temperatures above 70 °C. Precipitation of hydromagnesite resulted in a solution mainly containing (NH4)2SO4. The final step was the additive regeneration, with the decomposition of (NH4)2SO4 at ∼330 °C, and producing NH3 for the capture step and NH4HSO4 for the dissolution step.68,72
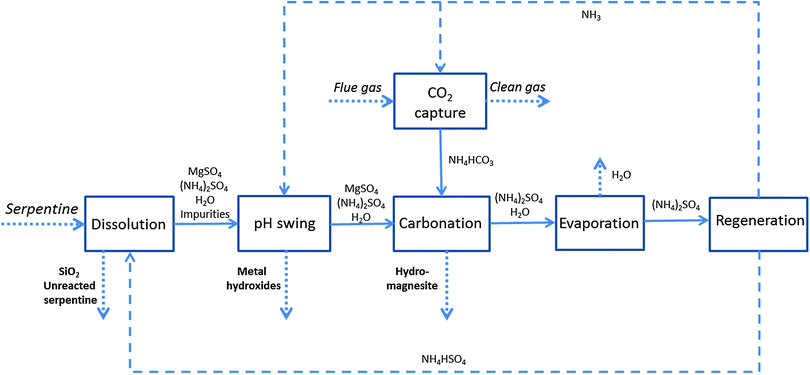 | ||
| Fig. 7 pH-swing CO2 mineral carbonation process with recyclable ammonium salts (modified from ref. 68). | ||
In a typical capture process, CO2 is first absorbed by chemicals (e.g. NH3) and then desorbed (to recover the sorbent) and compressed for transportation, where stripping and compression consumes about 70% of the total CCS energy consumption. Since CO2 captured as sodium carbonate/bicarbonate is directly used in the proposed mineral carbonation, there is no need for desorption and compression of CO2. This process as other pH swing processes is also able to separate three different products: silica, magnesite and iron oxide.56,66,68,106,114 This process could also be integrated with the chilled ammonia CO2 capture process, which has been demonstrated to capture more than 90% of CO2 (from 3–15% CO2 in flue gas)115 and an estimated energy penalty of 477 kW h per tCO2.116
The main drawback of the aqueous pH swing ammonium-based process is represented by the large amount of water that needs to be separated from the salts during the regeneration step. Based on their work, where a solid to liquid (S/L) ratio or 50 g L−1 was used, 50–56 tH2O were required to sequester 1 tCO2. Since water evaporation is a high energy penalty process, they attempted to reduce the water usage in the system.117 When the S/L ratio increased to 300 g L−1, 16 tH2O were required to sequester 1 tCO2. However, since the CO2 fixation efficiency decreased to 46.6%, a larger amount of reactants (serpentine and salts) were required. Moreover, the amount of water to be evaporated is still too high and alternative separation methods need to be investigated in order to make this process economically feasible.
A two-step process which also uses ammonium salts has been recently developed by Zevenhoven and co-workers.118,119 The scheme of this process is shown in Fig. 8. In the first step, Mg(OH)2 was produced from serpentinite and in the second step, Mg(OH)2 was carbonated in the dry phase. This process takes advantage of: (1) the higher reactivity of Mg(OH)2 compared to that of serpentinite and MgO and (2) the potential recovery of the heat of reaction released during the carbonation. In the Mg extraction step, a mixture of serpentinite was heated together with ammonium sulphate at a temperature of 450–550 °C. This resulted in the formation of magnesium sulphate, which was then dissolved in water. Adjusting the pH using ammonium hydroxide or ammonia led to precipitation of Mg(OH)2, while iron oxide was recovered as the by-product. Finally, Mg(OH)2 was carbonated in a fluidised bed at 20 bar and 500–550 °C. A 50% Mg conversion was achieved in 10 minutes.120 With this work, the authors attempted to reduce the high energy requirements for the regeneration steps of aqueous pH swing processes (water/salts separation by thermal evaporation) and also attempted the recovery of the carbonation heat. Although it is thermodynamically feasible to recover heat of reaction in the exothermic carbonation stage for the endothermic magnesium extraction, this has not yet been practically demonstrated. Moreover, modest recovery of magnesium (50–60%) is currently limiting this technology.121 Mg extraction from serpentine is carried out using ammonium sulphate acidic derivatives (ammonium bisulfate and sulfamic acid are formed at temperature >300 °C). However, sulfamic acid volatilizes and/or decomposes at a significant rate by 400 °C, which will affect the recovery of Mg.121,122
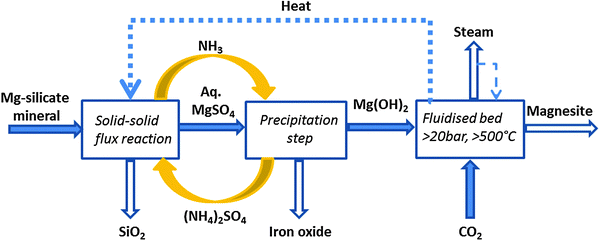 | ||
| Fig. 8 Åbo Akademi mineral carbonation process (modified from ref. 121). | ||
Hunwick123 developed a multistep method for capture and sequestration of CO2 from power station flue gases. This process is being developed by an Australian company, Integrated Carbon Sequestration Pty Ltd, after the proof-of-concept experiments carried out at Commonwealth Scientific and Industrial Research Organisation (CSIRO). In the first step, finely grinded (<40 μm) Mg/Ca silicates were mixed with ammonia to produce an aqueous slurry of 30% solids. Then, CO2 was absorbed into the slurry, which was pumped to a reactor at an elevated pressure of 100 bar and at a temperature of 225 °C so as to enhance the rate of a reaction between CO2 and serpentine. Magnesium carbonate was produced and the ammonia was finally regenerated.123 A pipeline reactor was proposed to transport the slurry produced at the mine to an underground chamber reactor, while another pipe reactor connected the power plant to the mine, where the reaction product would be stored. The proposed carbonation reactor was an underground chamber excavated from bed rock at a depth of 100 m sufficient to carbonation reactions to occur.96 Despite the fact that this approach looks promising, no data are available on its assessment, including feasibility and energy consumption for the proposed long distance transport to pump the slurries from/to the different locations need to be addressed. Moreover, serious problems could arise from pipeline corrosion as previously reported by O'Connor and co-workers.124
3.2 Technologies developed for waste materials
Some of the drawbacks of mineral carbonation of primary earth minerals could be avoided by using solid wastes generated from large scale industrial processes such as coal or oil shale fired power plant, solid waste incinerator, cement plant, steel and paper industry (Table 5) as a feedstock.125,126 This approach has a number of advantages: (1) these materials are often associated with CO2 point source emissions; (2) they tend to be chemically less stable than geologically derived minerals127 and thus require a lower degree of pre-treatment and less energy-intensive operating conditions to enhance carbonation yields;73,127 (3) waste materials could supply a readily available source of calcium or magnesium mineral matter (preferably in the form of CaO or Ca(OH)2) without the need for mining; they are typically fine-grained with high reactive surface areas (CKD, CBD, AODS); (4) hazardous waste can be reclassified through pH-neutralization and mineral transformation (MSWI, APC, asbestos tailings, RM, OS FA), and finally; (5) the end product of the sequestration step may be amendable for re-use in products such as road base or other construction material128 as well as pure and precipitated Ca or Mg-carbonates.126,129 On the other hand, the amount of industrial waste materials available is relatively limited and rather unpredictable due to developments in technology (changes in availability and chemical composition) and legislation issues.25 Currently, the research has focused on assessing and maximizing the storage of CO2 by optimizing the operating conditions including pressure, temperature, liquid-to-solid ratio, gas humidity, the gas flow rate, the liquid flow rate, particle size, and solid pretreatment.24,128,130,131| CaO (%) | MgO (%) | tCO2 (%) | Process | E CO2 (%) | Remarks | |
|---|---|---|---|---|---|---|
| Iron and steelmaking slags | ||||||
| BFS24,25,133 | 15–42 | 5–11 | 20–4425 | IAC: (1) step (T = 70 °C; CH3COOH); (2) step (T = 30 °C; P = 1 bar; NaOH)134 | 22.7 | +High CO2 sequestration capacity; +generated in large quantities; +generated near CO2 source; +carbonation improves mechanical and environmental parameters of slag; +possible applications: PCC; −must undergo milling, except AODS and LFS; −high T&P, US (€4000 per t slag135), or additives are needed for acceptable conversion; cost: €77 per t-CO2 net avoided (DAC)70; IAC with PCC production: 300 kW h per t-CO266 or €1990 per t-CaCO3 for chemicals only136 |
| DAC: P = 5 bar; L/S = 0.15; 100% CO2; t = 2 h137 | 7 | |||||
| BOFS25,133,138 | 34–56 | 2–6 | 29–5225 | DAC: T = 100 °C; P = 19 bar; L(DI)/S = 2; 100% CO2; t = 30 min; d < 38 μm139 | 25 | |
| DAC: T = 70 °C, P = 1 bar; L(DI)/S = 10; 100% CO2; t = 2 h131 | 22.9 | |||||
| DAC: T = 60 °C; P = 1.47 bar; L(DI)/S = 20; 100% CO2; t = 30 min140 | 28.9 | |||||
| DAC: T = 25 °C; P = 1 bar; L(CRW)/S = 20; t = 125 min; d < 44 μm141 | 28.3 | |||||
| IAC: (1) step (T = 80 °C, NH4Cl; ground d < 2000 μm); (2) step (13% CO2)66 | 16.2 | |||||
| IAC: (1) step (T = 30 °C; CH3COOH); (2) step (T = 30 °C; P = 1 bar; NaOH; 100% CO2)126 | 9 | |||||
| EAFS25,133,138,142 | 25–47 | 4–19 | 24–4825 | DAC: T = 20 °C; P = 1 bar; L(DI)/S = 10; 15% CO2; t = 40 h; d = 38–106 μm142 | 1.74 | |
| DAC: T = 20 °C; P = 1 bar; L(DI)/S = 10; 15% CO2; t = 65 min; d < 100 μm143 | 1.9–8.7 | |||||
| DAC: P = 5 bar; L/S = 0.15; 100% CO2; t = 2 h137 | 12 | |||||
| DAC: T = 50 °C; P = 3 bar; L/S = 0.4144 | 18 | |||||
| LFS142,143 | 42–58 | 6–15 | 42142 | DAC: T = 20 °C; P = 1 bar; L(DI)/S = 10; 15% CO2; t = 40 h; d = 38–106 μm142 | 24.7 | |
| DAC: T = 20 °C; P = 1 bar; L(DI)/S = 10; 15% CO2; t = 65 min143 | 4.6 | |||||
| AODS133,145 | 41–61 | 4–7.5 | 31–5425,135 | DAC: T = 50 °C; US; L(DI)/S = 10; 100% CO2; t = 4 h; d = 63–200 μm135 | 15.1 | |
| DAC: T = 50 °C; US; L(16.6 g L−1 MgCl)/S = 10; 10% CO2; t = 240 min145 | 27 | |||||
| DAC: T = 90 °C; P = 9 bar; L/S = 16; t = 120 min146 | 26.4 | |||||
| Cement wastes | ||||||
| CKD25,132,148,149 | 34–48 | 1–1.5 | 10–30 | DAC: ambient T&P; L/S = 0.33; 5–15% CO2, 8 h132 | 8–18 | +Generated in large quantities; +generated near CO2 source (CKD, CBD); +carbonated product can be reused in cement manufacturing, aggregates, to produce PCC etc.; +CKD, CBD has fine particle size; −waste cement needs to be ground; −low carbon sequestration capacities (CKD); cost: IAC with PCC production: $136–323 per t-CaCO3147 |
| DAC: ambient T, P = 2 bar; H2O to form paste; 100% CO2; t = 72 h149 | 10 | |||||
| CBD148,149 | 66 | 1 | 50 | DAC: ambient T, P = 2 bar; H2O to form paste; 100% CO2; t = 72 h149 | 25 | |
| Waste cement, RCA150,151 | 25–63 | 0.3–2 | 20 | DAC: T = 20 °C; P = 1–4 bar; L/S = 0.25–0.5;0.03–100% CO2; 0.8–100 h; d < 1.8 mm150 | 1.6–16.5 | |
| DAC: T = 20 °C; L(DI)/cement = 0.26; 20% CO2; t = 60 min151 | 8.9 | |||||
| IAC: (1) step (T = 50 °C; P = 30 bar; 100% CO2L/cement = 350; d = 10–200 μm; t = 10 min). (2) Step (T = 30 °C; 1 bar; t = 30 min)147 | ||||||
| MSWI ashes | ||||||
| MSWI BA25,130,149 | 22–53 | 2.8 | 25 | DAC: P = 3 bar; L/S = 0.3–034; 100% CO2, RH = 65%; t = 2.5 h; d < 710 μm152 | 3.2 | +Produced in large quantities (MSWI BA); +produced near CO2 source; +carbonation reduces pH and leaching of hazardous elements for safer landfill; +grinding not required; −low carbon sequestration capacity (MSWI BA) |
| DAC: ambient T, P = 2 bar; H2O to form paste; 100% CO2; t = 72 h149 | 4 | |||||
| APC residue25,152,153 | 36–60 | 1–2.5 | 50–58 | DAC: P = 3 bar; L/S = 0.2–0.3; 100% CO2, RH = 65%; t = 2.5 h; d < 212 μm152 | 7.3 | |
| DAC: T = 20–30 °C; L/S = 0.3; 20% CO2; t = 50–150 min; dmean = 66 μm153 | 8–12 | |||||
| DC: T = 650–500 °C; P = 1 bar, 10–50% CO267 | 25 | |||||
| T = 30–50 °C; P = 1–10 bar, 100% CO2, L/S = 0–0.667 | 25 | |||||
| Fuel combustion ashes | ||||||
| Coal FA25,154,155 | 1.3–10 | 1–3 | 6–9 | DAC: T = 20–60 °C; P = 10–40 bar; 100% CO2; L/S = 10; dmedian = 40 μm; 18 h154 | 2.6 | +Produced in large quantities (coal FA); +produced near CO2 source; +grinding usually not required; +high CO2 sequestration capacity (OS FA); −low CO2 sequestration capacity (coal FA); −waste available in few areas (OS FA); cost: $11–21 per t-CO2 at mineralization capacity of 0.1–0.2 tCO2 per t-FA.156 |
| DAC: T = 30 °C; 90 °C; P = 10–40 bar; L/S = 1–10; 100% CO2; ground d = 20–150 μm or <150 μm155 | 3.6–7.2 | |||||
| DAC: T = 90 °C; P = 40 bar; L/S = 1; bulk ash; t = 2 h157 | 6.5 | |||||
| NW: ambient T&P; wet deposited ash; t = 20 year157 | 6.8 | |||||
| Lignite FA | 27.5 | 6.5 | 43 | DAC: T = 75 °C, P = 1 bar; 10% CO2; L/S = 20; 4.5 h; d < 250 μm158 | 23 | |
| DAC: T = 30–80 °C; L/S = 40–80; NaCl 1–25 g L−1; pH = 5–9; 100% CO2; ground d = 30–125 μm; t = 10–50 min159 | 7.1 | |||||
| OS FA (PF, CFB)160,161 | 38–50 | 5–12 | 26–49 | DAC: ambient T&P; L/S = 10; 15% CO2; t = 65 min143 | 29 | |
| NW: ambient T&P; wet deposited ash; t = 8 w161 | 2.2 | |||||
| WA149 | 24–46 | 8–9 | 50 | DAC: ambient T, P = 2 bar; H2O to form paste; 100% CO2; t = 72 h149 | 8 | |
| Mine tailings | ||||||
| Asbestos tailings23,164 | 0.2 | 39 | 43 | DC: T = 375 °C; P = 1 bar; 56% CO2; 10% H2O; d = 37–75 μm; t = 5 h164 | 0.5 | +Carbonation destroys the asbestos nature (asbestos tailings, Ni tailings if chrysotile present); +grinding not required (asbestos and Ni tailings), +large quantities produced in localized areas; +carbonation stabilizes RM disposal; −low carbon sequestration ECO2; −too expensive to achieve high carbonate conversion (asbestos and Ni tailings); −bicarbonates generated instead of carbonates (RM); cost: $147 per t-CO2 for DAC of RM;162 IAC of Ni tailings with hydromagnesite production US$600–1600 per t-CO2 for chemicals only163 |
| Ni tailings23,165 | 3.4 | 21–40 | 43 | IAC: (1) step (T = 70 °C; L(4 M HCl, HNO3)/S = 10; t = 2 h; ground d < 0.5 mm); (2) step (T = 30 °C; 100% CO2; NaOH; t = 0.5 h)165 | 29 | |
| RM23,162,166 | 2–7 | <1 | 7–19 | DAC: T = 20 °C; P = 3.5 bar; L/S = 0.2–0.6; ground dmean = 30 μm; t = 12 h167 | 5.3 | |
| DAC: ambient T&P; 100% CO2; ground d = 0.1–160 μm; 3 carbonation cycles (each 5 h)162 | 7.2 | |||||
| DAC: ambient T&P; L/S = 10; 15% CO2; d < 1000 μm; t = 24 h166 | 4.15 | |||||
| Alkaline paper mill wastes | ||||||
| APMWA148,149,168 | 45–82 | 1–5 | 42–55 | DAC: T = 30 °C; P = 10 bar; L(DI)/S = 20; d = 15 μm; t = 2 h168 | 21.8 | +High carbon sequestration capacity; +grinding not required; −generated in small quantities |
| DAC: ambient T, P = 2 bar; H2O to form paste; 100% CO2; t = 72 h149 | 10–26 | |||||
The theoretical maximum CO2 uptake (tCO2 uptake, eqn (21)) of waste expressed in wt% was calculated using a modified Steinour formula;25,132ECO2 indicates the experimental CO2 uptake.
| tCO2 uptake = 0.785 × (%CaO − 0.53 × %CaCO3 − 0.7 × %SO3) + 1.091 × %MgO + 0.71 × %Na2O + 0.468 × (%K2O − 0.632 × %KCl) | (21) |
Table 5 presents a summary of inorganic waste materials (Furnace Slag (BFS), Electric Arc Furnace slag (EAFS), Basic Oxygen Furnace slag (BOFS) Cement Kiln Dust (CKD), Cement Bypass Dust (CBD), Recycled concrete aggregate (RCA), Municipal Solid Waste Incineration ash (MSWI), Air pollution control (APC) residue, Coal and Lignite Fly Ashes (FA), Wood ash (WA), Red Mud (RM), Mine Tailings and Alkaline Paper Mill Wastes Ash (APMWA)), which have been tested as mineral carbonation feedstocks; the % of CaO and MgO, the theoretical and experimental CO2 capture capacity and the different process conditions were investigated.
| CaO(s) + H2O(l) → Ca(OH)2(s) | (22) |
| Ca(OH)2(s) → Ca2+(aq) + 2OH−(aq) | (23) |
| CO2(g) + H2O(l) ↔ H2CO3(aq) ↔ H+(aq) + HCO3−(aq) | (24) |
| HCO3−(aq) + OH−(aq) ↔ CO32−(aq) + H2O(l) | (25) |
| Ca2+(aq) + CO32−(aq) → CaCO3(nuclei) → CaCO3(s) | (26) |
The aqueous carbonation of wastes, in which CaO is bound as a silicate (such as steel slags etc.), can in general be expressed using eqn (24), (27) and (28).21,139,170 Firstly CO2 dissolves in the aqueous phase resulting H+-ions (eqn (24)). Secondly, Ca (Mg) leaches from the mineral matrix due to a slightly acidic environment (eqn (27)). Finally, Ca (Mg) carbonate precipitates (eqn (28)). The rate and extent of calcium leaching were found to be inversely related to particle size and pH, and increased with increasing temperature, pressure and surface area.24,82,130,171
| Ca(or Mg) silicate(s) + 2H+(aq) → Ca2+(or Mg2+)(aq) + SiO2(s) + H2O(l) | (27) |
| Ca2+(or Mg2+)(aq) + HCO3−(aq) → Ca(or Mg)CO3 (s) + H+(aq) | (28) |
It has also been demonstrated that contaminated solids of cementitious nature can be rapidly remediated whilst binding CO2 in the process.172 Carbonation as a stabilization/solidification technique is a process which capsules toxic waste matter into solid bulk. The reaction products can cause rapid hardening. During carbonation of cementitious materials a sequence of individual steps occurs: (1) CO2 diffusion in air and (2) permeation through the solid is followed by (3) solvation of CO2(g) to CO2(aq), (4) hydration of CO2(aq) to H2CO3, (5) ionization of H2CO3 to H+, HCO3− and CO32−, (6) dissolution of cementitious phases (Ca3SiO5, Ca2SiO4) releasing Ca2+ and SiO42− ions, (7) nucleation of CaCO3 and calcium–silicate–hydrate gel, (8) precipitation of solid phases and (9) secondary carbonation by converting calcium–silicate–hydrate gel ultimately to silicate hydrate gel and CaCO3 (eqn (29)).24,172 The extent and rate of carbonation depend mainly on the diffusivity and reactivity of CO2, which in turn depend on the binder type and hydration degree as well as pore type and process conditions (CO2 partial pressure, relative humidity, temperature and pressure).172
| 3CaO·SiO2 + yH2O + (3 − x)CO2 → (3 − x)CaCO3 + xCaO·SiO2·yH2O + zCO2 → (x − z)CaO·SiO2·yH2O + zCaCO3 | (29) |
Generally, the waste carbonation reaction could occur in four routes: (1) conversion inside the solid particle, (2) CaCO3 crystallization on the surface, (3) CaCO3 precipitation in bulk solution, and (4) attachment on solid solution.24 According to Huntzinger et al.,132 Huijgen et al.127,139 and Uibu and Kuusik,173 the main mechanisms affecting the rate and extent of carbonation are transportation-controlled mechanisms such as CO2 and Ca2+-ions diffusion to/from reaction sites, boundary layer effects (diffusion across precipitate coatings on particle surface, dissolution of Ca(OH)2 at the particle surface), and pore blockage/precipitate coating. Typically, the classical shrinking core type model has been used for describing heterogeneous solid–fluid reactions for determination of the rate-limiting mechanism.132,174–176
In the next section, the carbonation of different inorganic waste materials will be discussed in detail.
Slags form as a result of interactions between process impurities (primarily silica) and lime at various stages of steel production.23,133 The main types of slags produced at steelmaking process are basic oxygen furnace slag (BOF) (62% of total steel slags), electric arc furnace slag (EAF) (29%), and ladle slag (LS) (9%).25,177 Blast furnace slag (BF) is generated as a by-product from iron production by melting the gangue of the ore, coke ashes and the siliceous and aluminous residues after the reduction and separation of iron from ore.25 Secondary processes for further refinement of stainless steel produce LS and argon oxygen decarburization slag (AOD).
Iron and steel slags consist mainly of Ca-, Mg-, Al-silicates and oxides in numerous combinations.133 Their annual total CO2 emissions are estimated to be up to 171 Mt of CO2,134 representing about 0.6% of global CO2 emissions from fuel combustion.23 In general steel-making slags require grinding as carbonation pre-treatment,23,48 but the cost of mining and transportation to CO2 emission sites can usually be avoided. Mineral carbonation of steel slag (Table 5) is in most cases carried out in a water slurry phase (L/S > 1 w/w) at ambient141–143 or elevated pressure and temperature.66,127,139,140,176,178 Santos et al. also used ultrasound (US) and/or additives (MgCl) to enhance the carbonation process.135,145 The CO2 uptake of slags depends on the operational parameters (temperature, pressure, particle size) similarly to the carbonation of natural Ca-silicates; but it is less energy demanding.139,142,176 As expected, the slags that contain free CaO as opposed to Ca-silicates were more reactive.142 Calcium from Ca-silicates was leached (eqn (27)) after rapid carbonation of free CaO (eqn (22)–(26)) and then carbonated (eqn (28)) and on the particles' surface. Ca diffusion through the solid matrix was the rate-limiting step due to the formation of a CaCO3 capsule and Ca-depleted silicate zone.23,139,176 The ECO2 values presented a very wide range (1.7–28.9%), depending on the type and composition of slag, as well as process conditions. Using elevated pressures and temperatures, additives and US treatment improved significantly the carbonation kinetics,25 but also increased costs (up to €4000 per t-slag).135 Particle size was also an important variable, as carbonation was significantly improved by using smaller fractions (38–106 μm).25,139,142,176 Estimations have shown that cost of slag (200 °C and 20 bar of pure CO2) was €77 per t-CO2 net avoided.70 Carbonation of Ca-carrying cementitious materials to sequester CO2 also resulted in the development of high early stage strength for building materials applications, achieving CO2 uptake of 7–12% in the process.137 A possibility to upgrade steel slags into products of high commercial value, such as high-purity precipitated CaCO3 (PCC), has also been addressed in several studies.66,126,133 A number of extraction agents including HNO3, H2SO4, NaOH,138 NH4Cl,66,106 and CH3COOH,126,133 CH3COONH4, NH4NO3106 NH4HSO4179 have been investigated for the indirect carbonation route. The use of HNO3 solution resulted in rapid extraction of Ca and Mg from BOF and EAF slags with CO2 sequestration capacity of 0.26–0.38 tCO2 per t-slag.138 In the case of NH4Cl (at 80 °C), 60% of Ca was extracted resulting in PCC of 98% purity. The CO2 sequestration capacity of 16% (0.16 tCO2 per t-slag) was achieved in the process with a total energy consumption of 300 kW h per t-CO2.66 Acetic acid extraction resulted in 31–86% carbonate conversion and PCC of 99.5–99.8% purity.126 Weaker acids, elevated temperatures and longer reaction times promoted selective Ca extraction. Production of high-purity PCC using the acetic acid route would cost €1990 per t-CaCO3 assuming that the byproduct sodium acetate could be sold for ∼€680 per t.136 A closed loop multi-step process was developed to extract Ca2+ from steel slag with NH4HSO4 solution to form solid CaSO4, which after pH adjustment and precipitation of impurities reacted with (NH4)2CO3 (from CO2 capture with NH3) to precipitate CaCO3.179,180 The carbonation efficiencies achieved by the latter process ranged from 59–74%.180
3.2.3.1 MSWI BA. MSWI BA is generally classified as a non-hazardous waste23,130,152 consisting mainly of silicates (SiO2, Ca2Al[AlSiO7]), sulfates (CaSO4, CaSO4·2H2O, Ca6Al2(SO4)3(OH)12·26H2O), carbonates (CaCO3), but also metal oxides, hydroxides (Ca(OH)2, Fe2O3, Fe3O4) and chlorides.25,130 As Ca and Mg contents of MSWI BA are typically too low for significant CO2 sequestration, the mineral carbonation technique is mainly applied to achieve a chemically stable structure with improved leaching behavior130,152 for different applications (e.g. secondary building material in road sub-bases, wind and noise barriers, etc.).23,183
Mineral carbonation of MSWI BA has been examined and compared to natural weathering to reduce alkalinity and trace metal mobility.152,184–186 Rate-controlling mechanisms and the effect of operating parameters, such as temperature, CO2 partial pressure, liquid to solid ratio (optimum L/S ∼ 0.3 w/w152,182), residence time, and particle size152,183 have also been investigated. MSWI BA has shown CO2 uptake on the order of 3.0–6.5 wt%130,149,152,183 (Table 5). Baciocchi et al.187 tested BA from refuse derived fuel (RDF) incineration with CO2 uptake ranging from 4–14% depending on particle size.
The mechanisms involved in the carbonation of these materials are complex. Although most of the studies have only considered Ca(OH)2 carbonation,71,183 it is likely that Ca- and Mg-silicates also take part in CO2 binding reactions. The dissolution of Ca from the solid matrix into the liquid phase and the diffusion of CO2 into the pores have been reported as the rate-limiting steps.130 The kinetics of CO2 uptake include the two following reaction steps: (1) an initial rapid CO2 uptake, which involves “faster” reacting minerals such as Ca(OH)2 and followed by (2) a decrease in the rate until an approximately constant value of CO2 uptake is achieved,152,183,184 which involves less-reactive Ca–Mg-silicates.139
3.2.3.2 APC residues. APC residues are formed in the process of the flue gas treatment and typically contain a mixture of fly ash, unburned carbon and unreacted lime. Due to the lime content (typically pH > 12), and high concentration of heavy metals (Zn, Pb, Cd, Cr, Cu, Hg, Ni), soluble salts and chlorinated compounds, APC residues are classified as a hazardous waste.23,25,152 High percentage of readily-active calcium hydroxides makes the carbonation of APC residues potentially suitable for CO2 sequestration.152,188 Also, the APC carbonation products present a pH value that meets the regulatory limits (pH < 9.5).172,189
The carbonation route for APC residues is more straightforward as compared to MSWI BA, since the main reactive species include Ca(OH)2 and CaClOH. The kinetics of CO2 uptake showed a similar trend as for MSWI BA, only with higher weight gains attributed to higher lime contents and larger specific surface areas.152 The ECO2 uptake of the APC residues ranges from 7 to 25 wt% (0.07–0.25 t-CO2 per t-APC)25,67,152,153,188 (Table 5). Baciocchi et al. compared dry (1 bar, 10–50% CO2, 350–500 °C) and wet routes (1–10 bar, 100% CO2, 30–50 °C, L/S = 0–0.6). Although both routes achieved a similar maximum conversion to carbonates (65%), corresponding to a potential CO2 storage capacity of 0.25 t per t-APC residue, the dry route presented faster reaction kinetics.67
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mt CO2 and 600 Mt fly ash (FA).23,25 About 30% of coal FA is utilized for construction materials.25 Coal FA is a fine powder (particle size typically 10–15 μm), whose composition varies depending on the mineral content of fuel. Generally, it consists of an amorphous aluminosilicate glass matrix (SixAlyOz) and recrystallized minerals, including quartz (SiO2), cristobalite (SiO2) and mullite (3Al2O32SiO2).25 The main components of bituminous coal FA are SiO2, Al2O3, Fe2O3, MgO (1–3%) and CaO (5–10%) and various amounts of unburned carbon.25
000 Mt CO2 and 600 Mt fly ash (FA).23,25 About 30% of coal FA is utilized for construction materials.25 Coal FA is a fine powder (particle size typically 10–15 μm), whose composition varies depending on the mineral content of fuel. Generally, it consists of an amorphous aluminosilicate glass matrix (SixAlyOz) and recrystallized minerals, including quartz (SiO2), cristobalite (SiO2) and mullite (3Al2O32SiO2).25 The main components of bituminous coal FA are SiO2, Al2O3, Fe2O3, MgO (1–3%) and CaO (5–10%) and various amounts of unburned carbon.25
FA from oil-shale (OS, low-grade fossil fuel) combustion has been investigated as a potential sorbent for mineral carbonation. OS FA contains 12–30% free CaO, depending on combustion regimes (PF or CFB).169 Also, potential CO2 sorbents are produced in power plants that co-fire wood and coal. The resulting wood ash (WA) contains about 45% CaO.149
The maximum CO2 sequestration potential of bituminous coal FA is relatively low ∼9 wt%,25,154 but it could be as high as 43–49% for Ca-rich lignite type coal or oil shale ashes143,158 (Table 5). Studies have mainly been focused on the direct aqueous carbonation route under mild process conditions with either water154,157,158,169 or brine155,190 as the reaction medium or by natural weathering over a longer period of time.157,161 A pilot scale mineral carbonation process was developed and tested by reacting coal FA with flue gases in a fluidized bed reactor at a 2120 MW coal-fired power plant in Point of Rocks, USA. A preliminary economic analysis of the process reported that 90% CO2 capture from a 532 MW power plant would cost about $11–21 per t-CO2 assuming a sequestration capacity of 0.1–0.2 t-CO2 per t-FA.156 According to general estimates, coal FA with an average CO2 sequestration capacity of 5% could sequester 0.25% of CO2 emissions from coal fired power plants.23,25,154
Waste cement is a by-product obtained from the aggregate recycling process, where waste concrete is pulverized and classified to separate the aggregate from the waste cement. According to Bobicki et al.,23 waste cement has a potential to store up to 61 Mt CO2 considering the annual waste concrete production of 1100 Mt from EU, USA and China together.23 However, the majority of waste cement is currently already reused in construction applications.25
Teramura et al.150 used a CO2-activated hardening process to produce building materials, where waste cement was mixed with water (50% H2O) before moulding it into bricks, curing with CO2 and drying overnight (with maximum ECO2 16.5%, at 100% CO2 and 4 bar, Table 5). Kashef-Haghighi and Ghoshal151 achieved a carbonation efficiency of 18% and an ECO2 of 8.9% by curing fresh concrete blocks in a flow-through reactor (20% CO2 in N2, 20 °C and 60 min). A small demonstration scale is planned for the technology of using point source CO2 emission to limit the need for heat and steam in the curing process in the production of precast concrete products.192
Katsuyama et al.147 and Iizuka et al.171 produced high purity CaCO3 using an indirect aqueous carbonation route for the extraction of Ca2+ from cement waste by pressurized CO2 (30 bar) and subsequent carbonation at reduced pressures (1 bar). The estimated costs per 1 metric tonne of CaCO3 were US$136 for desulfurization and US$323 for high-purity CaCO3 (market price of CaCO3 of about $400 per tCaCO3147).
3.2.6.1 Asbestos tailings. Production of 1 t of asbestos (4 Mt globally) generates ca. 20 t of tailings.23 The tailings from chrysotile processing are often associated with residual asbestos and, are therefore classified as hazardous wastes. Carbonation of asbestos tailings could be useful in several ways, as they contain up to 40% MgO, the mining and size reduction are already done and the asbestiform nature of the mineral is destroyed. Thus, both the remediation of a hazardous waste and the sequestration of CO2 could be achieved.82 The natural weathering of old tailing piles has been studied by several groups193,194 (Table 5). Wilson et al.193 estimated that the chrysotile in the tailing piles had carbonated approximately 0.3% per year. It was suggested that the CO2 sequestration efficiency could be enhanced by optimizing the surface area and particle size of the tailings, as well as bioleaching (adding microorganisms to increase solubilisation of alkaline earth metals).195 Larachi et al.164 investigated the direct carbonation in dry and humid (humidity 0–10%) environments over a range of temperatures (300–1200 °C) and low CO2 pressures and achieved a maximum carbonate conversion of 0.5% after 5 h at 375 °C in a moist atmosphere. Carbonation of Mg-rich wastes such as asbestos tailings requires elevated pressures and temperatures or pre-treatment, similar to serpentine treatment, in order to achieve higher carbonate conversions.
3.2.6.2 Nickel tailings. As high-grade sulphide deposits are almost depleted and laterites require more complex processing than sulphide ores, the nickel industry has focused on low-grade sulphide resources, often hosted in ultramafic rocks.23 Processing ultramafic ores generates vast quantities of Mg-rich tailings (40% MgO, Table 5). Valorizing these ultramafic tailings could make marginal nickel projects economically feasible.196 Teir et al.163,165 extracted magnesium from serpentinite (from stockpile nickel tailings mine) using a variety of acids (HCl, HNO3) (Table 5). The Mg-extracts were carbonated (carbonate conversion 94%) in a multistage process with a carbonate conversion of 94% and producing individual precipitates of silica, iron oxide and hydromagnesite of 93–99% purity.165 However, the estimated costs for chemicals were only US$600–1600 per tCO2.163 Integrating nickel mining operations with CO2 sequestration requires further developments to reduce costs and a carbon regulatory framework including a cap-and-trade scheme with sufficiently high carbon price.23
3.2.6.3 Red mud. Red mud (RM) is the caustic waste material of bauxite ore processing for alumina extraction. Producing 1 t of alumina generates 1.0–1.5 t of highly alkaline RM (70 Mt annually197).167 RM typically contains Fe2O3 (30–60%), Al2O3 (10–20%), SiO2 (3–50%), Na2O (2–10%), CaO (2–8%) and TiO2 (trace – 10%),162 which are present in portlandite (Ca(OH)2), sodium carbonate (Na2CO3), NaAl(OH)4, Na6[AlSiO4]6, crystalline hematite (Fe2O3), goethite (R-FeOOH), gibbsite (Al(OH)3), boehmite (γ-AlOOH), sodalite (Na4Al3Si3O12Cl), anastase (TiO2), rutile (TiO2), katoite (Ca3Al2SiO4(OH)12), gypsum (CaSO4·2H2O), and quartz (SiO2).166 Mineral carbonation of RM reduces its toxicity and leaching behavior in terms of long-term storage in addition to CO2 sequestration.167,197 Carbonated RM can also be used for various applications such as fertilizers, brick and tile industry, plastics industry, wastewater treatment and cement production.166 RM is generally carbonated via a direct process route at ambient temperatures and pressures162,166,167 and a sequestration capacity of 0.04–0.05 tCO2 per t RM has been reported (Table 5). The roughly calculated cost of CO2 sequestration is at US$147 per t-CO2.162 At Kwinana in Western Australia, Alcoa operates a residue carbonation plant, where gaseous CO2 from a nearby ammonia plant is contacted with RM, reducing the pH of the slurry to a less hazardous level and capturing in the process 0.030-0.035 tCO2 per t of RM.192
As the primary source of alkalinity in RM is NaOH, the main carbonation products are Na2CO3 and NaHCO3.162 Soluble Na-carbonates provide a less permanent CO2 storage than solid Ca–Mg-carbonates because of their solubility. In order to provide a more permanent CO2 storage option, RM was mixed with brine solution (solution of hydrated Ca- and Mg-chlorides) prior to carbonation.197,198 It has been estimated that over 100 Mt of CO2 have been sequestered in RM through the natural weathering of historically produced RM (6 Mt annually).199 By utilizing appropriate technologies for incorporating binding cations into RM, approximately 6 Mt of additional CO2 could be sequestered whilst RM is also remediated.199
4. Product utilisation
The effective development of utilization routes for the materials produced by mineral carbonation could help to make this technology economically viable and facilitate its deployment. Based on the information discussed in the previous sections, processes that produce multiple separated products are preferred in terms of products utilisation, due to high purity required by the market. Therefore, processes where cations are extracted from the feed material in a separate step (indirect processes) may be suitable for controlling the morphology and particle size of precipitated products for high-value applications, compared to direct mineralisation technologies.200,201 It has been shown that indirect processes are generally able to separate silica, magnesium/calcium carbonate and iron hydroxides with purities as high as 90% by switching the pH of the solution from acidic to basic.10,72,200,202 The dissolution of silicates selectively removes Mg/Ca and other elements from the mineral matrix, leaving behind silica in amorphous phase with particle in the range of tens of microns and purity of about 80%.163,200 Precipitated hydromagnesite (Mg5(CO3)4(OH)2 4H2O) with purity >93 wt% can be produced by indirect processes.72,163,201 Also, 99 wt% pure calcite with particles >5 μm have been produced by carbonation of wollastonite under mild conditions (10 bar, 100 °C).200 However, purity required to access high-value markets is difficult to be achieved without extra purification steps.10Applications of carbonate products can be divided into low-end high-volume and high-end low-volume uses. For the MC products to be commercially used there are specifications and quality criteria that must be met (e.g. particle size, distribution and low level of contaminants). Construction and filler applications seem to be the most appropriate for silica and carbonate products, respectively, while feedstock for iron/steel works may represent the natural pathway for iron oxides from MC. Among the low end applications, MC products as liming agents to buffer the acidity of soils are promising, but require the MC products to be free from potential pollutants that might derive from particular flue gas or mineral wastes converted into carbonates. Also, land reclamation from the sea in coastal areas and mine reclamation using silica, magnesium and calcium carbonates are other possible low-tech high-volume applications.10
High-end applications usually require stringent specifications. Mono-disperse nano-particles uniform in size, shape and composition have a wide number of applications in industry, such as catalysts, chromatography, ceramics, pigments, pharmacy, photographic emulsions, etc.203
MC can be used to produce silica in the amorphous phase and with particles smaller than <30 μm, which could serve as a pozzolanic cement replacement material or as a filler.204 Silica from MC, for deoxydiser in steel making, circuit boards, ceramix matrix composites, semiconductors should reach very high purity (SiO2 > 98.5%; Fe2O3 < 0.1%, Al2O3 < 0.15%).10,205 Similar purity is expected for ceramics applications, while slightly lower purity would be required for use a refractory material (95% SiO2) and iron and steel making (90% SiO2). It is unlikely that MC products can reach purity levels required for silicon applications without additional post-processing. Amorphous silica, which is a fine powder and it is currently considered a high quality reactive cement additive, may represent the most likely application for amorphous silica from MC.10
Calcium carbonate is extensively used as a novel functional material in several fields such as plastics, rubber, paint, printing ink, weaving, toothpaste, make-up, and foodstuffs. Calcium carbonate is a product in MC processes that use inorganic wastes or calcium silicates, such as wollastonite.
An interesting perspective to filler technology is the development of nano-sized, high performance, and low cost fillers from calcium carbonates in the form of ground calcium carbonate (GCC) and precipitated calcium carbonate (PCC).10 Calcium carbonate can precipitate in six different forms, namely amorphous calcium carbonate (ACC), hexahydrate calcium carbonate (HCC), monohydrate calcium carbonate (MCC) and the polymorphs calcite, aragonite and vaterite, which have the trigonal, orthorhombic, and hexagonal crystal system, respectively.206 For the PCC applications, several physical and chemical properties, such as particle size average and distribution, morphology, specific surface area, polymorph or the chemical purity are very important in determining the potential market.207 The different polymorphs of CaCO3 can have different functions as additives. For example, dispersion can be increased if cubic CaCO3 is added as an addition in paint; acicular or rod-like CaCO3 has a reinforcing effect on rubber and plastics; and spherical CaCO3 has a significant impact on the brightness and transparency of ink.208
By controlling the initial concentration of the reagents, stirring speed, pH, type and amount of additives, and other reaction conditions, CaCO3 with different polymorphs, morphologies, and grain sizes can be obtained. For example, different CaCO3 polymorphs were generated by changing the carbonation time or after aging.208
Particle sizes and morphologies of precipitated CaCO3 varied from rhombohedral (15–35 nm) to scalenohedral (400 nm in diameter and 2 μm in length) upon changing the operating variables, CaO concentration, the CO2 flow rate and surfactants concentration.203 Addition of the ethyltrimethyl ammonium bromide cationic surfactant (2%) produced narrow size rounded particle morphologies either rhombohedral or spherical and a limited amount of agglomerate.203
Finally, enzymes such as Carbonic Anhydrase (CA) have been used to enhance carbonation efficiency and modify the properties of the reaction products. Mesoporous alumina synthesized from the egg shell membrane and pore-expanded SBA-15 was used as a template to immobilise CA.209,210 The carbonation capacity of alumina immobilized CA was found to be 25% lower compared to that obtained in the presence of free CA.209 Favre et al. reported that at higher pH, calcite and vaterite were observed while at lower pH, only calcite was favoured.210 Another biomimetic complex (Co-BBP) that mimics the catalytic activity of carbonic anhydrase (CA) in mineral carbonation was prepared by the coordination of cobalt(II) with 2,6-bis(2-benzimidazolyl) and was encapsulated into a metal organic framework (Co-BBP@Tb-MOF). The biomimetic catalyst enhanced CO2 hydration and calcium carbonate (CaCO3) crystallization as CA. The metal organic framework was determined by the CaCO3 morphology, resulting in the formation of circular plate structures.211
Producing precipitated calcium carbonate (PCC) from wastes can contribute to the reduction of wastes that contain high calcium content, such as steel slag can be utilized as PCC if calcium is selectively extracted prior to carbonation to fulfill the requirements of purity and crystal shape. Zevenhoven and co-workers selectively extracted calcium from the slag with an aqueous solution of ammonium salt (NH4NO3, CH3COONH4 or NH4Cl) producing PCC from the steel slag derived calcium rich solution with properties comparable to the PCC produced by conventional methods. However, a very small liquid ratio (5 g L−1) was required to get high dissolution efficiency (73%), rendering this method expensive because of the large reactor volumes required.212 Also, calcium carbonate powder produced from steel slag presented inferior brightness compared to traditional PCC (mainly due to iron and manganese content) resulting in a decrease of the market value of the alternative product. Despite this, the separation of iron oxide before the carbonation stage can enhance the quality of PCC produced by this method.200,201,213 Recently, an innovative synthesis of the goethite–calcite nanocomposite has been proposed. This synthesis involved the sequential precipitation of (1) nanosized acicular goethite (a-FeOOH); (2) the instantaneous precipitation of portlandite (Ca(OH)2) by adding the CaCl2 salt to a goethite alkaline suspension (2NaOH + CaCl2 = Ca(OH)2 + 2NaCl) and; (3) sub-micrometric calcite precipitation by injection of CO2 into a goethite–portlandite alkaline suspension (Ca(OH)2 + CO2 = CaCO3 + H2O).214 The precipitated nanocomposite had a surface area of around 92 m2 g−1 when synthesized at 30 °C and 45 m2 g−1, when synthesized at 70 °C.
It has to be mentioned that other methods have been proposed to convert CO2 into chemicals and fuels. Compared to utilisation of MC products as construction or filling materials, which could in theory absorb Gt of CO2, industrial utilization of CO2 as solvent and reactant amounts to only 0.5 wt% (128 Mt per year) of the total anthropogenic CO2 emissions every year, so that it may not necessarily help mitigate the greenhouse effect considering energy input and carbon circulation.215 Catalysed hydrogenation or photocatalytic and electrocatalytic conversion of CO2 to hydrocarbons has been extensively reviewed.215–217 Even if technologies have been developed for large-scale CO2 hydrogenation to methanol or methane, their deployment is mainly limited by the high price of renewable hydrogen. Instead, significant technical and catalytic advances are still required for the large-scale use of electro- and photocatalytic routes, due to their current low energy efficiency and productivity.216
An indicative order of magnitude of the current and potential future CO2 consumption is presented in Table 6. EOR and urea yield boosting are technologies already in use. MC technologies, algae cultivation and potentially ECBM could utilize flue gas directly and therefore would not require a conventional capture plant to deliver a concentrated CO2 stream.192 A semi-quantitative ranking process identified mineralisation technologies (mineral carbonation and concrete curing), EOR and algae cultivation having the greatest potential to accelerate alternative forms of CCS. This assessment considered a series of 14 criteria including the CO2 emissions in the act of reusing it.192
| CO2 uses | Existing (future) CO2 demand (Mt per h) |
|---|---|
| Enhanced oil recovery | 30–300 (<300) |
| Urea | 5–30 (<30) |
| Food and beverage | ∼17 (35) |
| Water treatment | 1–5 (<5) |
| Other | 1–2 (<6) |
| Enhanced coal bed | |
| Methane recovery | (30–300) |
| CO2 concrete curing (MC) | (30–300) |
| Algae cultivation | (>300) |
| Mineralisation (MC) | (>300) |
| Red mud stabilisation (MC) | (5–30) |
| Baking soda (MC) | <1 |
| Liquid fuels (methanol, formic acid) | (>600) |
5. Summary and remarks
It is generally accepted that to reduce the level of CO2 emitted in the atmosphere, a portfolio of different and complementary technologies such as renewables, change in energy uses and CCS has to be employed. Mineral carbonation has the potential to sequester billions of tonnes of CO2, but the current costs are too high for a widespread deployment of this technology. This work reviews the current state of mineral carbonation routes and the role they can play in decreasing the emissions of CO2.5.1 MC options comparison
In situ MC has great potential in terms of volume of CO2 which could be permanently fixed within the hosting rocks as solid carbonates thus reducing the risk of potential seepage from the storage site. There is a large availability of minerals which can react in situ with the injected CO2, both onshore and offshore and often close to anthropogenic sources of CO2. In situ MC can also be beneficial for the worldwide development of storage projects. Abundant onshore and offshore basalts and peridotites are available for in situ low temperature mineralisation. The largest layered onshore basalt formations are located in India (provinces of Deccan Traps), USA (Columbia River basalts), Russia (Siberian Traps) and UAE/Oman.218 The current limits of in situ carbonation are due to the slow pace of the process and the need for artificial ways of enhancement of the chemical reactions which require a large amount of energy. Identifying specific sites where natural characteristics such as geothermal gradients are favourable to the carbonation process may reduce the associated costs.Ex situ MC presents intrinsic materials handling issues, due to the large mineral requirements and associated reaction products, which result in large process scale (larger than actual power plant materials handling), and it seems to be only employable to existing small-medium emitters. MC may be suitable to large emitters if the new plants are designed with the required infrastructures. Since for small-medium emitters, geologic sequestration may not be an economically viable option, and there are no commercialized processes that specifically address this technology gap, MC may target this market. Large ultramafic rock deposits within a 100–200 km radius of power/industrial plants emitting over 1 Mt per year CO2 are available in South Africa, China, Russia, Kazakhstan,219 New South Wales in Australia,93 USA and Europe.82 However, not all these resources are easily accessible. Mg-bearing silicates such as serpentine and olivine represent the most suitable mineral resources, while other Mg-silicates and Ca-silicates are less attractive due to their low Mg content and/or low availability.
Moreover, a number of large scale industrial wastes can be considered as feedstock for CO2 mineralisation. Regardless of several benefits, such as avoiding costs for mining and transport, the current CO2 mineralization technologies developed for wastes still cannot compete with geological storage in terms of potential quantity and cost of sequestrated CO2. The ones that appear to carbonate easily under mild conditions (contain free lime, do not require additional grinding, bind CO2 effectively even from dilute flue gases etc.) and have a high carbon sequestration capacity (APC residues, APMW, OS FA) are only available locally or in too small quantities to make a global impact. However, especially in countries that lack geological storage, these options should be considered (for instance OS FA could capture 10–12% of CO2 emitted from OS based heat and power sector161).
Overall, the processes that are attracting major attention (Fig. 9) and that seem to be viable at this point have in common the potential production of sellable products, the co-removal of different pollutants from the flue gas and process integration essential to lower the costs. The conceptual integration of high temperature and pressure industrial mineral carbonation facility into a developing mine site has been recently demonstrated to be feasible at an operating cost of ∼$83 per tCO2.220
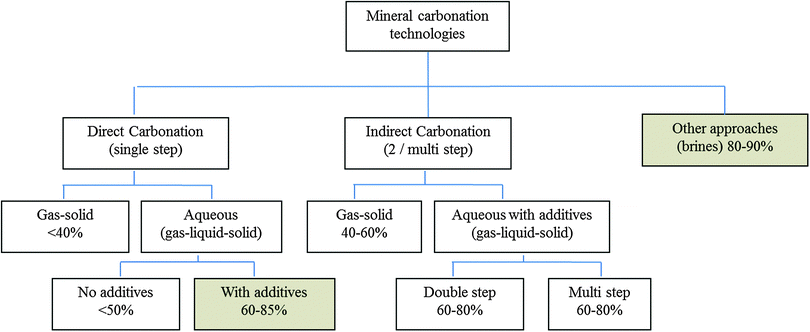 | ||
| Fig. 9 Mineral carbonation process routes. In dark the most promising technologies at the current state of research and development.8 | ||
Direct gas-solid processes, which require temperatures up to 500 °C and fine grinding of minerals (5–35 μm), achieve low capture efficiency and are not viable on the industrial scale at the current scale of development. On the contrary, it is well documented in the literature that the presence of water considerably enhances the reaction rate in the carbonation process.9 Feedstock pre-treatment by fine grinding, thermal activation and chemicals in direct aqueous carbonation processes shows significant improvements in CO2 capture efficiency (up to 85% with pure CO2 stream).82 Meanwhile, the regeneration and recyclability of additives (NaOH, NaHCO3) still need to be addressed. The NETL modified processes proposed by Brent and Shell make use of the low grade heat from power plants and from the serpentine thermal-activation to decrease the overall energy consumption. However, no public data are available to estimate the potential deployment and costs associated with these processes.
Multistep aqueous indirect processes in the presence of additives are also able to reach high carbonation efficiency using mild process conditions and short residence time as a result of faster reaction kinetics in the presence of additives. However, the energy intensive chemical regeneration step is slowing the development of this group of technologies. Also, the use of catalytic enzymes such as carbonic anhydrase is unlikely to be effective due to their instability and very high costs. At the current state of the art, indirect routes seem to be still too expensive to be competitive as CCS technology for large deployment.
5.2 Demonstration projects
So far, mineral carbonation has been implemented only in a few demonstration plants: the first is the Calera process, in the gas fired Moss Landing plant (USA), which has been running for about two years. The plant showed the technical capacity to capture CO2 (30 kt per year) from a 10 MW power generator at 90% efficiency, with an associated energy penalty of 10–40%. However, the potential impact on water balances and hydrology from extraction and reinjection of brines and the conclusion that the tested brines (technically unsuitable), sea water (too costly) and alkaline wastes (limited availability) render this process unsuitable for operations at a significant scale.98Another brine based process (Skyonic) is approaching the commercialization stage. Skyonic is currently retrofitting Capitol's cement mill (San Antonio, USA) owned by Capitol Aggregates. This process directly processes flue gas and produces hydrochloric acid, bleach, chlorine, and hydrogen. In terms of large deployment, an evident drawback of this process is going to be the scale of products generated compared to current markets.
Similarly, only a few projects based on inorganic wastes have moved to the commercial or even small-scale demonstration phase. For example, a pilot scale mineral carbonation process that uses coal FA has been installed at a 2120 MW coal-fired power plant to reduce CO2, SO2 and Hg emissions in Point of Rocks, USA.156,221 Also, accelerated carbonation has been applied for the commercial production of aggregates from APC residues222 and in a residue carbonation plant for Red Mud stabilization at Kwinana in Western Australia.96
Carbonation of Red Mud has been run by Alcoa since 2007 locking 70 ktCO2 per year generated in a nearby ammonia plant.192 However, ∼30 t red mud per tCO2 is used, which is about ten times the typical rate of serpentine rock usage. Also, this technology requires a concentrated and preferably high pressure source of CO2 (85% pure) to be located in reasonable proximity to an alumina refinery.223–225 The CarbFix demonstration project (in situ MC), where 5% CO2 in water has been injected in porous basalts near the continental margins, has recently shown that it is feasible to sequester more than 80% of CO2 injected in less than 1 year at 20–50 °C.226 This mineral trapping pathway avoids one of the major drawbacks associated to geological storage in sedimentary basins, since CO2 dissolved in water is not buoyant and also offers a storage potential one order of magnitude higher than the potential CO2 emissions from burning all fossil fuel resources.226
5.3 MC cost assessment
One of the major challenges for CCS including MC projects is the cost. Recently, an estimated transport and storage cost of ∼$17 per tCO2, which is about double of the cost associated to geological storage in sedimentary basins, has been associated to in situ MC in basaltic rocks.226 Therefore, the total cost of in situ MC will be in the range 72–129 per tCO2 (considering a CO2 capture cost of $55–112 per tCO2), which is by far larger than recent European carbon market CO2 price of ∼$7 per tCO2. However, it has to be pointed out that geological storage costs do not take into account potential long term monitoring costs due to the un-reactivity of dry CO2 in sedimentary rocks. Also, the in situ MC option drastically reduces potential leakages.226Due to lack of commercial applications, mineral carbonation cost estimates reported in literature are roughly based on laboratory or pilot scale experiments. As expected, the main energy and cost penalties are related to plant size, pre-treatment (grinding feedstock and thermal-treatment), operating conditions (mixing, high temperature/pressure), additives (extraction of reactive species) and separation/disposal of the reaction products.
The most reliable ex situ mineral carbonation cost evaluation still remains that calculated by the NETL extensive work, since feasibility studies from the other promising technologies are not available. On the basis of these calculations, sequestration costs for a direct process with pre-treatments were estimated to be 50, 90 and 210 $ per tCO2 for olivine, wollastonite and serpentine, respectively.82 Recent developments in the NETL process estimated an overall mineral carbonation process cost of A$ 70 per tCO2 avoided, where the direct use of thermal heat instead of electrical energy, coupled to partial dehydroxylation with heat integration led to a 63% decrease in energy requirement for thermal-activation.91–93
Huijgen et al.70 estimated a sequestration cost (based on depreciation of investments and variable and fixed operating costs) of €102 per tCO2 net avoided for wollastonite.
The cost of direct aqueous carbonation of inorganic wastes such as concrete waste and steel slag was estimated to be in the range of US$8–104 per t-CO2, depending on the operation conditions (spray trickle bed systems in air227 or at 200 °C, 20 bar, 100% CO270).
Using the indirect aqueous carbonation route with production of value-adding products such as high-purity PCC or hydromagnesite from EAF slags or serpentine would require chemicals (HCl, HNO3, CH3COOH, NaOH) for a cost of $600–4500 per t-CO2 if not regenerated. An acid (HCl) extraction route technology assessment was performed by IEA GHG (2000), concluding that the calculated cost of €179 per tCO2 avoided made this approach unattractive. A similar conclusion was reached by Teir et al.163 using HCl/HNO3 for the dissolution of the feedstock and NaOH in the precipitation step. They found that the regeneration of the chemicals used would emit 2.6–3.5 times the amount of CO2 bound in the carbonation process.136,163 CO2 sequestration using IAC of cement waste at 30 bar and 50 °C would require $136–323 per t-CaCO3, depending on product purity.147
As already discussed, these costs are still higher compared to geological storage cost.228 However, costs of CCS by geological storage in industrial plants such as refineries are less well known and could lead to higher costs due to their complexity (e.g. Mongstad in Norway). For example, capture costs applied to chemicals, fertilizers, refineries and gas fuelled plants could be up to $235 per tCO2 due to geographic location, production/operating specifics and new technology versus retrofit capture situations.229 Also, MC does not present the same uncertainties of geological storage in terms of potential leakages and potential long term monitoring costs. In order to compete with geological storage, CO2 sequestration by MC must offer some additional economic benefits including remediation or stabilization of the hazardous wastes such as asbestos tailings, nickel tailings, and red mud, MSWI and power plant ashes or production of value adding products (building materials, feedstock for existing processes). Recently, the production of artificial aggregates from CO2 carbonation has been demonstrated by a UK based spin-out company of the University of Greenwich, Carbon8. These aggregates have been further used to produce a carbon neutral building block (CO2, sand, cement) manufactured by Lignacite, UK.
Overall, the global low and high-value market for the raw commodities, primarily cement additives, fillers and iron ore feedstock which could be produced by rock and/or industrial waste mineralisation, is about 27.5 Gt and can be easily flooded assuming 10% of the global CO2 emissions sequestered by MC. However, carbonation technologies, which produce building materials or aggregates, still need to be demonstrated at a scale sufficient to prove their commercial viability on a large scale.230 From the technologies reviewed above, it emerges that the processes that have advanced at the demonstration phase are those that use alkalinity generated by electrolysis of brines, saltwater or alkaline wastes as feedstock. This can be explained by the similarities of these processes to conventional technologies that use brines, by the flexibility in terms of feedstocks (different alkaline sources such as varies brines, sea water, NaOH) and products and by the by the compatibility of the produced materials to existing markets (e.g. aggregates and bricks for Carbon8/Ligancite, feedstock for the Solvay process for Skyonic). Table 7 summarises the primary benefits from the use of CO2 in terms of CO2 avoided, energy required to obtain the carbonation products and market values. Although these “advanced” carbonation processes are viewed as an attractive concept, beneficial uses for carbon dioxide are very far from the scale of anthropogenic emissions of this greenhouse gas and therefore suggest to maintain primary focus on large-scale capture and geologic storage and further develop the carbonation concepts able to produce materials for the larger construction market. However, the CO2 sequestration potential of wastes remains marginal on a global scale of CO2 emissions.
| Carbonation process | Amount of CO2 utilised | Value of by-products ($ per tCO2) | Energy penalty for by-product process (%) | CO2 emissions avoided | Products market | Market size (billion $ per year) |
|---|---|---|---|---|---|---|
| Skyonic | Cl2: 14 Mt per year; Na2CO3: 20 Mt per year; H2: 836 Mt per year | Na2CO3: ∼300 $ per t, H2: ∼10 $ per t, Cl2: 240 $ per t | 20 | 2.9 t per tCO2; captured | Solvay process (Na2CO3 or CaCO3) | 3.4–9 |
| Calera | Sand and aggregate market: 1500 Mt per year; cement: 24 Mt per year | Aggregate: 7 $ per t, cement: 100 $ per t | 8–28 | 0.5 t per tCO2 captured | CaCO3 for cement, aggregates | 21 |
| Alcoa | 2–23 Mt per year | 10–300 $ per t | n.a. | n.a. | n.a. | ∼500 |
5.4 Final remarks
Despite the large resources available for CO2 sequestration and the clear advantages over geological storage, the costs of both in situ and ex situ MC are currently too high for a large deployment of the technology and new systems are being investigated to attempt to overcome the unchanged technology challenges: 1-process energy economics, 2-chemical reaction rates and 3-materials handling issue (for ex situ carbonation). The current technology research and development gaps that have to be addressed to enhance the understanding on mineral carbonation and its deployment are as follows:• Scarce representative raw materials comparison
• Processes performance data incomplete and inaccurate
• MC integration with point source not well explored
• Incomplete information on cost/energy balance for thermal activation
• Insufficient knowledge of indirect carbonation fundamentals
• Insufficient knowledge of carbonation fundamentals using flue gas
• Lack of assessed reactor technology options and cost studies. A more systematic approach in costing the process should be addressed for comparison purpose
• Process scale and materials handling issue not well explored
• Scarce data on the environmental impact of large mining operations
While it may not be a complete solution in itself for large emitters (excluding the favourable cases where for example a large deposit of silicates is closely located to a large emitter), ex situ mineral carbonation with inorganic wastes could be part of an integrated approach to carbon sequestration, which combines remediation of hazardous wastes such as asbestos tailings and use of readily available fine industrial wastes such as EAF and cement-kiln dusts to meet CO2 emission goals. On the contrary, in situ carbonation may be viable for large scale emitters if the current limitations are overcome. However, at these MC technology costs, its deployment as CCS option requires strong financial incentives.
Acknowledgements
The authors thank the Energy Technologies Institute (ETI) that commissioned and funded the work as part of its CCS programme. Also, the authors thank the Centre for Innovation in Carbon Capture and Storage, Heriot-Watt University (EPSRC Grant No. EP/F012098/2) for support and logistics.References
- P. Falkowski, R. J. Scholes, E. Boyle, J. Canadell, D. Canfield, J. Elser, N. Gruber, K. Hibbard, P. Högberg, S. Linder, F. T. MacKenzie, B. Moore, T. Pedersen, Y. Rosenthal, S. Seitzinger, V. Smetacek and W. Steffen, Science, 2000, 290, 291–296 CrossRef CAS PubMed.
- IPCC, ed. R. K. Pachauri and A. Reisinger, IPCC, Geneva, Switzerland, 2007, p. 104.
- S. Pacala and R. Socolow, Science, 2004, 305, 968–972 CrossRef CAS PubMed.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Ferńandez, M.-C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7, 130–189 CAS.
- B. Praetorius and K. Schumacher, Energy Policy, 2009, 37, 5081–5093 CrossRef.
- A. P. Hallenbeck and J. R. Kitchin, Ind. Eng. Chem. Res., 2013, 52, 10788–10794 CrossRef CAS.
- W. Seifritz, Nature, 1990, 345, 486 CrossRef.
- J. Sipilä, S. Teir and R. Zevenhoven, Åbo Akademi University report VT 2008-1, 2008.
- IPCC, ed. B. Metz, O. Davidson, H. C. de Coninck, M. Loos and L. A. Meyer, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2005, p. 442.
- A. Sanna, M. R. Hall and M. M. Maroto-Valer, Energy Environ. Sci., 2012, 5, 7781–7796 CAS.
- G. Holmes and D. W. Keith, Philos. Trans. R. Soc., A, 2012, 370, 4380–4403 CrossRef CAS PubMed.
- K. Z. House, A. C. Baclig, M. Ranjan, E. A. van Nierop, J. Wilcox and H. J. Herzog, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(51), 20428–20433 CrossRef CAS PubMed.
- D. S. Goldberg, K. S. Lackner, P. Han, A. L. Slagle and T. Wang, Environ. Sci. Technol., 2013, 47, 7521–7529 CAS.
- M. D. Eisaman, K. Parajuly, A. Tuganov, C. Eldershaw, N. Chang and K. A. Littau, Energy Environ. Sci., 2012, 5, 7346–7352 CAS.
- G. H. Rau, Environ. Sci. Technol., 2011, 45, 1088–1092 CrossRef CAS PubMed.
- P. Renforth and T. Kruger, Energy Fuels, 2013, 27, 4199–4207 CrossRef CAS.
- S. S. Salek, R. Kleerebezem, H. M. Jonkers, G.-J. Witkamp and M. C. M. van Loosdrecht, Trends Biotechnol., 2013, 31, 139–146 CrossRef CAS PubMed.
- M. Verduyn, H. Boerrigter, R. Oudwater, G.A.F. van Mossel, 5th Trondheim CCS, 16–17th June 2009.
- M. Verduyn, H. Geerlings, G. van Mossel and S. Vijayakumari, Energy Procedia, 2011, 4, 2885–2892 CrossRef.
- H. Yan, J. Y. Zhang, Y. C. Zhao and C. G. Zheng, Sci. China: Technol. Sci., 2013, 56, 2219–2227 CrossRef CAS.
- A. Olajire, J. Pet. Sci. Eng., 2013, 109, 364–392 CrossRef CAS.
- R. Zevenhoven, J. Fagerlund and J. K. Songok, Greenhouse Gases: Sci. Technol., 2011, 1, 48–57 CrossRef CAS.
- E. R. Bobicki, Q. Liu, Z. Xu and H. Zeng, Prog. Energy Combust. Sci., 2012, 38, 302–320 CrossRef CAS.
- S.-Y. Pan, E. E. Chang and P.-C. Chiang, Aerosol Air Qual. Res., 2012, 12, 770–791 CAS.
- A. Sanna, M. Dri, M. R. Hall and M. Maroto-Valer, Appl. Energy, 2012, 99, 545–554 CrossRef CAS.
- A. Kirchofer, A. Becker, A. Brandt and J. Wilcox, Environ. Sci. Technol., 2013, 47, 7548–7554 CAS.
- A.-h. A. Park, P. Kelemen, J. Matter, G. Gadikota, Geo-Chemo-Mechanical Studies for Permanent Storage of CO2 in Geologic Formations, DE-FE0002386, Columbia University, New York, U.S. Department of Energy National Energy Technology Laboratory Carbon Storage R&D Project Review Meeting, August 21–23, 2012, http://www.netl.doe.gov/.
- R. Berner, A. Lasaga and R. Garrels, Am. J. Sci., 1983, 283, 641–683 CrossRef CAS.
- S. Gislason, E. Oelkers, E. Eiriksdottir, M. Kardjilov, G. Gisladottir, B. Sigfusso, A. Snorrason, S. Elefsen, J. Hardardottir, P. Torssander and N. Pskarsson, Earth Planet. Sci. Lett., 2009, 277, 213–222 CrossRef CAS.
- B. M. Reinhardt, Schweiz. Mineral. Petrogr. Mitt., 1969, 49, 1–30 CAS.
- F. Boudier and R. G. Coleman, J. Geophys. Res., 1981, 86, 2573–2592 CrossRef CAS.
- P. Kelem and J. Matter, PNAS, 2008, 105/45, 17295–17300 CrossRef.
- A. Paukert, J. Matter, P. Kelemen, E. Shock and J. Havig, Chem. Geol., 2012, 330/331, 86–100 CrossRef.
- G. D. Nicholls, Mineral. Mag., 1965, 34/268, 373–388 Search PubMed.
- C. Dessert, B. Dupre', J. Gaillardet, L. Francois and C. Allegre, Chem. Geol., 2003, 202, 257–273 CrossRef CAS.
- E. Rosenthal, J. Hydrol., 1987, 89/3–4, 329–352 CrossRef.
- J. Matter and T. Takahashi, Geochem., Geophys., Geosyst., 2007, 8/2, 1–19 Search PubMed.
- K. House, D. Schrag, C. Harvey and K. Lackner, PNAS, 2006, 103, 12291–12295 CrossRef CAS PubMed.
- P. Olsen, D. Kent, B. Cornet, W. Witte and R. Schlische, Geol. Soc. Am. Bull., 1996, 108/1, 40–77 Search PubMed.
- B. McGrail, A. Spane, E. Sullivan, D. Bacon and G. Hund, Energy Procedia, 2011, 4, 5653–5660 CrossRef.
- N. Zakharova, D. Goldberg, C. Sullivan, M. Herron and J. Grau, Geochem., Geophys., Geosyst., 2012, 13/11, 1–22 Search PubMed.
- T. Flaathen, S. Gislason, E. Oelkers and A. Sveinbjornsdottir, Appl. Geochem., 2009, 24, 463–474 CrossRef CAS.
- S. Gislason, D. Wolff-Boenisch, A. Stefansson, E. Olelkers, E. Gunnlaugsson, H. Sigurdardottir, B. Sigfusson, W. Broecker, J. Matter, M. Stute, G. Axelsson and T. Fridriksson, Int. J. Greenhouse Gas Control, 2010, 4, 537–545 CrossRef CAS.
- J. Matter and P. Kelemen, Nat. Geosci., 2009, 2, 837–841 CrossRef CAS.
- J. Matter, S. Broecker, S. Gislason, E. Gunnlaugsson, E. Oelkers, M. Stute, H. Sigurdardottir, A. Stefansson, H. Alfredsson, E. Aradottir, G. Axelsson, B. Sigfusson and D. Wolff-Boenisch, Energy Procedia, 2011, 4, 5579–5585 CrossRef CAS.
- B. Sigfusson, 8th CO2GeoNet Open Forum, S. Servolo, Italy 2013.
- D. Becker, EOS, Trans., Am. Geophys. Union, 1998, 79, 369–378 Search PubMed.
- D. Goldberg, T. Takahashi and A. Slagle, PNAS, 2008, 105/29, 9920–9925 CrossRef PubMed.
- P. Olsen, J. Smoot, P. Le Tourneau, Fieldtrip for the 11th Annual Continental Scientific Drilling Workshop, Arlington, 2007, 1–45.
- D. Folger, J. Hathaway, R. Christopher, P. Valentine and S. Poag, U.S. Geol. Surv. Circ., 1978, 773, 1–28 Search PubMed.
- T. Maguire, R. Sheridan and R. Volkert, Geodynamics, 2004, 37, 457–485 CrossRef.
- D. Goldberg, D. Kent and P. Olsen, PNAS, 2010, 107/4, 1327–1332 CrossRef PubMed.
- D. Goldberg and A. Slagle, Energy Procedia, 2009, 1, 3675–3682 CrossRef CAS.
- W. K. O'Connor, D. C. Dahlin, G. E. Rush, S. J. Gerdemann, L. R. Penner and D. N. Nilsen, Albany Research Centre. DOE/ARC–TR-04-002, USA, 2005.
- O. Levenspiel, Chemical Reaction Engineering, John Wiley & Sons, New York, 2nd edn, 1972 Search PubMed.
- S. Teir, H. Revitzer, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Int. J. Miner. Process., 2007, 83, 36–46 CrossRef CAS.
- T. A. Haug, R. A. Kleiv and I. A. Munz, Appl. Geochem., 2010, 25, 1547–1563 CrossRef CAS.
- M. Hänchen, S. Krevor, M. Mazzotti and K. Lackner, Chem. Eng. Sci., 2007, 62, 6412–6422 CrossRef.
- M. Hänchen, V. Prigiobbe, G. Storti, T. M. Seward and M. Mazzotti, Geochim. Cosmochim. Acta, 2006, 70, 4403–4416 CrossRef.
- T. A. Haug, I. A. Munz and R. A. Kleiv, Energy Procedia, 2011, 4, 5029–5036 CrossRef CAS.
- W. K. O'Connor, D. N. Nielsen, S. J. Gerdemann, G. E. Rush, R. P. Waltera and P. C. Turner, 18th Annual International Pittsburgh coal conference, Newcastle, Australia, 2001.
- B. Bonfils, C. Julcour-Lebigue, F. Guyot, F. Bodénan, P. Chiquet and F. Bourgeois, Int. J. Greenhouse Gas Control, 2012, 9, 334–346 CrossRef CAS.
- D. Daval, I. Martinez, J. Corvisier, N. Findling, B. Goffé and F. Guyot, Chem. Geol., 2009, 262, 262–277 CrossRef.
- G. J. Stockmann, D. Wolff-Boenisch, S. R. Gislason and E. H. Oelkers, Chem. Geol., 2013, 337–338, 56–66 CrossRef CAS.
- R. M. Santos and T. Van Gerven, Greenhouse Gases: Sci. Technol., 2011, 1, 287–293 CrossRef CAS.
- S. Kodama, T. Nishimoto, N. Yamamoto, K. Yogo and K. Yamada, Energy, 2008, 33, 776–784 CrossRef.
- R. Baciocchi, G. Costa, A. Polettini, R. Pomi and V. Prigiobbe, Energy Procedia, 2009, 1, 4851–4858 CrossRef CAS.
- X. Wang and M. M. Maroto-Valer, Fuel, 2011, 90, 1229–1237 CrossRef CAS.
- W. K. O'Connor, D. C. Dahlin, D. N. Nilsen, R. P. Walters, P. C. Turner, Proceedings of the 25th International Technical Conference on Coal Utilization & Fuel Systems, Clear Water, FL, 2000.
- W. J. J. Huijgen, R. N. J. Comans and G.-J. Witkamp, Energy Convers. Manage., 2007, 48, 1923–1935 CrossRef CAS.
- S. Arickx, T. Van Gerven and C. Vandecasteele, J. Hazard. Mater., 2006, B137, 235–243 CrossRef PubMed.
- X. Wang and M. M. Maroto-Valer, ChemSusChem, 2011, 4, 1291–1300 CrossRef CAS PubMed.
- W. J. J. Huijgen and R. N. J. Comans, ECN-C-03016 ECN-C-03-016, Energy Research Centre of the Netherlands, 2003.
- K. S. Lackner, D. P. Butt and C. H. Wendt, Energy Convers. Manage., 1997, 38, S259–S264 CrossRef CAS.
- H. F. DaCosta, M. Fan and A. T. Russell, 20100221163, 2010.
- S. Kwon, M. Fan, H. F. M. DaCosta and A. G. Russell, J. Environ. Sci., 2011, 23, 1233–1239 CrossRef CAS.
- S. Kwon, PhD dissertation, Georgia Institute of Technology, 2011.
- J. H. Kwak, J. Z. Hu, D. W. Hoyt, J. A. Sears, C. Wang, K. M. Rosso and A. R. Felmy, J. Phys. Chem., 2010, 114, 4126–4134 CAS.
- J. H. Kwak, J. Z. Hu, R. V. F. Turcu, K. M. Rosso, E. S. Ilton, C. Wang, J. A. Sears, M. H. Engelhard, A. R. Felmy and D. W. Hoyt, Int. J. Greenhouse Gas Control, 2011, 5, 1081–1092 CrossRef CAS.
- W. J. J. Huijgen, G. J. Ruijg, R. N. J. Comans and G.-J. Witkamp, Ind. Eng. Chem. Res., 2006, 45, 9184–9194 CrossRef CAS.
- T. A. Haug, Dissolution and carbonation of mechanically activated olivine: Investigating CO2 sequestration possibilities, PhD thesis, Norwegian University of Science and Technology, Trondheim, March 2010, ISBN 978-82-471-1961-7, http://www.diva-portal.org/smash/get/diva2:303780/FULLTEXT01.pdf.
- S. J. Gerdemann, W. K. O'Connor, D. C. Dahlin, L. R. Penner and H. Rush, Environ. Sci. Technol., 2007, 41, 2587–2593 CrossRef CAS PubMed.
- O. S. Pokrovsky and J. Schott, Geochim. Cosmochim. Acta, 1999, 63, 881–897 CrossRef CAS.
- W. J. J. Huijgen and R. N. J. Comans, ECN-C-05-022, Energy Research Centre of The Netherlands, Petten, The Netherlands, 2005.
- W. K. O'Connor, D. C. Dahlin, G. E. Rush, S. J. Gerdemann, L. R. Penner and R. P. Nielsen, Albany Research Center, DOE/ARC-TR-04-002, Albany, OR, USA, 2004.
- S. J. Gerdemann, D. C. Dahlin, W. K. O'Connor, 6th International Conference on Green House Gas Control Technologies, Kyoto, Japan, 2002.
- M. Fabian, M. Shopska, D. Paneva, G. Kadinov, N. Kostova, E. Turianicova, J. Briancin, I. Mitov, R. A. Kliev and P. Balaz, Miner. Eng., 2010, 23, 616–620 CrossRef CAS.
- P. Balaz, E. Turianicova, M. Fabian, R. A. Kliev, J. Briancin and A. Obut, Int. J. Miner. Process., 2008, 88, 1–6 CrossRef CAS.
- Chizmeshya et al., 2nd Annual Conference on Carbon Capture & Sequestration, Pittsburgh, 2002.
- P. Raschman, A. Fedoročková and G. Sučik, Hydrometallurgy, 2013, 139, 149–153 CrossRef CAS.
- H. Boerrigter, WO2008142025 A2, 2008.
- A. Fedoročkova, M. Hreus, P. Raschman and G. Sučik, Miner. Eng., 2012, 32, 1–4 CrossRef.
- R. D. Balucan, B. Z. Dlugogorski, E. M. Kennedy, I. V. Belovac and G. E. Murch, Int. J. Greenhouse Gas Control, 2013, 17, 225–239 CrossRef CAS.
- A. Sanna, X. Wang, A. Lacinska, M. Styles, T. Paulson and M. M. Maroto-Valer, Miner. Eng., 2013, 49, 135–144 CrossRef CAS.
- G. F. Brent, 20090305378, 2009.
- GCCS, Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide, P. Brinckerhoff, 2011, p. 279.
- Calera, 2013, Calera process, http://www.calera.com/index.php/technology/the_science/.
- S. O. Andersen, D. Zaelke, O. Young, H. Ahmadzai, F. Anderson, M. Atkinson, E. Carson, R. J. Carson, S. Christensen, J. S. J. Van Deventer, S. Hanford, V. Hoenig, A. Miller, M. Molina, L. Price, V. Ramanathan, H. Tope, J. Wilkinson, M. Yamabe, Scientific Synthesis of Calera Carbon Sequestration and Carbonaceous By-Product Applications, Consensus Findings of the Scientific Synthesis Team, January 2011, http://www.igsd.org/climate/documents/Synthesis_of_Calera_Technology_Jan2011.pdf.
- B. R. Constantz, et al., US8062418 B2, 2011 Search PubMed.
- B. R. Constantz, et al., US2009001020 A1, 2009 Search PubMed.
- J. D. Jones, US Pat., 7,727,374, Skyonic Corporation, 2010 Search PubMed.
- Skyonic, 2013, http://skyonic.com/wp-content/uploads/2010/02/Skyonic-Groundbreaking-Release-September-30-2013.pdf.
- J. I. Drever and L. L. Stillings, Colloids Surf., A, 1997, 120, 167–181 CrossRef CAS.
- J. Declercq, O. Bosc and E. H. Oelkers, Appl. Geochem., 2013, 39, 69–77 CrossRef CAS.
- A. A. Olsen and J. D. Rimstidt, Geochim. Cosmochim. Acta, 2008, 72, 1758–1766 CrossRef CAS.
- S. Eloneva, A. Said, C.-J. Fogelholm and R. Zevenhoven, Appl. Energy, 2012, 90, 329–334 CrossRef CAS.
- I. A. Munz, J. Kihle, Ø. Brandvoll, I. Manchenbach, J. W. Carey, T. A. Haug, H. Johansen and N. Eldrup, Energy Procedia, 2009, 1, 4891–4898 CrossRef CAS.
- P. C. Lin, C. W. Huang, C. T. Hsiao and H. Teng, Environ. Sci. Technol., 2008, 42, 2748–2752 CrossRef CAS PubMed.
- M. M. Maroto-Valer, D. J. Fauth, M. E. Kuchta, Y. Zhang and J. M. Andrésen, Fuel Process. Technol., 2005, 86, 1627–1645 CrossRef CAS.
- S. C. M. Krevor and K. S. Lackner, Energy Procedia, 2009, 1, 4867–4871 CrossRef CAS.
- S. C. M. Krevor and K. S. Lackner, Int. J. Greenhouse Gas Control, 2011, 5, 1073–1080 CrossRef CAS.
- J. Baldyga, M. Henczka and K. Sokolnicka, Mater. Lett., 2010, 64, 702–704 CrossRef CAS.
- A.-H. A. Park, R. Jadhav and L.-S. Fan, Can. J. Chem. Eng., 2003, 81, 885–890 CrossRef CAS.
- A.-H. A. Park and L.-S. Fan, Chem. Eng. Sci., 2004, 59, 5241–5247 CrossRef CAS.
- E. Gal, WO/2006/022885, 2006.
- A. Kothandaraman, PhD thesis, Massachusetts Institute of Technology, 2010, http://sequestration.mit.edu/pdf/Anusha_Kothandaraman_thesis_June2010.pdf.
- X. Wang and M. M. Maroto-Valer, Energy, 2013, 51, 431–438 CrossRef CAS.
- J. Fagerlund, E. Nduangu and R. Zevenhoven, Energy Procedia, 2011, 4, 4993–5000 CrossRef CAS.
- J. Fagerlund, E. Nduangu, I. Romão and R. Zevenhoven, Energy, 2012, 41, 184–191 CrossRef CAS.
- J. Fagerlund, J. Highfield and R. Zevenhoven, RSC Adv., 2012, 2, 10380–10393 RSC.
- J. Highfield, H. Q. Lim, J. Fagerlund and R. Zevenhoven, RSC Adv., 2012, 2, 6535–6541 RSC.
- E. Nduagu, T. Björklöf, J. Fagerlund, J. Wärnå, H. Geerlings and R. Zevenhoven, Miner. Eng., 2012, 30, 75–87 CrossRef CAS.
- R. Hunwick, AU2008/000232, 2008.
- W.K. O'Connor, D. C. Dahlin, D. C. Nilsen, S. J. Gerdemann, G. E. Rush, L. R. Penner, R. P. Walters, P. C. Turner, 27th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 2002.
- J.-H. Wee, Appl. Energy, 2013, 106, 143–151 CrossRef CAS.
- S. Eloneva, S. Teir, J. Salminen, C.-J. Fogelholm and R. Zevenhoven, Ind. Eng. Chem. Res., 2008, 47, 7104–7111 CrossRef CAS.
- W. J. J. Huijgen and R. N. J. Comans, Environ. Sci. Technol., 2006, 40, 2790–2796 CrossRef CAS PubMed.
- D. N. Huntzinger, J. S. Gierke, L. L. Sutter, S. K. Kawatrad and T. C. Eisele, J. Hazard. Mater., 2009, 168, 31–37 CrossRef CAS PubMed.
- O. Velts, M. Uibu, J. Kallas and R. Kuusik, J. Hazard. Mater., 2011, 195, 139–146 CrossRef CAS PubMed.
- G. Costa, R. Baciocchi, A. Polettini, R. Pomi, C. D. Hills and P. J. Carey, Environ. Monit. Assess., 2007, 135, 55–75 CrossRef CAS PubMed.
- E. E. Chang, C.-H. Chen, Y.-H. Chen, S.-Y. Pan and P.-C. Chiang, J. Hazard. Mater., 2011, 186, 558–564 CrossRef CAS PubMed.
- D. N. Huntzinger, J. S. Gierke, K. Kawatra, T. C. Eisele and L. L. Sutter, Environ. Sci. Technol., 2009, 43, 1986–1992 CrossRef CAS PubMed.
- S. Teir, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Energy, 2007, 32, 528–539 CrossRef CAS.
- S. Eloneva, S. Teir, J. Salminen, C.-J. Fogelholm and R. Zevenhoven, Energy, 2008, 33, 1461–1467 CrossRef CAS.
- R. M. Santos, D. François, G. Mertens, J. Elsen and T. Van Gerven, Appl. Therm. Eng., 2013, 57, 154–163 CrossRef CAS.
- S. Eloneva, Doctoral Dissertation Doctoral Dissertation, Aalto University School of Science and Technology, 2010. http://https://aaltodoc.aalto.fi/bitstream/handle/123456789/4876/isbn9789526034577.pdf?sequence=1.
- S. Monkman and Y. Shao, J. Mater. Civ. Eng., 2006, 18, 768–776 CrossRef CAS.
- F. J. Doucet, Miner. Eng., 2009, 23, 262–269 CrossRef.
- W. J. J. Huijgen, G.-J. Witkamp and R. N. J. Comans, Environ. Sci. Technol., 2005, 39, 9676–9682 CrossRef CAS PubMed.
- E. E. Chang, S.-Y. Pan, Y.-H. Chen, C.-S. Tan and P.-C. Chiang, J. Hazard. Mater., 2012, 227–228, 97–106 CrossRef CAS PubMed.
- E. E. Chang, A.-C. Chiu, S.-Y. Pan, Y.-H. Chen, C.-S. Tan and P.-C. Chiang, Int. J. Greenhouse Gas Control, 2013, 12, 382–389 CrossRef CAS.
- D. Bonenfant, L. Kharoune, S. Sauve, R. Hausler, P. Niquette, M. Mimeault and M. Kharoune, Ind. Eng. Chem. Res., 2008, 47, 7610–7616 CrossRef CAS.
- M. Uibu, R. Kuusik, L. Andreas and K. Kirsimäe, Energy Procedia, 2011, 4, 925–932 CrossRef CAS.
- R. Baciocchi, G. Costa, E. Di Bartolomeo, A. Polettini and R. Pomi, Greenhouse Gases: Sci. Technol., 2011, 1, 312–319 CrossRef CAS.
- R. M. Santos, M. Bodor, P. N. Dragomir, A. G. Vraciu, M. Vlad and T. Van Gerven, Miner. Eng., 2014, 59, 71–81 CrossRef CAS.
- R. M. Santos, J. Van Bouwel, E. Vandevelde, G. Mertens, J. Elsen and T. Van Gerven, Int. J. Greenhouse Gas Control, 2013, 17, 32–45 CrossRef CAS.
- Y. Katsuyama, A. Yamasaki, A. Iizuka, M. Fujii, K. Kumagai and Y. Yanagisawa, Environ. Prog., 2005, 24, 162–170 CrossRef CAS.
- P. J. Gunning, C. D. Hills and P. J. Carey, Waste Manage., 2009, 29, 2722–2729 CrossRef CAS PubMed.
- P. J. Gunning, C. D. Hills and P. J. Carey, Waste Manage., 2010, 30, 1081–1090 CrossRef CAS PubMed.
- S. Teramura, N. Isu and K. Inagaki, J. Mater. Civ. Eng., 2000, 12, 288–293 CrossRef CAS.
- S. Kashef-Haghighi and S. Ghoshal, Ind. Eng. Chem. Res., 2009, 49, 1143–1149 CrossRef.
- M. Fernández Bertos, X. Li, S. J. R. Simons, C. D. Hills and P. J. Carey, Green Chem., 2004, 6, 428–436 RSC.
- J. Sun, M. Fernandez Bertos and S. J. R. Simons, Energy Environ. Sci., 2008, 1, 370–377 CAS.
- G. Montes-Hernandez, R. Perez-Lopez, F. Renard, J. M. Nieto and L. Charlet, J. Hazard. Mater., 2009, 161, 1347–1354 CrossRef CAS PubMed.
- M. G. Nyambura, G. W. Mugera, P. L. Felicia and N. P. Gathura, J. Environ. Manage., 2011, 92, 655–664 CrossRef CAS PubMed.
- K. J. Reddy, S. John, H. Weber, M. D. Argyle, P. Bhattacharyya, D. T. Taylor, M. Christensen, T. Foulke and P. Fahlsing, Energy Procedia, 2011, 4, 1574–1583 CrossRef CAS.
- G. N. Muriithi, L. F. Petrik, O. Fatoba, W. M. Gitari, F. J. Doucet, J. Nel, S. M. Nyale and P. E. Chuks, J. Environ. Manage., 2013, 127, 212–220 CrossRef CAS PubMed.
- M. Back, M. Kuehn, H. Stanjek and S. Peiffer, Environ. Sci. Technol., 2008, 42, 4520–4526 CrossRef CAS PubMed.
- M. C. Mayoral, J. M. Andrés and M. P. Gimeno, Fuel, 2013, 106, 448–454 CrossRef CAS.
- R. Kuusik, M. Uibu and K. Kirsimäe, Oil Shale, 2005, 22, 407–420 CAS.
- M. Uibu, M. Uus and R. Kuusik, J. Environ. Manage., 2009, 90, 1253–1260 CrossRef CAS PubMed.
- R. C. Sahu, R. K. Patel and B. C. Ray, J. Hazard. Mater., 2010, 179, 29–34 Search PubMed.
- S. Teir, S. Eloneva, C.-J. Fogelholm and R. Zevenhoven, Appl. Energy, 2009, 86, 214–218 CrossRef CAS.
- F. Larachi, I. Daldoul and G. Beaudoin, Geochim. Cosmochim. Acta, 2010, 74, 3051–3074 CrossRef CAS.
- S. Teir, R. Kuusik, C.-J. Fogelholm and R. Zevenhoven, Int. J. Miner. Process., 2007, 85, 1–15 CrossRef CAS.
- D. Bonenfant, L. Kharoune, S. b. Sauve, R. Hausler, P. Niquette, M. Mimeault and M. Kharoune, Ind. Eng. Chem. Res., 2008, 47, 7617–7662 CrossRef CAS.
- V. S. Yadav, M. Prasad, J. Khan, S. S. Amritphale, M. Singh and C. B. Raju, J. Hazard. Mater., 2010, 176, 1044–1050 CrossRef CAS PubMed.
- R. Perez-Lopez, G. Montes-Hernandez, J. M. Nieto, F. Renard and L. Charlet, Appl. Geochem., 2008, 23, 2292–2300 CrossRef CAS.
- M. Uibu, O. Velts and R. Kuusik, J. Hazard. Mater., 2010, 174, 209–214 CrossRef CAS PubMed.
- A. A. Olajire, Energy, 2010, 35, 2610–2628 CrossRef CAS.
- A. Iizuka, M. Fujii, A. Yamasaki and Y. Yanagisawa, Ind. Eng. Chem. Res., 2004, 43, 7880–7887 CrossRef CAS.
- B. Fernández Bertos, S. J. R. Simons, C. D. Hills and P. J. Carey, J. Hazard. Mater., 2004, B112, 193–205 CrossRef PubMed.
- M. Uibu and R. Kuusik, Oil Shale, 2009, 26, 40–58 CrossRef CAS.
- D. K. Lee, Chem. Eng. J., 2004, 100, 71–77 CrossRef CAS.
- S.-M. Shih, C. u.-S. Ho, Y.-S. Song and J.-P. Lin, Ind. Eng. Chem. Res., 1999, 38, 1316–1322 CrossRef CAS.
- S. N. Lekakh, C. H. Rawlins, D. G. C. Robertson, V. L. Richards and K. D. Peaslee, Metall. Mater. Trans. B, 2008, 39, 125–134 CrossRef.
- C. S. Gahan, M. L. Cunha and Å. Sandström, Hydrometallurgy, 2009, 95, 190–197 CrossRef CAS.
- J. K. Stolaroff, G. V. Lowry and D. W. Keith, Energy Convers. Manage., 2005, 46, 687–699 CrossRef CAS.
- M. Dri, A. Sanna and M. M. Maroto-Valer, Fuel Process. Technol., 2013, 113, 114–122 CrossRef CAS.
- M. Dri, A. Sanna and M. M. Maroto-Valer, Appl. Energy, 2014, 113, 515–523 CrossRef CAS.
- L. Wang, Y. Jin and Y. Nie, J. Hazard. Mater., 2010, 174, 334–343 CrossRef CAS PubMed.
- X. Li, M. Fernandez Bertos, C. D. Hills, P. J. Carey and S. Simon, Waste Manage., 2007, 27, 1200–1206 CrossRef CAS PubMed.
- E. Rendek, G. Ducom and P. Germain, J. Hazard. Mater., 2006, B128, 73–79 CrossRef PubMed.
- T. Van Gerven, E. Van Keer, S. Arickx, M. Jaspers, G. Wauters and C. Vandecasteele, Waste Manage., 2005, 25, 291–300 CrossRef CAS PubMed.
- J. A. Meima, R. D. van der Weijden, T. T. Eighmy and R. N. J. Comans, Appl. Geochem., 2002, 17, 1503–1513 CrossRef CAS.
- A. Polettini and R. Pomi, J. Hazard. Mater., 2004, B113, 209–215 CrossRef PubMed.
- R. Baciocchi, G. Costa, E. Lategano, C. M. Polettini, R. Pomi, P. Postorino and S. Rocca, Waste Manage., 2010, 30, 1310–1317 CrossRef CAS PubMed.
- R. Baciocchi, A. Polettini, R. Pomi, V. Prigiobbe, V. Von Zedwitz and A. Steinfeld, Energy Fuels, 2006, 20, 1933–1940 CrossRef CAS.
- H. Ecke, Waste Manage., 2003, 23, 631–640 CrossRef CAS PubMed.
- Y. Soong, D. L. Fauth, B. H. Howard, R. J. Jones, D. K. Harrison, A. L. Goodman, M. L. Gray and E. A. Frommell, Energy Convers. Manage., 2005, 47, 1676–1685 CrossRef.
- D. N. Huntzinger and T. D. Eatmon, J. Cleaner Prod., 2009, 17, 668–675 CrossRef CAS.
- Accelerating the uptake of CCS: industrial use of captured carbon dioxide, Parsons Brinckerhoff and Global CCS Institute, 2011. http://www.globalccsinstitute.com/publications/accelerating-uptake-ccs-industrial-use-captured-carbon-dioxide.
- S. A. Wilson, G. M. Dipple, I. M. Power, J. M. Thom, R. G. Anderson, M. Raudsepp, J. E. Gabites and G. Southam, Econ. Geol., 2009, 104, 95–112 CrossRef CAS.
- H. C. Oskierski, B. Z. Dlugogorski and G. Jacobsen, Chem. Geol., 2013, 358, 156–169 CrossRef CAS.
- Y. W. Chiang, R. M. Santos, A. Monballiu, K. Ghyselbrecht, J. A. Martens, M. L. T. Mattos, T. V. Gerven and B. Meesschaert, Miner. Eng., 2013, 48, 116–125 CrossRef CAS.
- M. Hitch, S. M. Ballantyne and S. R. Hindle, Int. J. Min., Reclam. Environ., 2009, 24, 64–79 CrossRef.
- R. Dilmore, P. Lu, D. Allen, Y. Soong, S. Hedges, J. K. Fu, C. L. Dobbs, A. Degalbo and C. Zhu, Energy Fuels, 2007, 22, 343–353 CrossRef.
- M. Johnston, M. W. Clark, P. McMahon and N. Ward, J. Hazard. Mater., 2010, 182, 710–715 CrossRef CAS PubMed.
- C. Si, Y. Ma and C. Lin, J. Hazard. Mater., 2013, 244–245, 54–59 CrossRef CAS PubMed.
- S. Teir, J. Kettle, A. Harlin, J. Sarlin, Proceedings of the 3rd International Conference on Accelerated Carbonation for Environmental and Materials Engineering, ACEME10, Turku, Finland, Nov. 29–Dec. 1, 2009, 63–74.
- S. Teir, S. Eloneva and R. Zevenhoven, Energy Convers. Manage., 2005, 46, 2954–2979 CrossRef CAS.
- A.-H. Park and L.-S. Fan, US7,722,842 B2, 2010.
- S. M. El-Sheikh, S. El-Sherbiny, A. Barhoum and Y. Deng, Colloids Surf., A, 2013, 422, 44–49 CrossRef CAS.
- P. Gronchi, T. De Marco and L. Cassar, US6,716,408 B1, 2004.
- A. Sanna, in Fossil Fuels: Sources, Environmental Concerns and Waste Management Practices, ed. R. Kumar, Nova Science Pub. Inc., New York, 2013, ch. 6, pp. 199–208 Search PubMed.
- R. E. F. Lindeboom, I. Ferrer, J. Weijma and J. B. van Lier, Water Res., 2010, 47, 3742–3751 CrossRef PubMed.
- C. Y. Tai and F. B. Chen, AIChE J., 1998, 1790–1798 CrossRef CAS.
- G. Li, Z. Li and H. Ma, Int. J. Miner. Process., 2013, 123, 25–31 CrossRef CAS.
- S. Wanjari, C. Prabhu, N. Labhsetwar and S. Rayalu, J. Mol. Catal. B: Enzym., 2013, 93, 15–22 CrossRef CAS.
- N. Favre, M. L. Christ and A. C. Pierre, J. Mol. Catal. B: Enzym., 2009, 60, 163–170 CrossRef CAS.
- P. C. Sahoo, Y. N. Jang and S. W. Lee, J. Cryst. Growth, 2013, 373, 96–101 CrossRef CAS.
- A. Said, H.-P. Mattila, M. Järvinen and R. Zevenhoven, Appl. Energy, 2012, 112, 765–771 CrossRef.
- M. Faatz, F. Gröhn and G. Wegner, Adv. Mater., 2004, 16, 996–1000 CrossRef CAS.
- G. Montes-Hernandez, F. Renard, R. Chiriac, N. Findling, J. Ghanbaja and F. Toche, Chem. Eng. J., 2013, 214, 139–148 CrossRef CAS.
- W. Wang, S. Wang, X. Ma and J. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazábal and J. Peréz-Ramírez, Energy Environ. Sci., 2013, 6, 3112–3135 CAS.
- J. Qiao, Y. Liu, F. Hong and J. Zhang, Chem. Soc. Rev., 2014, 43, 631–675 RSC.
- W. S. Broecker, Elements, 2008, 4, 4295–4297 Search PubMed.
- F. Bodénan, F. Bourgeois, C. Petiot, T. Augé, B. Bonfils, C. Julcour, F. Guyot, A. Boukary, J. Tremosa, A. Lassin, P. Chiquet, 4th International Conference on Accelerated Carbonation for Environmental and Materials Engineering, 10–12 Apr 2013, Leuven, Belgium.
- M. Hitch and G. M. Dipple, Miner. Eng., 2012, 39, 268–275 CrossRef CAS.
- K. J. Reddy, B. Reynolds and M. D. Argyle, in Proceedings of the 4th international Conference on Accelerated Carbonation for Environmental and Materials Engineering, Leuven, Belgium, 2013, pp. 175–183.
- P. J. Gunning, C. D. Hills and P. J. Carey, in Proceedings of the 4th International Conference on Accelerated Carbonation and Materials Engineering Leuven, Belgium, 2013, pp. 185–192.
- G. Jones, G. Joshi, M. Clark and D. McConchie, Environ. Chem., 2006, 3, 297–303 CrossRef CAS.
- B. E. H. Jones and R. J. Haynes, Crit. Rev. Environ. Sci. Technol., 2011, 41, 271–315 CrossRef CAS.
- D. J. Cooling, in Paste 2007—Proceedings of the Tenth International Seminar on Paste and Thickened Tailings, Australian Center for Geomechanics, ed. A. B. Fourie and R. J. Jewell, Perth, Australia, 2007, pp. 3–15 Search PubMed.
- S. R. Gislason and E. H. Oelkers, Science, 2014, 344, 373–374 CrossRef CAS PubMed.
- J. K. Stolaroff, G. V. Lowry and D. W. Keith, Energy Convers. Manage., 2005, 46, 687–699 CrossRef CAS.
- GCCSI, Strategic Analysis of the Global Status of Carbon Capture and Storage Report 2: Economic Assessment of Carbon Capture and Storage Technologies. 2009. Global CCS Institute, GPO Box 828, Canberra ACT 2601 Australia.
- IMC, 2008, Alberta CO2 Capture Cost Survey and Supply Curve, prepared for the Alberta CCS Development Council. Ian Murray and Co. Ltd. http://www.canadiancleanpowercoalition.com/.
- CSLF, 2012, Annual Meeting Documents Book, CSLF-T-2012-10, 4 September 2012, Perth, Australia.
| This journal is © The Royal Society of Chemistry 2014 |