Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective nucleation of iron phthalocyanine crystals on micro-structured copper iodide
Luke A.
Rochford
*a,
Alexandra J.
Ramadan
b,
Sandrine
Heutz
b and
Tim S.
Jones
a
aDepartment of Chemistry, The University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK. E-mail: l.rochford@warwick.ac.uk
bDepartment of Materials, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
First published on 20th October 2014
Abstract
Morphological and structural control of organic semiconductors through structural templating is an efficient route by which to tune their physical properties. The preparation and characterisation of iron phthalocyanine (FePc)–copper iodide (CuI) bilayers at elevated substrate temperatures is presented. Thin CuI(111) layers are prepared which are composed of isolated islands rather than continuous films previously employed in device structures. Nucleation in the early stages of FePc growth is observed at the edges of islands rather than on the top (111) faces with the use of field emission scanning electron microscopy (FE-SEM). Structural measurements show two distinct polymorphs of FePc, with CuI islands edges nucleating high aspect ratio FePc crystallites with modified intermolecular spacing. By combining high substrate temperature growth and micro-structuring of the templating CuI(111) layer structural and morphological control of the organic film is demonstrated.
Introduction
Control of morphology and crystal structure in organic semiconductor films affords the ability to tune their physical and electronic properties for specific device applications.1–3 Inorganic thin films have been widely employed as structural modification layers for the improvement of device parameters.4,5 A significant example of this methodology is the insertion of an evaporated copper(I) iodide layer to alter the structure of a planar metallo-phthalocyanine (MPc) epilayer.6 The effect of such a structural modification has been observed in organic electronic devices7 and explored using theoretical simulations.8 Our recent work has elucidated the structure of the templating effect of highly crystalline films of CuI on both planar9 and non-planar MPc molecules.The crystal structure of planar Pcs is inherently anisotropic due to face-to-face packing motifs leading to short intermolecular distances within stacks and larger inter-stack spacings.10 Structurally templating allows control of which of these spacings is predominantly observed in or out of the substrate plane. This in turn allows favorable charge transport pathways to be aligned in specific device architectures composed of crystalline thin films of MPc molecules.
In previous work on CuI templating we employed a continuous, highly crystalline, (111) oriented CuI layer and observed its influence on FePc layers grown at ambient substrate temperatures. Here we report the preparation and characterization of evaporated iron phthalocyanine (FePc)–CuI bilayer thin films at elevated substrate temperatures. The CuI films employed here are composed of isolated islands of CuI rather than continuous films, produced through deliberate micro-structuring induced by increasing substrate temperature during CuI growth. Crystallization sites in the early stages of FePc growth are observed at the edges of islands rather than on the top (111) faces. This leads to the growth of high aspect ratio needle-like crystallites in contrast to the continuous films previously observed in ambient temperature preparation of FePc on continuous CuI(111) films.9 XRD patterns of the films produced using this methodology cannot be indexed using the single crystal structure of FePc, and the presence of a new structural polymorph is suggested.
Experimental details
Copper iodide (Sigma Aldrich, UK) was used as received and evaporated from a home-built evaporator at 340 °C at a rate of 0.5 Å s−1 as measured by a calibrated quartz crystal microbalance. FePc (Sigma Aldrich, UK) was triply purified by thermal gradient sublimation and the resulting crystals were used for growth from a home-built evaporator at 375 °C at a rate of 0.2 Å s−1.All films were grown in a custom-built ultra-high vacuum (UHV) chamber with a base pressure of 3 × 10−9 mbar in which organic and inorganic materials were sublimed onto substrates held at elevated temperature. The substrate temperature was measured using a K-type thermocouple mounted close to the sample and calibrated using an optical pyrometer (IRCON®). Substrates were 10 × 10 mm pieces of thermally oxidised silicon (100) single crystal (IDB technologies, UK) cleaned in acetone, Decon-90®–deionised water mix and isopropanol. These were dried in a stream of dry nitrogen and UV-ozone cleaned before being loaded into vacuum.
Thin film XRD patterns were obtained using a PANalytical X'Pert Pro MRD diffractometer with monochromatic Cu Kα1 radiation. Electron micrographs were recording using field emission scanning electron microscopy (FE-SEM, Zeiss Supra 55VP) with no additional film coating step. Atomic force microscopy (AFM) images were recorded using an Asylum research MFP-3D in AC mode (tapping mode) using Olympus AC240-TS silicon tips.
Results
High substrate temperatures are known to induce larger crystallite sizes in crystalline films of planar MPcs.11 To establish the behavior of FePc at high substrate temperatures, film growth was performed on clean SiO2 substrates. When FePc was grown at a substrate temperature of 180 °C to a thickness of 50 nm largely continuous films with interlocking grains were produced (Fig. 1). Angular holes and voids are observed as previously seen in high temperature growth on single crystal sapphire,12 and the structure is consistent with a highly crystalline (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) oriented (from comparison to CCDC no. 996880) layer by XRD.
) oriented (from comparison to CCDC no. 996880) layer by XRD.
 | ||
| Fig. 1 (a) AFM topography image (b) XRD pattern and (c) SEM image of 50 nm FePc–SiO2 film grown at a substrate temperature of 180 °C. A schematic of the FePc molecule is shown in (d). | ||
CuI films were then prepared for use as structural templating layers for high substrate temperature FePc film deposition. As previously reported, CuI thin films grow with their (111) plane oriented parallel to the substrate plane at ambient and elevated temperatures on SiO2 substrates.9 At high growth temperatures (Tsub = 180 °C), 15 nm thick films consist of isolated islands with predominantly hexagonal growth habits (Fig. 2). The height of these islands is up to 70 nm and a distribution of island heights is present with bare substrate between the crystallites. These micro-structured films were used at their growth temperature (180 °C) as substrates for the subsequent growth of FePc films.
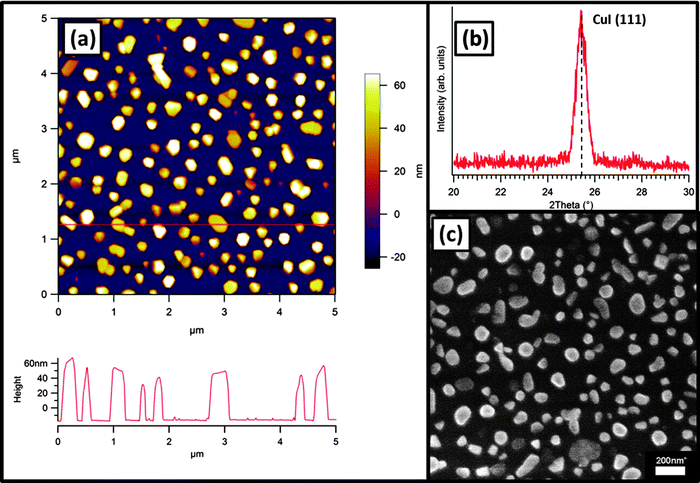 | ||
| Fig. 2 (a) AFM image with corresponding cross-section (corresponds to line in image) (b) XRD pattern and (c) SEM image of 15 nm CuI(111)–SiO2 film grown at a substrate temperature of 180 °C. | ||
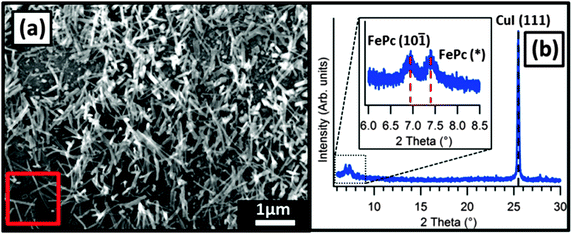
In 10 nm thick FePc films on CuI a sparse covering of high aspect ratio FePc needles (through which the underlying CuI(111) islands were visible) was observed (Fig. 3). A large spread in the lengths of these features was observed from SEM images and the largest dimension was approximately 1 μm. The shorter dimensions were more uniform with a value of approximately 50 nm for all of the needles present in this film. On closer inspection the distribution of these features across the surface was not random and seemed to be related to the morphology of the underlying CuI(111) islands. Crystals nucleated at the edges, rather than on the faces, of the CuI(111) islands and grew faster in one direction producing needle-like morphology; crystallites also seem to be protruding away from the substrate. Structural analysis by X-ray diffraction was attempted for this film but no peaks corresponding to FePc were observed due to the small amount of material (and therefore diffracting planes) present.
Thicker films were then grown (50 nm FePc) to observe the evolution of the film morphology. The length of the needle-like FePc crystals clearly increases with deposition time (longest observed dimension of approximately 4 μm) suggesting that they continue to grow throughout the deposition process rather than exclusively favoring nucleation of more crystallites (Fig. 4). A single growth direction is not evident as might be expected if the crystallites adopted a fixed orientation with respect to the CuI(111) faces. Areas with similar appearance to FePc films grown on bare SiO2 are also evident and exhibit the same angular voids as previously mentioned. This suggests that the growth of FePc still occurs in the voids between the CuI(111) islands (which are composed of clean SiO2) in the same (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) orientation already discussed.
) orientation already discussed.
Discussion
Previous studies have shown that when grown at ambient substrate temperature on a polycrystalline CuI(111) surface FePc molecules adopt a “lying down” orientation with the molecular plane parallel to the substrate surface.9 The CuI(111) surface studied here is different as the overall coverage of the surface by the overlayer is far lower, consisting of hexagonal crystallites surrounded by bare SiO2. As shown in SEM images, nucleation of FePc occurs at the edges of the CuI islands, which is not possible on complete CuI(111) films due to lack of exposed crystallite edges. The nature of these edges is therefore intrinsically important in the nucleation process of the FePc overlayer. Under ambient conditions CuI adopts a zinc blende face centred cubic (FCC) unit cell, as such the crystallite edges should correspond to the (100), (001) and (010) Miller planes. In this system the (100) and (001) planes are equivalent resulting in two possible orientations for the edges of the CuI crystallites, classified as {100}.Planar MPcs have been shown to exhibit multiple polymorphs, the most common of those found in thin films typically termed α and β, with substrate temperatures below 200 °C leading to the α-polymorph.13 Increasing growth temperature or post-growth annealing have been shown produce the β polymorph.14 The tilt angle between MPc molecules is inherently different in the films composed of each polymorph. High temperature growth leads to a reduction of the distances between molecular columns, manifested in XRD patterns through equivalent peaks shifting to slightly higher angles than those of the α-polymorph. The additional FePc peak present at 2θ = 7.4° (FePc(*), Fig. 4(b)) in the high-temperature templated film, which cannot be interpreted with previously reported FePc structures (CCDC no. 996880 and Kirner et al.15) and may therefore similarly be attributed to a new polymorph with reduced edge-to-edge intermolecular spacing. The central atom of the MPc influences the observed unit cell, but despite this the overall effects of the α-to-β transition upon XRD patterns are the same so comparison between planar phthalocyanines remains appropriate. Similar XRD patterns were previously observed in films of the β-polymorphs of the parent metal-free phthalocyanine (H2Pc)13 and copper(II) phthalocyanine (CuPc).16 While polymorphism in FePc has been suggested in the literature17 no structural data containing atomic positions suitable for XRD pattern simulation can account for diffraction at 2θ = 7.4°. The change in peak position is, however, consistent with a polymorph transition in FePc although full structural analysis is not possible from such a small number of peaks.
Conclusion
Structural analysis suggests that the FePc grown on an incomplete CuI(111) layer consists of two distinct polymorphs of FePc. Edges of the CuI islands act as nucleation sites for the growth of high aspect ratio FePc crystallites which contain intermolecular spacings which cannot be indexed with a single FePc crystal structure. The change in d-spacing evident from the difference in peak position between the two FePc peaks is consistent with the presence of multiple polymorphs in other MPcs. The morphology of these crystallites is unlike that of FePc grown at high substrate temperatures on SiO2 and at ambient temperature on complete CuI(111) layers. This confirms that the combination of increased substrate temperature and the presence of the edges of CuI(111) islands is responsible for the growth of the polymorphic crystals.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Funding sources
LAR and TSJ acknowledge support from the Engineering and Physical Sciences Research Council (EPSRC), UK (Grant no. EP/H021388/1). AJR and SH thank the EPSRC for financial support via the Doctoral Training Centre in Plastic Electronics (Grant no. EP/G037515/1).Acknowledgements
LAR and TSJ acknowledge support from the Engineering and Physical Sciences Research Council, UK (Grant no. EP/H021388/1). AJR and SH thank the EPSRC for financial support via the Doctoral Training Centre in Plastic Electronics (Grant no. EP/G037515/1). The PANalytical MRD diffractometer used in this research was obtained through the Science City Advanced Materials Project: Creating and Characterizing Next Generation Advanced Materials, with support from Advantage West Midlands (AWM) and part funded by the European Regional Development Fund (ERDF).References
- S. Heutz, R. Cloots and T. S. Jones, Appl. Phys. Lett., 2000, 77, 3938 CrossRef CAS PubMed.
- P. Sullivan, T. S. Jones, A. J. Ferguson and S. Heutz, Appl. Phys. Lett., 2007, 91, 233114 CrossRef PubMed.
- W. Wu, L. A. Rochford, S. Felton, Z. Wu, J. L. Yang, S. Heutz, G. Aeppli, T. S. Jones, N. M. Harrison and A. J. Fisher, J. Appl. Phys., 2013, 113, 013914 CrossRef PubMed.
- Y. Zhou, T. Taima, T. Miyadera, T. Yamanari, M. Kitamura, K. Nakatsu and Y. Yoshida, Nano Lett., 2012, 12, 4146–4152 CrossRef CAS PubMed.
- S. R. Forrest, Chem. Rev., 1997, 97, 1793–1896 CrossRef CAS PubMed.
- C. H. Cheng, J. Wang, G. T. Du, S. H. Shi, Z. J. Du, Z. Q. Fan, J. M. Bian and M. S. Wang, Appl. Phys. Lett., 2010, 97, 083305 CrossRef PubMed.
- T.-M. Kim, H. J. Kim, H.-S. Shim, M.-S. Choi, J. W. Kim and J.-J. Kim, J. Mater. Chem. A, 2014, 2, 8730 CAS.
- B. P. Rand, D. Cheyns, K. Vasseur, N. C. Giebink, S. Mothy, Y. Yi, V. Coropceanu, D. Beljonne, J. Cornil, J.-L. Brédas and J. Genoe, Adv. Funct. Mater., 2012, 22, 2987–2995 CrossRef CAS.
- L. A. Rochford, D. S. Keeble, O. J. Holmes, G. J. Clarkson and T. S. Jones, J. Mater. Chem. C, 2014, 2, 6056 RSC.
- A. Hoshino, Y. Takenaka and H. Miyaji, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 393–403 Search PubMed.
- J. E. S. Kim, E. Lim, K. Lee, D. Cha and B. Friedman, Appl. Surf. Sci., 2003, 205, 274–279 CrossRef.
- C. Miller, A. Sharoni, G. Liu, C. Colesniuc, B. Fruhberger and I. Schuller, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104113 CrossRef.
- S. M. Bayliss, S. Heutz, G. Rumbles and T. S. Jones, Phys. Chem. Chem. Phys., 1999, 1, 3673–3676 RSC.
- S. Heutz, S. M. Bayliss, R. L. Middleton, G. Rumbles and T. S. Jones, J. Phys. Chem. B, 2000, 104, 7124–7129 CrossRef CAS.
- J. F. Kirner, W. Dow and W. R. Scheidt, Inorg. Chem., 1976, 15, 1685–1690 CrossRef CAS.
- S. Heutz, C. Mitra, W. Wu, A. J. Fisher, A. Kerridge, M. Stoneham, A. H. Harker, J. Gardener, H.-H. Tseng, T. S. Jones, C. Renner and G. Aeppli, Adv. Mater., 2007, 19, 3618–3622 CrossRef CAS.
- A. S. Milev, N. Tran, G. S. Kamali Kannangara, M. A. Wilson and I. Avramov, J. Phys. Chem. C, 2008, 112, 5339–5347 CAS.
| This journal is © the Owner Societies 2014 |