Open Access Article
Open Access ArticleA face-sharing bi-icosahedral model for Al23−†
K.
Koyasu
ab and
T.
Tsukuda
*ab
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: tsukuda@chem.s.u-tokyo.ac.jp
bElements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, Katsura, Kyoto 615-8520, Japan
First published on 28th August 2014
Abstract
A face-sharing bi-icosahedral motif is proposed as a candidate structure of the magic cluster, Al23−, on the basis of DFT calculations. The structure can be viewed as a quasi-molecule made of two Al13 (D3d) superatoms with an open electronic configuration via constructive overlap of 1F and 2P superatomic orbitals. A face-sharing tri-icosahedral motif is also predicted for Al33−.
Introduction
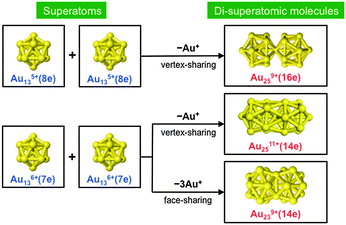
A cluster of simple metals can be viewed as a “superatom” since valence electrons confined in a jellium-like potential field are accommodated in a series of quantized orbitals. These superatomic orbitals (SAOs) are named 1S, 1P, 1D, 2S, 1F, 2P, 1G, 2D, 3S, 1H, 1I, etc., in order of energy, where S, P, D, F, G, H, and I represent the angular momenta, L = 0, 1, 2, 3, 4, 5, and 6, respectively.1 The superatom concept has been verified experimentally and successfully used to explain the magic numbers of clusters of s-electron (ref. 1) metals, such as Na (ref. 2) and Au,3,4 and trivalent Al.5 For example, the well-known magic cluster, Al13−, can be viewed as a superatom with an icosahedral motif and a closed electronic shell with 40 valence electrons.5 With the prospect that superatoms will serve as the building blocks of novel nanomaterials, considerable effort has been made to develop Au superatoms protected by ligands, such as thiolates (RS) and phosphines (PR3).6,7 For example, Au113+(8e)8,9 and Au135+(8e)10–13 superatoms protected by ligands have been isolated. Their high stability has been explained by an extended version of the superatomic concept that takes into account electron transfer from the Au cores to the ligands.14An interesting challenge is to construct a new class of pseudo-molecules from superatoms (superatomic molecules) by their direct connection or partial fusion.15 So far, three types of ligand-protected Au superatomic molecules with bi-icosahedral motifs have been synthesized (Chart 1). The Au259+(16e) core of [Au25(SR)5(PR′3)10Cl2]2+ comprises two Au135+(8e) superatoms with the closed electronic configuration (1S)2(1P)6 by sharing a vertex atom.16 The Au239+(14e) core of Au38(SR)24 (ref. 17) and the Au2511+(14e) core of Au25(SR)11 (ref. 18) are constructed from two Au136+(7e) superatoms with an open electronic configuration of (1S)2(1P)5 by sharing a facet and vertex, respectively. Yang and Cheng developed the super valence bond (SVB) model to explain the bonding of two superatoms in Au239+(14e) and found similarities with the bonding in F2.19,20 The production of poly-Au13 icosahedra with different bonding schemes has been theoretically proposed.21,22
In contrast, Al13-based superatomic molecules corresponding to those in Chart 1 have not been synthesized so far, although mass spectrometry has demonstrated the formation of a variety of superatomic complexes, including Al13Na,23 Al13I−,24 Al13X (X = H, Au, and alkali metals),25 Al13(OAl)n,26 Cs+B@Al12−, and F−P@Al12+.27 Assemblies of endohedral Al clusters, X@Al12–Y@Al12 (X–Y = Si–Si, B–P, Al–P), were predicted theoretically.28 The present work is focused on the structure of one of the magic clusters Al23−, which also exhibits high stability towards O2 like Al13−.5 Previous experimental and theoretical studies have proposed that Al23− has an octupolar-deformed shape (structure 2 in Fig. 1)29,30 and a closed electronic configuration. We propose using DFT calculations the possibility of a face-sharing bi-icosahedral motif for Al23− and the generalized bonding scheme of multiple Al13 units based on the structure of Al23−.
Calculation procedure
The fully optimized structures, formation energies, and SAO structures of face-sharing bi-icosahedral Al23− and tri-icosahedral Al33− were obtained at the B3LYP/6-311++G** level using the Gaussian09 package.31 As references, we also optimized the structures of Al23− with deformed octupolar29 and fcc-based motifs30 and Al13− with the icosahedral (Ih) motif. Frequency analysis of the obtained structures showed no imaginary values indicating that they are local minima in energy. The SAOs were visualized by using the Gaussview program with the isovalue of 0.020, otherwise noted. The nature of SAOs of the Al13− (D3d) moiety of bi-icosahedral Al23− (3) was studied by monitoring how the energies of the SAOs were changed during the optimization to Al13− (Ih).Results and discussion
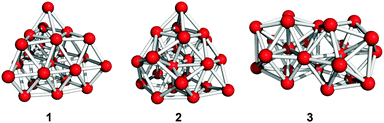
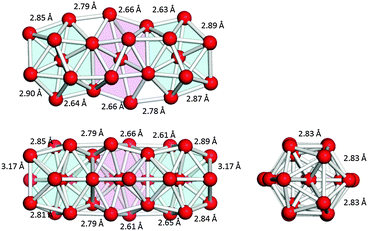
Fig. 1 shows the geometries of three optimized Al23− structures (1–3) in the singlet electronic state. Vibrational analysis confirmed that the geometries are local minima. The atomic coordinates of 1–3 are given in Tables S1–S3 (ESI†), respectively. Structures 1 and 2 are similar to structures reported previously.29,30 Newly-found structure 3 retains a face-sharing bi-icosahedral motif. Fig. 2 shows the structure of 3 in the third angle projection and the optimized structure of Al13− (Ih). The average Al–Al bond lengths at the joint (3.44 Å) and the facets at both ends (3.17 Å) are significantly elongated compared with that (2.82 Å) of Al13− (Ih). The symmetry of the Al13 moiety of 3 was reduced to D3d.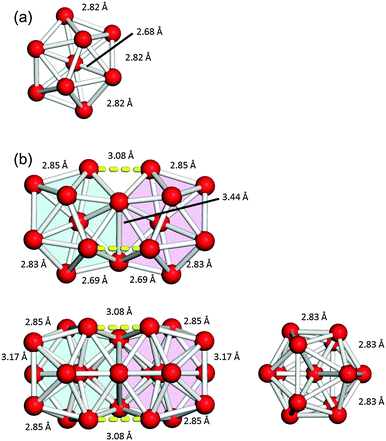 | ||
| Fig. 2 The structures of (a) Al13− (Ih) and (b) Al23− (3) in the third angle projection. Three bonds between the two Al13 units in 3 are shown by yellow dashed lines. | ||
Table 1 compares the relative stability of 1–3. Structures 1 and 2 have comparable stability,29 whereas structure 3 is less stable than 1 and 2 by 0.97 eV. Nevertheless, we cannot exclude the possibility that structure 3 is produced under certain experimental conditions given that structure 3 is isolated from 1 and 2 by significant energy barriers. Table 1 also lists the vertical detachment energy (VDE), which is defined as the energy difference between the neutral and anionic states at the optimized structures of the anion. The VDE values of 1–3 are comparable to the experimental value (3.57 eV).29
Energy diagrams of SAOs of 1–3 are shown in Fig. 3. Large HOMO–LUMO gaps for all the structures (Table 1) indicate that their high stability originates from their closed electronic shells. The electronic structures of 1 and 2 are similar and the energy diagrams of 1 and 2 indicate that they can be viewed as superatoms with nearly spherical morphologies and a closed electronic configuration of (1S)2(1P)6(1D)10(2S)2(1F)14(2P)6(1G)18(2D)10(3S)2. In contrast, the electronic structure of 3 is significantly different from those of 1 and 2. The occupied SAOs of 3 are named #116–#150 in order of energy (Fig. 3) and their shapes are visualized in Fig. S1 and S2 (ESI†). In the following, we attempt to interpret the electronic structure of 3 in the framework of the SVB model19 rather than that of a deformed (elongated) superatom. According to the SVB model,19 the face-sharing bi-icosahedral Al23− (3) is constructed from two Al134+(35e) superatoms with an open electronic configuration,
| Al23−(70e) = 2 × Al134+(35e) − 3Al3+. | (1) |
The physical meaning of removing 3Al3+ nuclei is the reduction of the electron confinement volume. To gain an insight into the bonding scheme between two Al134+ superatoms in Al23− (3), we consider how the SAOs of 3 are constructed from those of Al134+ by comparing their shapes. In the following, Al13− with the same geometry (D3d) as Al23− (3) was considered as the building unit, instead of Al134+. The nature of the SAOs of Al13− (D3d) was characterized by correlation with those of Al13− (Ih) (Fig. S3, ESI†), which was established by following the evolution of the SAOs over the course of structural optimization from Al13− (D3d) to Al13− (Ih) (Fig. S4–S6, ESI†).
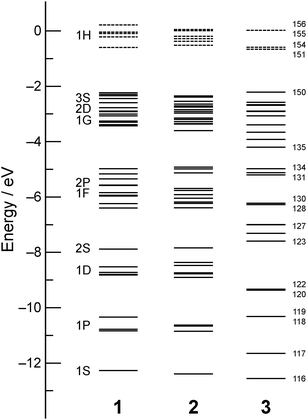 | ||
| Fig. 3 Energy diagram of SAOs of 1–3. The solid and dotted lines represent occupied and unoccupied orbitals, respectively. | ||
The correlation between the SAOs of 3 (#116–#150) and those of Al13− (D3d) is summarized in Table 2. The construction of the low-lying SAOs (Fig. S1, ESI†) is straightforward. For example, SAOs #116 and #117 are assigned to bonding and anti-bonding orbitals, respectively, constructed by interactions of SAO #66 of Al13− with 1S nature (Table 2 and Fig. S3, ESI†). SAOs #118–#134 and #143 are constructed from the SAO of Al13− with 1P, 1D, and 2S nature. The high-lying SAOs #135–#150 except #143 (Fig. S2, ESI†) are made from the SAOs of Al13− with 1F and 2P nature (Table 2 and Fig. S3, ESI†). In 3, SAOs #142, #145, and #146 with bonding nature are occupied whereas their anti-bonding counterparts #151, #155, and #156 are unoccupied (Table 2). The occupation of SAOs #142, #145, and #146 is responsible for the bonding interaction between the two Al13 superatoms. SAOs #142, #145, and #146 are constructed from the SAO of Al13− with 2P, 1F, and 1F nature, respectively, as shown in Fig. 4. The bond order (BO) for Al23− (3) is estimated to be 3 according to the definition in conventional VB theory. Note that the BO value for Al23− (3) is larger than that (BO = 1) of Au239+ (Chart 1).
| SAO of Al23− (3)a | SAO of Al13− (D3d)c | |
|---|---|---|
| Bonding | Anti-bonding | |
| a The SAOs in brackets are unoccupied orbitals. The notations in parentheses (1Σ, 2Σ, …) indicate orbital symmetries numbered in the order of energy in the same symmetry. b LUMO of Al23− (3). c The orbital symmetries shown in parentheses are those of the corresponding SAOs of Al13− (Ih) (Fig. S3, ESI). | ||
| 116 (1Σ) | 117 (2Σ) | 66 (1S) |
| 118, 119 (1Π) | 121, 122 (2Π) | 67, 68 (1Px,y) |
| 120 (3Σ) | 123 (4Σ) | 69 (1Pz) |
| 124, 125 (1Δ) | 128, 129 (2Δ) | 70, 71 (1D) |
| 126, 127 (3Π) | 132, 133 (4Π) | 72, 73 (1D) |
| 130 (5Σ) | 131 (6Σ) | 74 (2S) |
| 134 (7Σ) | 143 (9Σ) | 75 (1Dz) |
| 136, 137 (3Δ) | 149, 150 (4Δ) | 76, 77 (1F) |
| 135 (1Φ) | 141 (3Φ) | 78 (1F) |
| 138 (2Φ) | 144 (4Φ) | 79 (1F) |
| 139, 140 (5Π) | 147, 148 (7Π) | 80, 81 (2Px,y) |
| 145, 146 (6Π) | [155, 156] (8Π) | 82, 83 (1F) |
| 142 (8Σ) | [151]b (10Σ) | 84 (2Pz) |
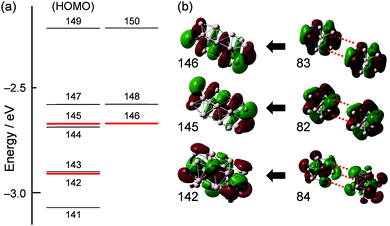 | ||
| Fig. 4 (a) Energy diagram of SAOs of Al23− (3). (b) Schematic representation of the bonding interaction between two SAOs of Al13− (D3d). | ||
We can rewrite eqn (1) as follows by considering Al13−(40e) as a superatomic unit,
| Al23−(70e) = [2 × Al13−(40e) − 3Al3+] − 10e = Al2311−(80e) − 10e. | (2) |
This indicates that two Al13−(40e) superatoms are bonded by sharing 10 electrons for each superatomic bond. Counterintuitively, the BO of Al23− (3) is not 5, but 3. This suggests that 8 and 2 electrons are removed from anti-bonding and bonding orbitals of a putative Al2311−(80e), respectively. By extending this idea, we can predict a series of sizes for the linear polymers of Al13(40e) which are connected by sharing the facet as follows,
| Al33−(100e) = 3 × Al13−(40e) − 2 × 3Al3+ − 2 × 10e | (3) |
| Al43−(130e) = 4 × Al13−(40e) − 3 × 3Al3+ − 3 × 10e | (4) |
Interestingly, it was reported that Al33− is stable against etching by O2.32 The stability of Al33−(100e) cannot be explained by the number of effective valence electrons since superatomic shell closure under spherical potential is accomplished at n* = 92 and 106. Thus, formation of a linear trimer is a possible explanation for the chemical stability of Al33−. To test this hypothesis, we conducted structural optimization of Al33− with a tri-icosahedral motif. We obtained a local minimum structure as shown in Fig. 5. The atomic coordinates of the optimized Al33− are given in Table S4 (ESI†).
Conclusions
DFT calculations allowed us to propose face-sharing bi- and tri-icosahedral motifs as possible structures of the magic clusters, Al23− and Al33−. Constructive overlap of 1F and 2P superatomic orbitals of deformed Al13 (D3d) units is responsible for the bonding of two Al13 superatoms. Fabrication of these unique Al clusters is a future challenge required to establish this building-up principle and to develop new functional materials with a ubiquitous element.Acknowledgements
This research was financially supported by the Funding Program for Next Generation World-Leading Researchers (NEXT Program) (GR-003), the “Elements Strategy Initiative for Catalysts & Batteries (ESICB),” supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank Dr Hironori Tsunoyama (Keio Univ.) and Ms Tomomi Watanabe (The Univ. of Tokyo) for their contribution at the initial stage of this research.Notes and references
- W. A. de Heer, Rev. Mod. Phys., 1993, 65, 611 CrossRef CAS.
- W. D. Knight, K. Clemenger, W. A. de Heer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Phys. Rev. Lett., 1984, 52, 2141 CrossRef CAS.
- K. J. Taylor, C. L. Pettiette-Hall, O. Cheshnovsky and R. E. Smalley, J. Chem. Phys., 1992, 96, 3319 CrossRef CAS PubMed.
- L.-M. Wang and L.-S. Wang, Nanoscale, 2012, 4, 4038 RSC.
- R. E. Leuchtner, A. C. Harms and A. W. Castleman, Jr., J. Chem. Phys., 1989, 91, 2753 CrossRef CAS PubMed.
- R. Jin, Nanoscale, 2010, 2, 343 RSC.
- P. Maity, S. Xie, M. Yamauchi and T. Tsukuda, Nanoscale, 2012, 4, 4027 RSC.
- Y. Yanagimoto, Y. Negishi, H. Fujihara and T. Tsukuda, J. Phys. Chem. B, 2006, 110, 11611 CrossRef CAS PubMed.
- B. S. Gutrath, U. Englert, Y. Wang and U. Simon, Eur. J. Inorg. Chem., 2013, 2002 CrossRef CAS PubMed.
- C. E. Briant, B. R. C. Theobald, J. W. White, L. K. Bell, D. M. P. Mingos and A. J. Welch, J. Chem. Soc., Chem. Commun., 1981, 201 RSC.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754 CrossRef CAS PubMed.
- M. C. Zhu, M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883 CrossRef CAS PubMed.
- Y. Shichibu and K. Konishi, Small, 2010, 6, 1216 CrossRef CAS PubMed.
- M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck and H. Häkkinen, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9157 CrossRef CAS PubMed.
- J. Nishigaki, K. Koyasu and T. Tsukuda, Chem. Rec. DOI:10.1002/tcr.201402011 , in press.
- Y. Shichibu, Y. Negishi, T. Watanabe, N. K. Chaki, H. Kawaguchi and T. Tsukuda, J. Phys. Chem. C, 2007, 111, 7845 CAS.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280 CrossRef CAS PubMed.
- J. Nishigaki, S. Yamazoe, S. Kohara, A. Fujiwara, W. Kurashige, Y. Negishi and T. Tsukuda, Chem. Commun., 2014, 50, 839 RSC.
- L. Cheng, C. Ren, J. Zhang and X. Yang, Nanoscale, 2013, 5, 1475 RSC.
- Y. Yuan, L. Cheng and J. Yang, J. Phys. Chem. C, 2013, 117, 13276 CAS.
- K. Nobusada and T. Iwasa, J. Phys. Chem. C, 2007, 111, 14279 CAS.
- D. Jiang, K. Nobusada, W. Luo and R. L. Whetten, ACS Nano, 2009, 3, 2351 CrossRef CAS PubMed.
- A. Nakajima, K. Hoshino, T. Naganuma, Y. Sone and K. Kaya, J. Chem. Phys., 1991, 95, 7061 CrossRef CAS PubMed.
- D. E. Bergeron, P. J. Roach, A. W. Castleman, Jr., N. O. Jones and S. N. Khanna, Science, 2005, 307, 231 CrossRef CAS PubMed.
- Y. J. Ko, A. Shakya, H. Wang, A. Grubisic, W. Zheng, M. Götz, G. Ganteför, K. H. Bowen, P. Jena and B. Kiran, J. Chem. Phys., 2010, 133, 124308 CrossRef PubMed.
- T. Watanabe and T. Tsukuda, J. Phys. Chem. C, 2013, 117, 6664 CAS.
- M. Akutsu, K. Koyasu, J. Atobe, N. Hosoya, K. Miyajima, M. Mitsui and A. Nakajima, J. Phys. Chem. A, 2006, 110, 12073 CrossRef CAS PubMed.
- T. Iwasa and A. Nakajima, J. Phys. Chem. C, 2013, 117, 21551 CAS.
- J. Akola, M. Manninen, H. Häkkinen, U. Landman, X. Li and L.-S. Wang, Phys. Rev. B, 2000, 62, 13216 CrossRef CAS.
- A. Aguado and J. M. López, J. Chem. Phys., 2009, 130, 064704 CrossRef PubMed.
- M. J. Frisch, et al., Gaussian 09, Revision C.01 Search PubMed, (see ESI† for full citation).
- R. L. Hettich, J. Am. Chem. Soc., 1989, 111, 8582 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full citation of ref. 31, full list of atomic coordinates and orbital energy levels with SAOs of the three isomers of Al23− and Al33−, and the energy diagram with SAOs of distorted Al13−. See DOI: 10.1039/c4cp03199g |
| This journal is © the Owner Societies 2014 |