Excimer formation in cofacial and slip-stacked perylene-3,4:9,10-bis(dicarboximide) dimers on a redox-inactive triptycene scaffold†
Abstract
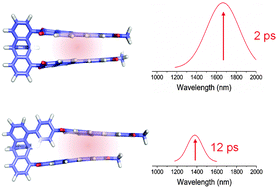
Excitation energy transfer in perylene-3,4:9,10-bis(dicarboximide) (PDI) aggregates is of interest for light-harvesting applications of this strongly absorbing and π–π stacking chromophore. Here we report the synthesis and characterization of two PDI dimers in which the chromophores are covalently linked by a redox-inactive triptycene bridge in orientations that are cofacial (1) and slip-stacked along their N–N axes (2). Femtosecond transient absorption experiments on 1 and 2 reveal rapid exciton delocalization resulting excimer formation. Cofacial π–π stacked dimer 1 forms a low-energy excimer state absorption (λmax = 1666 nm) in τ = ∼2 ps after photoexcitation. Inserting a phenyl spacer on the bridge to generate a slip-stacked PDI–PDI geometry in 2 results in a less stable excimer state (λmax = 1430 nm), which forms in τ = ∼12 ps due to decreased electronic coupling. The near-infrared (NIR) excimer absorption of cofacial dimer 1 is ∼120 meV lower in energy than that of slip-stacked dimer 2, further highlighting electronic differences between these states.

 Please wait while we load your content...
Please wait while we load your content...